Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft
Abstract
:1. Introduction
2. Results
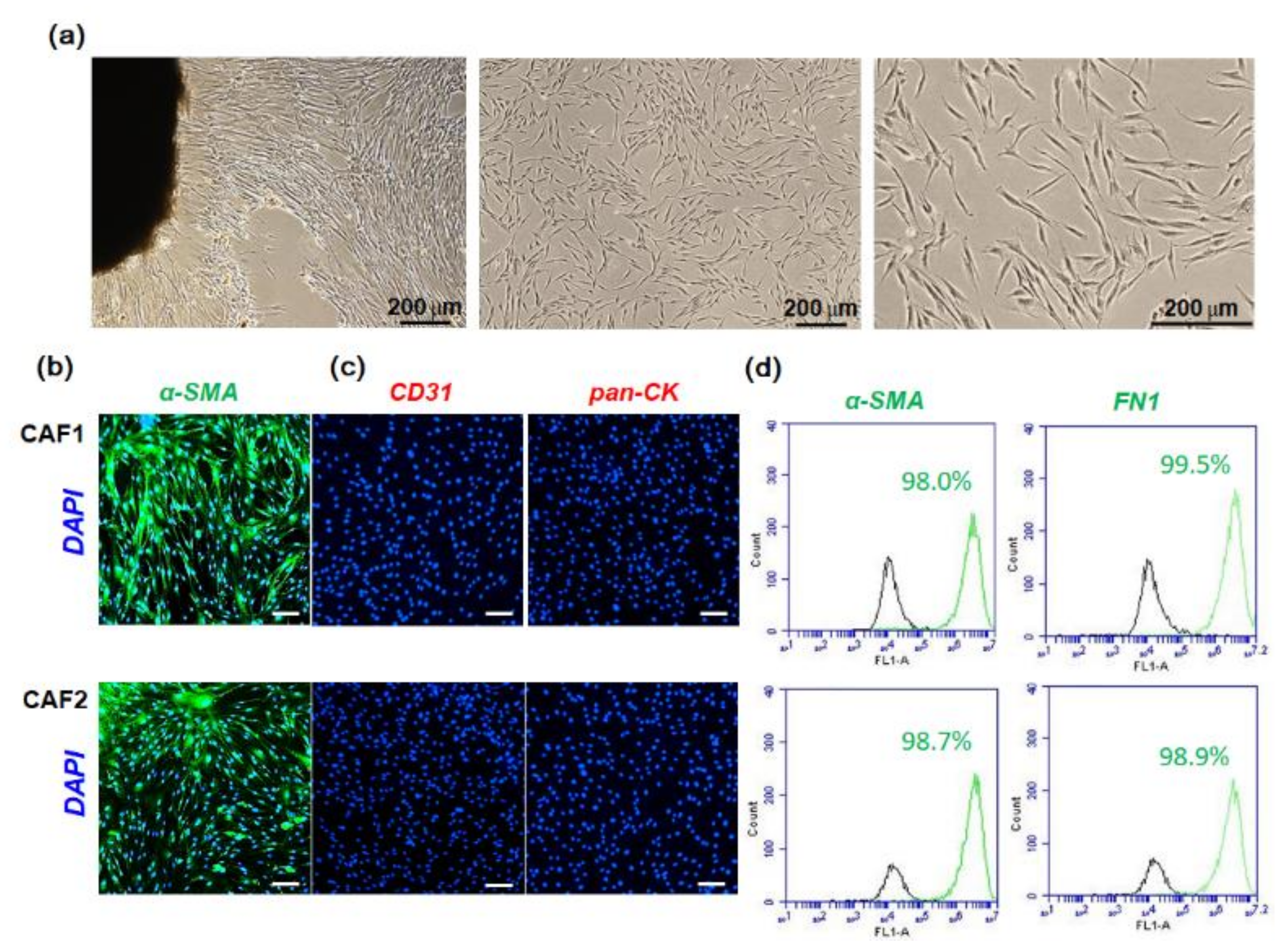
2.1. Characterization of Fibroblast Cells Cultured from HNSCC Tissues
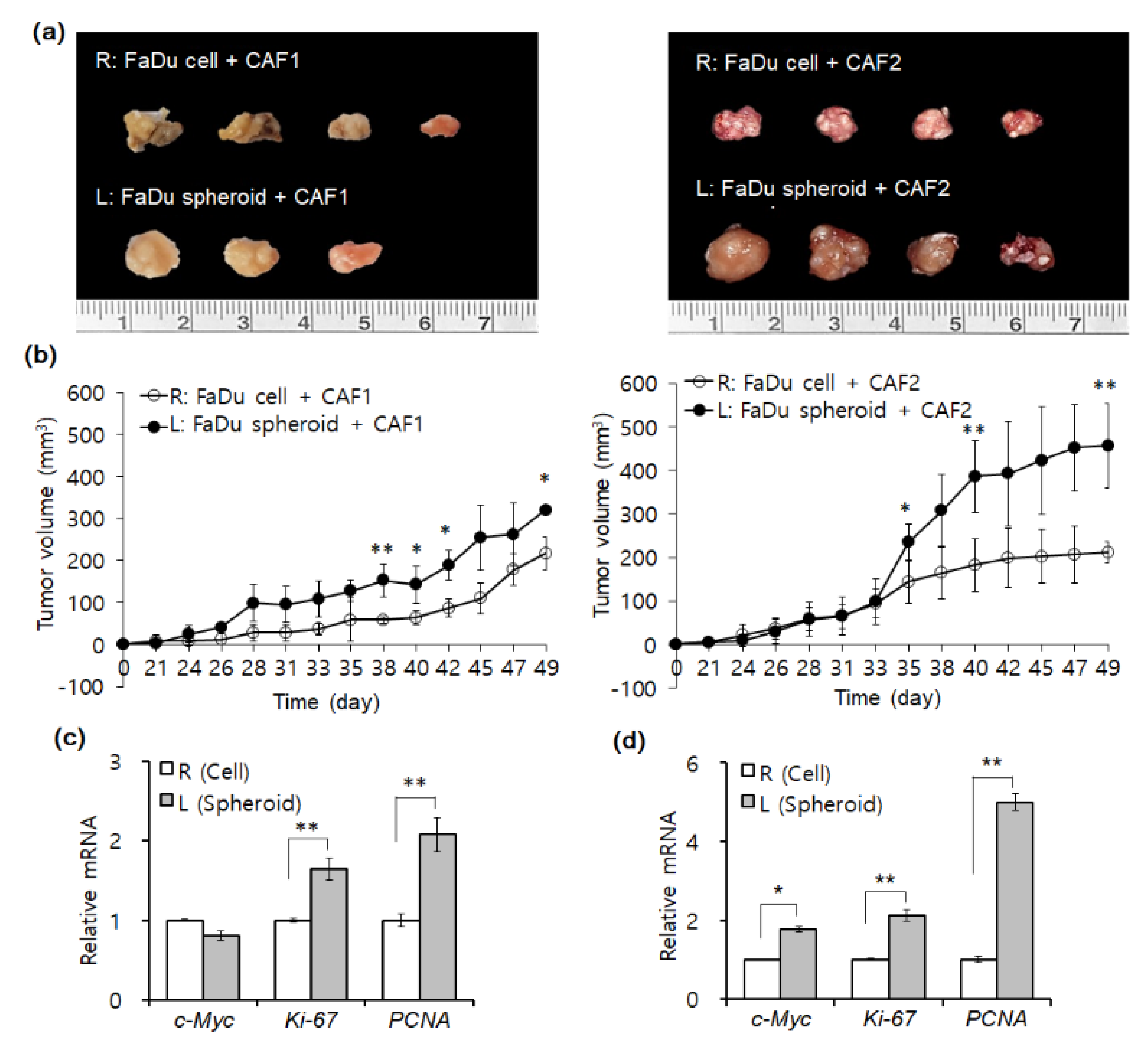
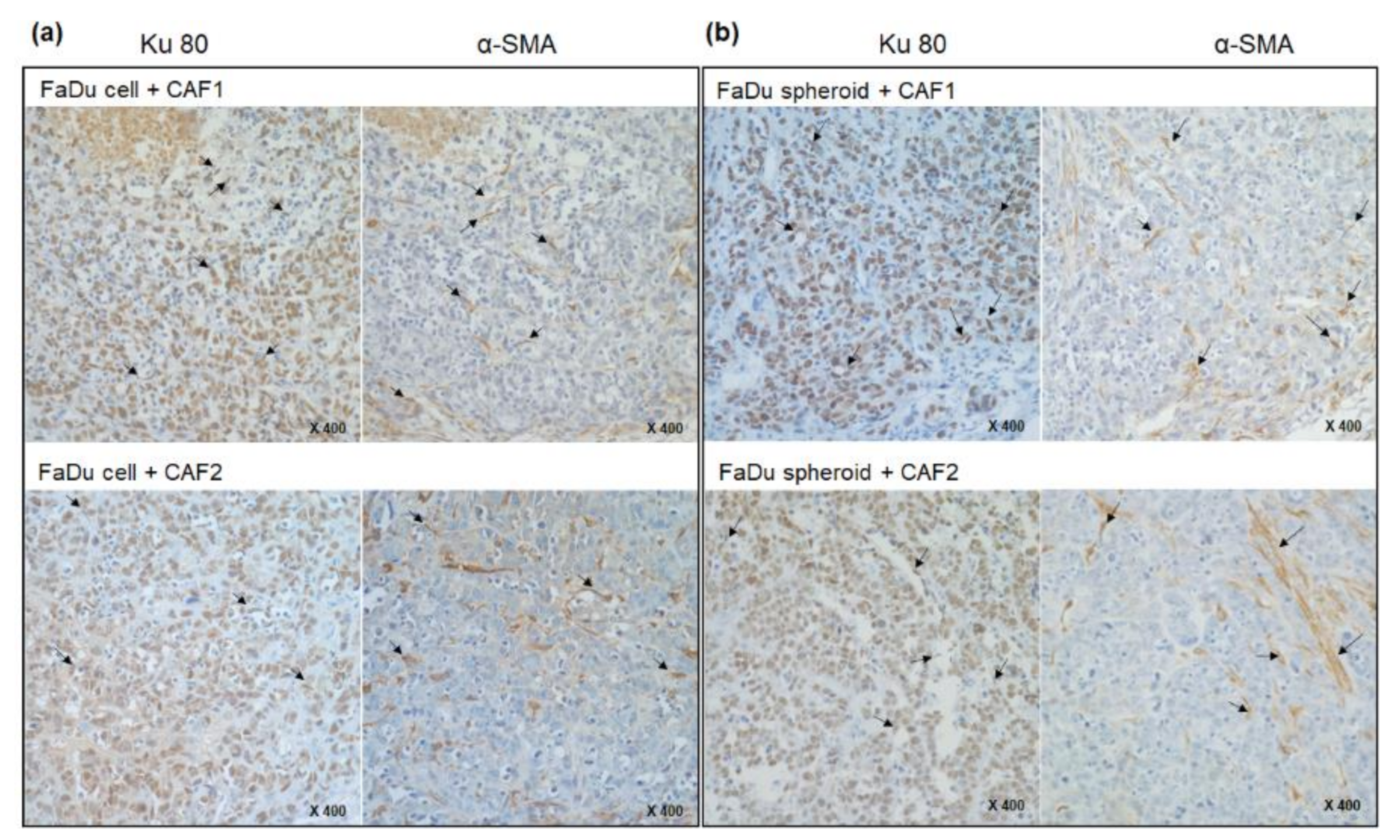
2.2. Mouse Tumorigenesis from FaDu Monolayer Cells and 3D Spheroids
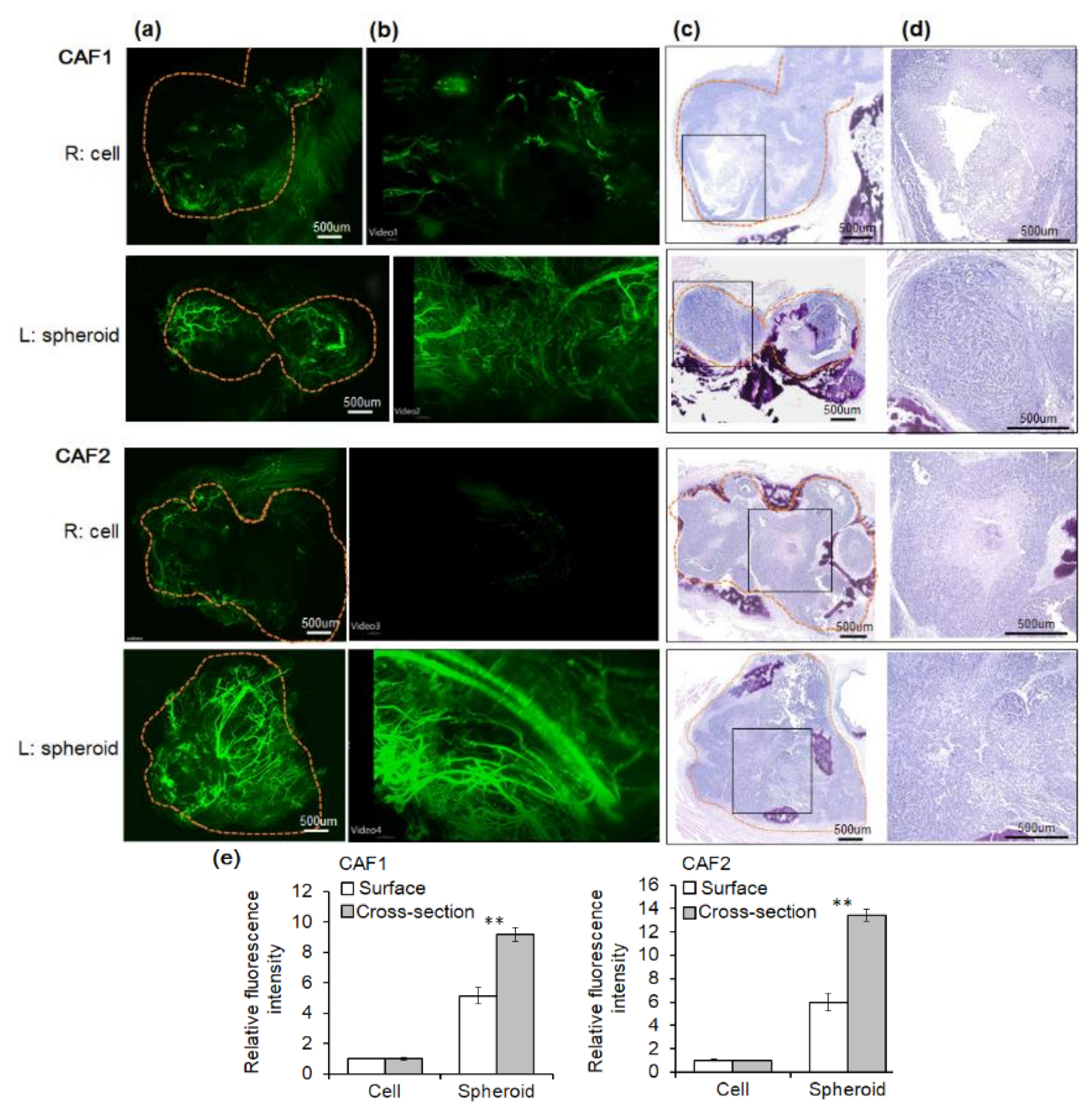
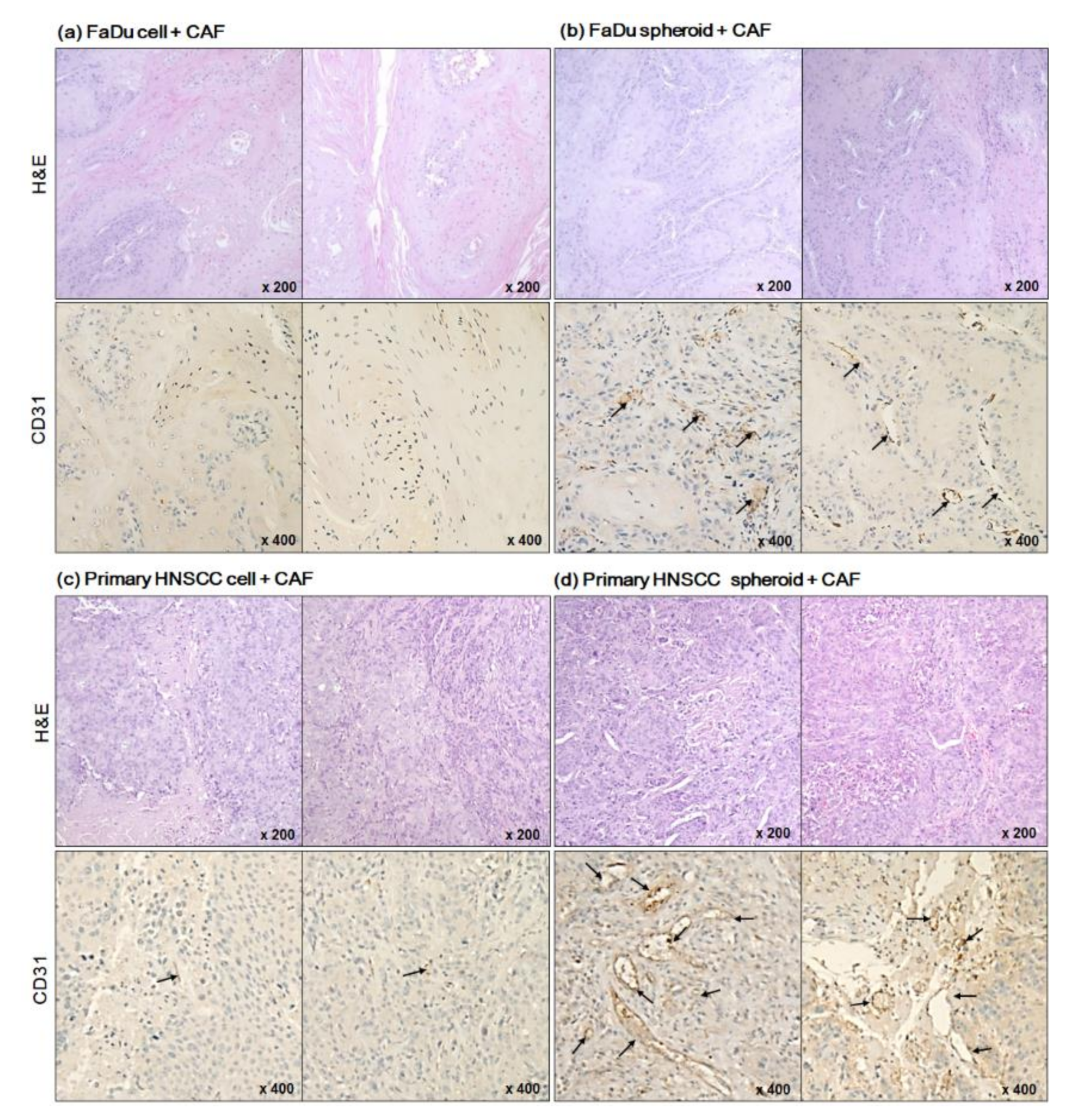
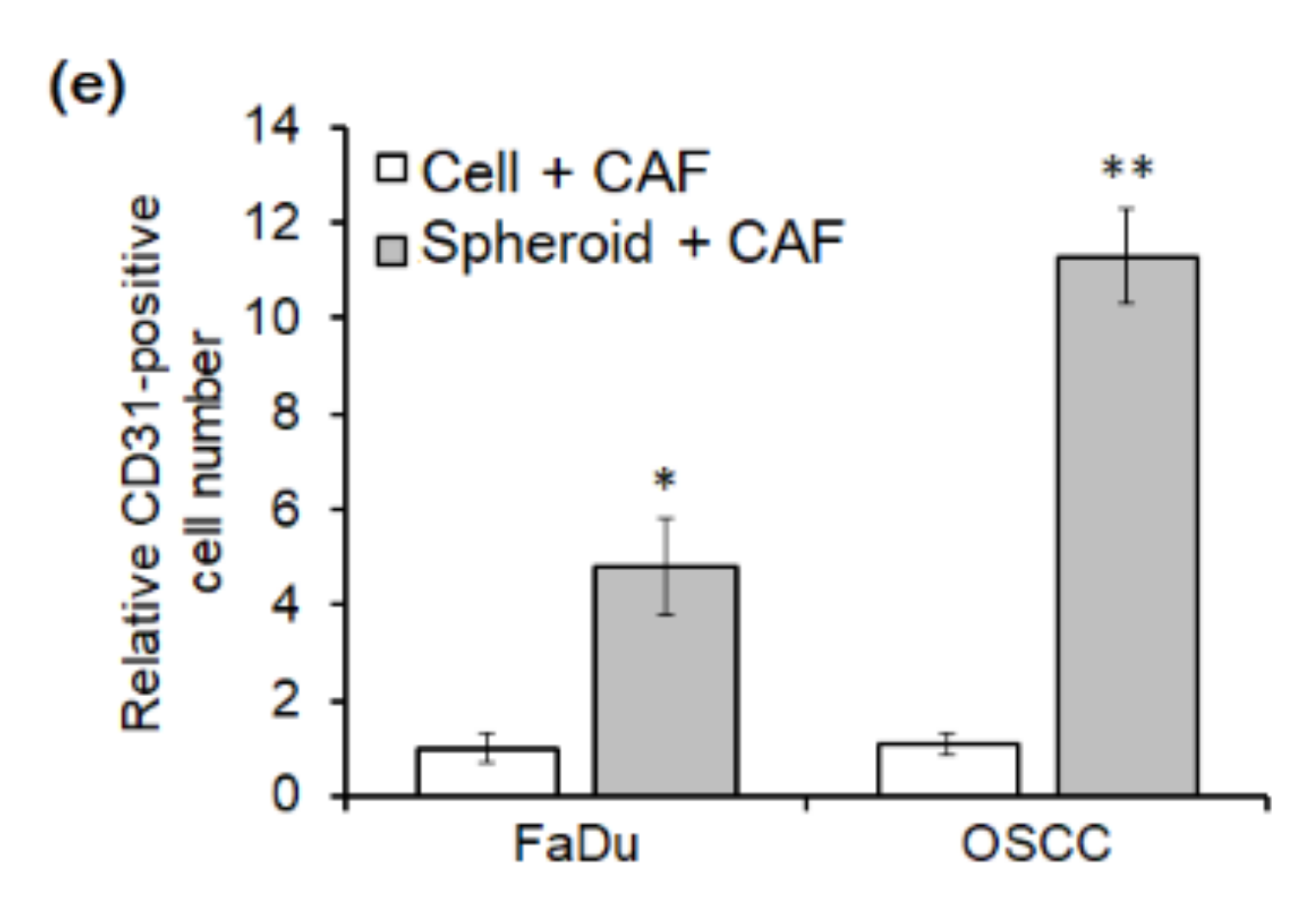
2.3. Angiogenesis of Mouse Xenograft from FaDu Monolayer Cells and 3D Spheroids
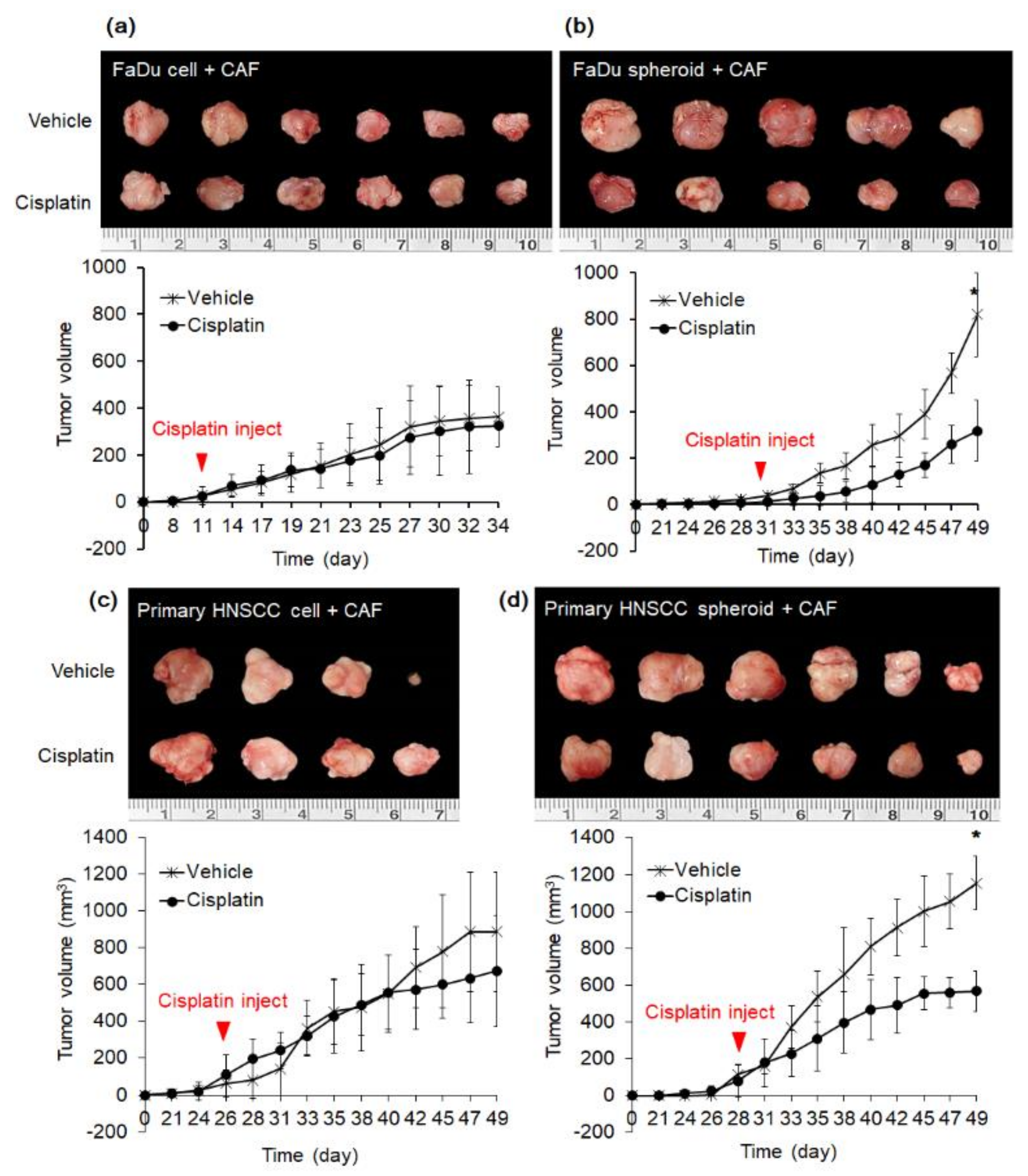
2.4. Effect of Cisplatin on Mice Tumor from FaDu and Primary HNSCC Cells
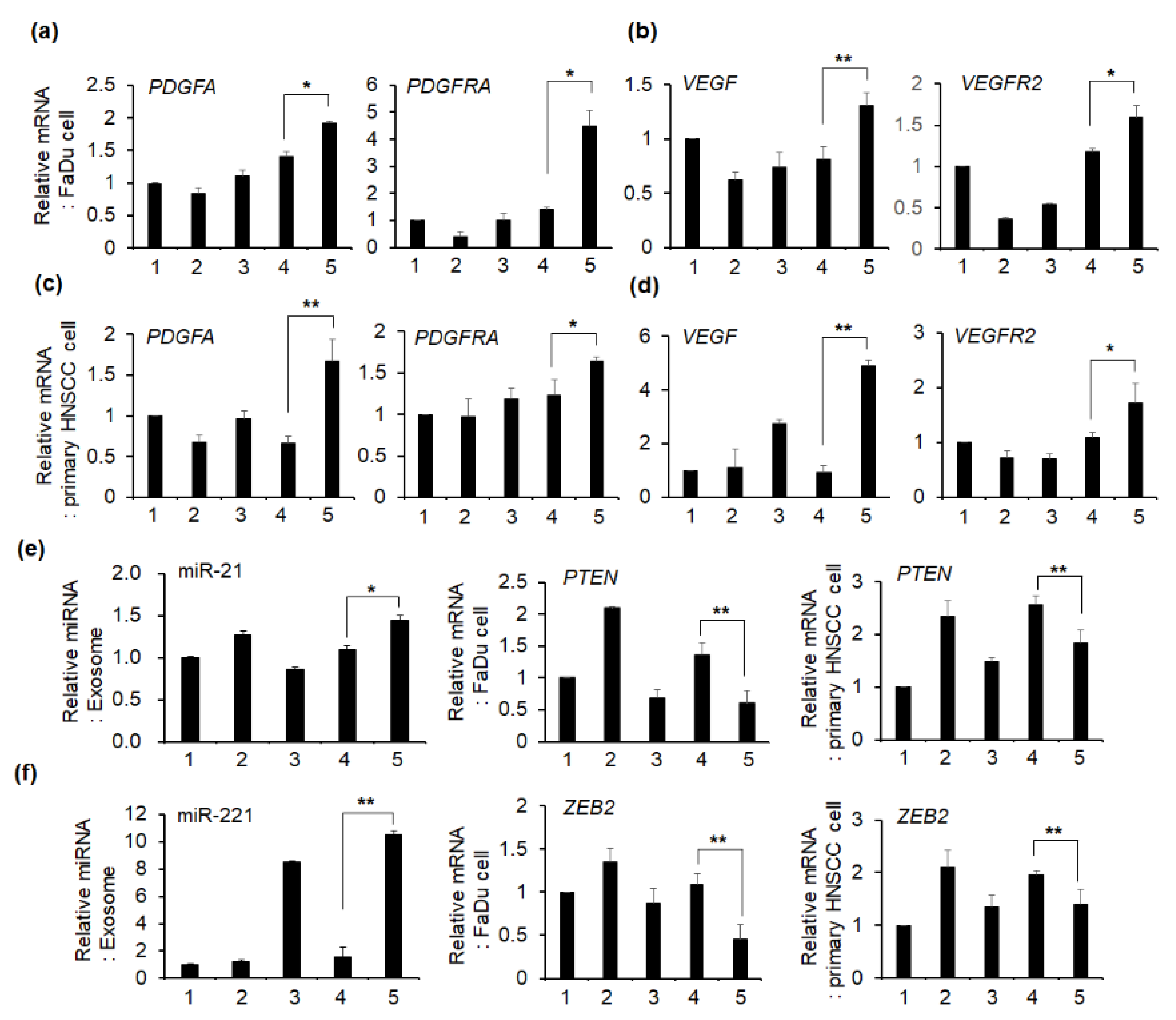
2.5. Effect of Exosomes on the mRNA Expression of Angiogenesis-Related Genes in HNSCC Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Fibroblast Primary Culture from Fresh HNSCC Tissues
4.3. FaDu and Primary oral Squamous Carcinoma Cell Culture and Spheroid Formation
4.4. Mouse Xenograft Model
4.5. Tissue Clearing and Blood Vessel Staining
4.6. Real-Time Polymerase Chain Reaction (qPCR) of mRNA and miRNA
4.7. Cisplatin Effect on Mice Tumor Derived from FaDu and HNSCC Primary Cell
4.8. Exosome Isolation and Characterization
4.9. Effect of Exosome on the mRNA Expression Related to Blood Vessel Formation in Cancer Cells
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Galitis, E.; Droukas, V.; Tzakis, M.; Psarras, V.; Galiti, D.; Kyrodimos, E.; Trichas, M.; Psyrri, A.; Papadogeorgakis, N.; Kouri, M.; et al. Trismus and Reduced Quality of Life in Patients With Oral Squamous Cell Carcinoma, Who Received Post-Operative Radio-Therapy Alone or Combined With Chemotherapy. J. Clin. Oncol. 2017, 8, 29–36. [Google Scholar]
- Maggiore, R.; Zumsteg, Z.S.; BrintzenhofeSzoc, K.; Trevino, K.M.; Gajra, A.; Korc-Grodzicki, B.; Epstein, J.B.; Bond, S.M.; Parker, I.; Kish, J.A.; et al. The Older Adult With Locoregionally Advanced Head and Neck Squamous Cell Carcinoma: Knowledge Gaps and Future Direction in Assessment and Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 868–883. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, E.M.F.; Vermeulen, L.; Fessler, E.; Medema, J.P. Cancer Heterogeneity–A Multifaceted View. EMBO Rep. 2013, 14, 686–695. [Google Scholar]
- Albini, A.; Sporn, M.B. The Tumour Microenvironment as a Target for Chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef]
- Tomes, L.; Emberley, E.; Niu, Y.; Troup, S.; Pastorek, J.; Strange, K.; Harris, A.; Watson, P.H. Necrosis and Hypoxia in Invasive Breast Carcinoma. Breast Cancer Res. Treat. 2003, 81, 61–69. [Google Scholar] [CrossRef]
- Lekshmi, A.; Varadarajan, S.N.; Lupitha, S.S.; Indira, D.; Mathew, K.A.; Nair, A.C.; Nair, M.; Prasad, T.; Sekar, H.; Gopalakrishnan; et al. A Quantitative Real-Time Approach for Discriminating Apoptosis and Necrosis. Cell Death Discov. 2017, 3, 16101. [Google Scholar] [CrossRef] [Green Version]
- Ismerim, A.B.; Xavier, F.C.A.; Cangussu, M.C.T.; Ramalho, L.M.P.; Agra, I.M.G.; Santos, J.N.D. Useful Histological Findings in Incisional Biopsies of Oral Squamous Cell Carcinoma. Srp. Arh. Celok. Lek. 2016, 144, 384–390. [Google Scholar] [CrossRef]
- Pires, F.R.; Ramos, A.B.; Oliveira, J.B.; Tavares, A.S.; Luz, P.S.; Santos, T.C. Oral Squamous Cell Carcinoma: Clinicopathological Features From 346 Cases From a Single Oral Pathology Service During an 8-Year Period. J. Appl. Oral. Sci. 2013, 21, 460–467. [Google Scholar] [CrossRef]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.S.; Ho, M.; Takayama, S. High-Throughput 3D Spheroid Culture and Drug Testing Using a 384 Hanging Drop Array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Leidig, T.; Rodday, B.; Lindemann, C.; Mueller-Klieser, W. Test System for Trifunctional Antibodies in 3D MCTS Culture. J. Biomol. Screen. 2009, 14, 980–990. [Google Scholar] [CrossRef] [Green Version]
- Massa, A.; Varamo, C.; Vita, F.; Tavolari, S.; Peraldo-Neia, C.; Brandi, G.; Rizzo, A.; Cavalloni, G.; Aglietta, M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers 2020, 12, 2308. [Google Scholar] [CrossRef]
- Jung, H.R.; Kang, H.M.; Ryu, J.W.; Kim, D.S.; Noh, K.H.; Kim, E.S.; Lee, H.J.; Chung, K.S.; Cho, H.S.; Kim, N.S.; et al. Cell Spheroids with Enhanced Aggressiveness to Mimic Human Liver Cancer In Vitro and In Vivo. Sci. Rep. 2017, 7, 10499. [Google Scholar]
- Bhowmick, N.A.; Neilson, E.G.; Moses, L.H. Stromal Fibroblasts in Cancer Initiation and Progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Sadlonova, A.; Bowe, D.B.; Novak, Z.; Mukherjee, S.; Duncan, V.E.; Page, G.P.; Frost, A.R. Identification of Molecular Distinctions Between Normal Breast-Associated Fibroblasts and Breast Cancer-Associated Fibro-Blasts. Cancer Microenviron. 2009, 2, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, A.; Osawa, Y.; Nagaki, M.; Saji, S.; Moriwaki, H.; Bouvet, M.; Hoffman, R.M. Identification of Molecular Distinctions Between Normal Breast-Associated Fibroblasts and Breast Cancer-Associated Fibro-Blasts. J. Cell. Biochem. 2011, 112, 949–953. [Google Scholar] [CrossRef]
- Olumi, A.F.; Grossfeld, G.D.; Hayward, S.W.; Carroll, P.R.; Tlsty, T.D.; Cunha, G.R. Carcinoma-Associated Fibroblasts Direct Tumor Progression of Initiated Human Prostatic Epithelium. Cancer Res. 1999, 59, 5002–5011. [Google Scholar]
- Peltanova, B.; Liskova, M.; Gumulec, J.; Raudenska, M.; Polanska, H.H.; Vaculovic, T.; Kalfert, D.; Grega, M.; Plzak, J.; Betka, J.; et al. Sensitivity to Cisplatin in Head and Neck Cancer Cells Is Significantly Affected by Patient-Derived Cancer-Associated Fi-Broblasts. Int. J. Mol. Sci. 2021, 22, 1912. [Google Scholar] [CrossRef]
- Liao, J.K.; Zhou, B.; Zhuang, X.M.; Zhuang, P.L.; Zhang, D.M.; Chen, W.L. Cancer-Associated Fi Broblasts Confer Cisplatin Resistance of Tongue Cancer via Autophagy Activation. Biomed. Pharmacother. 2018, 97, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Molecular Biomarkers of Cancer Stem/Progenitor Cells Associated With Progression, Metastases, and Treatment Resistance of Aggressive Cancers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 234–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, N.; Yerneni, S.S.; Razzo, B.M.; Whiteside, T.L. Exosomes from HNSCC Promote Angiogenesis through Reprogramming of Endothelial Cells. Mol. Cancer Res. 2018, 16, 1798–1808. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Guo, H.; Wang, X.; Zhu, X.; Yan, M.; Wang, X.; Xu, Q.; Shi, J.; Lu, E.; Chen, W.; et al. Exosomal miR-196a Derived From Cancer-Associated Fibroblasts Confers Cisplatin Resistance in Head and Neck Cancer Through Targeting CDKN1B and ING5. Genome Biol. 2019, 20, 12. [Google Scholar] [CrossRef]
- Micke, P.; Ostman, A. Exploring the Tumour Environment: Cancer-Associated Fibroblasts As Targets in Cancer Therapy. Expert. Opin. Ther. Targets 2005, 9, 1217–1233. [Google Scholar] [CrossRef]
- Liu, L.Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.F.; Lai, L.; Jiang, B.H. MiR-21 Induced Angiogenesis Through AKT and ERK Activation and HIF-1alpha Expression. PLoS ONE 2011, 6, e19139. [Google Scholar]
- Chen, Y.; Banda, M.; Speyer, C.L.; Smith, J.S.; Rabson, A.B.; Gorski, D.H. Regulation of the Expression and Activity of the Antiangiogenic Homeobox Gene GAX/MEOX2 by ZEB2 and microRNA-221. Mol. Cell. Biol. 2010, 30, 3902–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, D.; Addadi, Y.; Kalchenko, V.; Harmelin, A.; Kunz-Schughart, L.A.; Neeman, M. In Vivo Imaging of the Systemic Recruitment of Fibroblasts to the Angiogenic Rim of Ovarian Carcinoma Tumors. Cancer Res. 2007, 67, 9180–9189. [Google Scholar] [CrossRef] [Green Version]
- Aref, A.R.; Huang, R.Y.; Yu, W.; Chua, K.N.; Sun, W.; Tu, T.Y.; Bai, J.; Sim, W.J.; Zervantonakis, I.K.; Thiery, J.P.; et al. Screening Therapeutic EMT Blocking Agents in a Three-Dimensional Microenvironment. Integr. Biol. 2013, 5, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Majety, M.; Pradel, L.P.; Gies, M.; Ries, C.H. Fibroblasts Influence Survival and Therapeutic Response in a 3D Co-Culture Model. PLoS ONE 2015, 10, e0127948. [Google Scholar] [CrossRef]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Condron, C.M.; Toomey, D. Tumour Hypoxia, Chemotherapeutic Resistance and Hypoxia-Related Therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef]
- Meijer, T.W.; Kaanders, J.H.; Span, P.N.; Bussink, J. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism To Improve Radiotherapy Efficacy. Clin. Cancer Res. 2012, 18, 5585–5594. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Kwak, H.S.; Kang, M.H.; Park, Y.Y.; Jeong, G.S. Fibroblast-Associated Tumour Microenvironment Induces Vascular Structure-Networked Tumouroid. Sci. Rep. 2018, 8, 2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilad, A.A.; Israely, T.; Dafni, H.; Meir, G.; Cohen, B.; Neeman, M. Functional and Molecular Mapping of Uncoupling Between Vascular Permeability and Loss of Vascular Maturation in Ovari-An Carcinoma Xenografts: The Role of Stroma Cells in Tumor Angiogenesis. Int. J. Cancer 2005, 117, 202–211. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.Q.; Liu, J.L.; Tian, L. Exosomes in Tumor Microenvironment: Novel Transporters and Biomarkers. J. Transl. Med. 2016, 14, 297. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, Y.; Zou, L.; Zhu, Z. Role of Exosomes in Crosstalk Between Cancer-Associated Fibroblasts and Cancer Cells. Front Oncol. 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L. Potential Roles of Tumor-Derived Exosomes in Angiogenesis. Expert Opin. Ther. Targets 2018, 22, 409–417. [Google Scholar] [CrossRef]
- Giusti, I.; Monache, S.D.; di Francesco, M.; Sanita, P.; D’Ascenzo, S.; Gravina, G.L.; Festuccia, C.; Dolo, V. From Glioblastoma to Endothelial Cells Through Extracellular Vesicles: Messages for Angiogenesis. Tumour Biol. 2016, 37, 12743–12753. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Tai, S.K.; Yang, M.H. Snail-Overexpressing Cancer Cells Promote M2-Like Polarization of Tumor-Associated Macrophages by Delivering MiR-21-Abundant Exosomes. Neoplasia 2018, 20, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, T.; He, R.; Ren, J.; Yao, H.; Wang, K.; Zhu, L.; Xu, Y. Exosomes From 3D Culture of Marrow Stem Cells Enhances Endothelial Cell Proliferation, Migration, and Angiogenesis via Activation of the HMGB1/AKT Pathway. Stem Cell Res. 2020, 50, 102122. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.; Li, K.; Lopez, X.M.; Blanchard, S.; Barbot, P.; Rorive, S.; Decaestecker, C.; Pochet, R.; Bohl, D.; Lepore, A.C.; et al. Immunohistochemical Toolkit for Tracking and Quantifying Xenotransplanted Human Stem Cells. Regen. Med. 2014, 9, 437–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kang, H.S.; Lee, Y.J.; Lee, H.J.; Yun, J.; Shin, J.H.; Lee, C.W.; Kwon, B.M.; Hong, S.H. EGR1-Dependent PTEN Upregulation by 2-Benzoyloxycinnamaldehyde Attenuates Cell Invasion and EMT in Colon Can-Cer. Cancer Lett. 2014, 349, 35–44. [Google Scholar] [CrossRef] [PubMed]
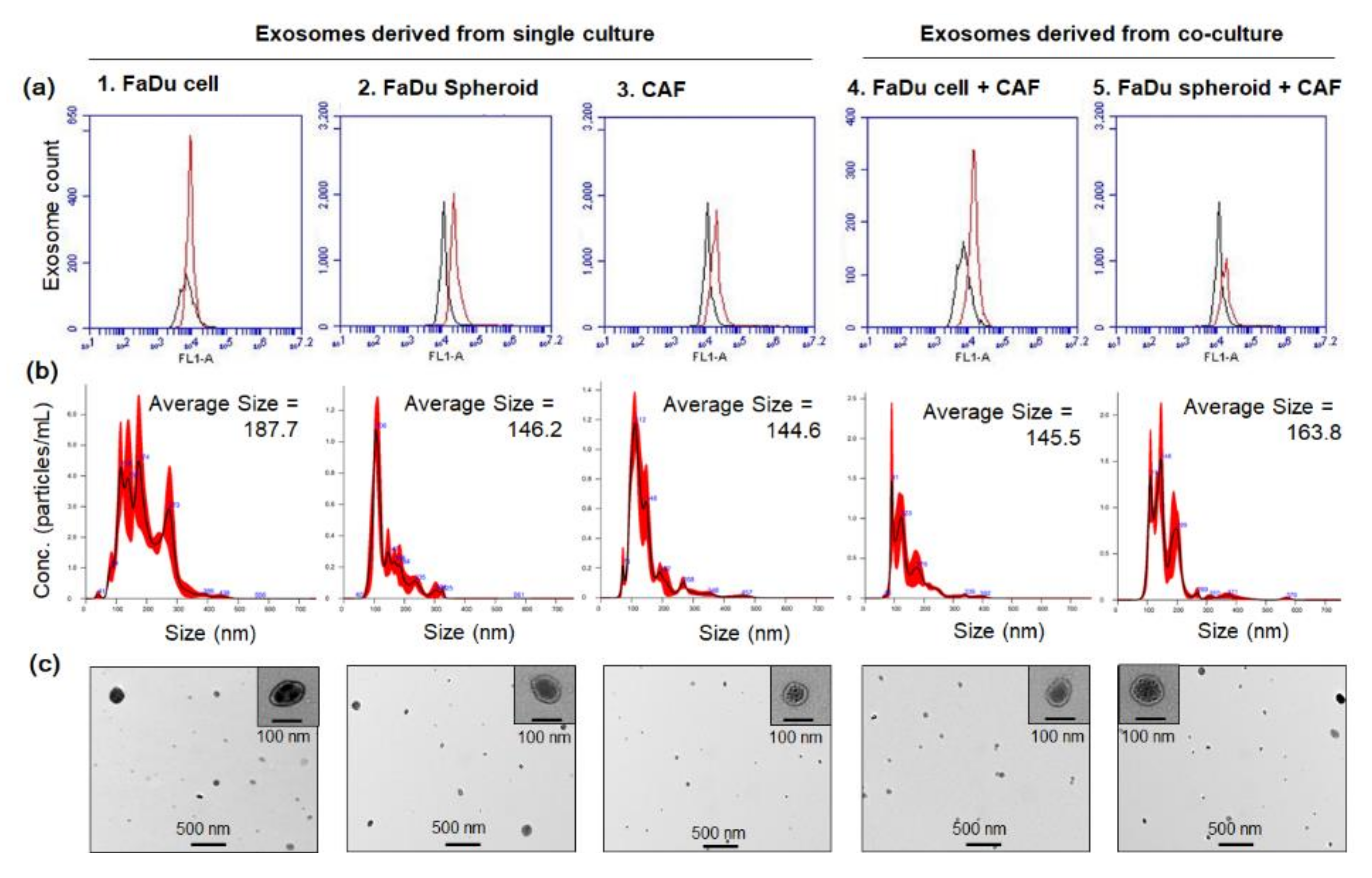
| Exosome Isolated from | Concentration (Particles/mL/2 × 106 Cells) | p-Value | ||
|---|---|---|---|---|
| Single culture | CAF | 1.50 × 108 ± 1.88 × 107 | ||
| FaDu cell | 6.29× 108 ± 4.78 × 107 | p > 0.05 | p < 0.05 | |
| FaDu spheroid | 5.47 × 108± 8.84 × 107 | |||
| Co-culture | FaDu cell + CAF | 8.21 × 108± 9.52 × 107 | p < 0.05 | |
| FaDu spheroid + CAF | 11.1 × 108 ± 1.13 × 107 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-Y.; Kang, S.H.; Oh, S.Y.; Lee, K.Y.; Lee, H.-J.; Gum, S.; Kwon, T.-G.; Kim, J.-W.; Lee, S.-T.; Hong, Y.J.; et al. Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. Int. J. Mol. Sci. 2021, 22, 8245. https://doi.org/10.3390/ijms22158245
Choi S-Y, Kang SH, Oh SY, Lee KY, Lee H-J, Gum S, Kwon T-G, Kim J-W, Lee S-T, Hong YJ, et al. Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. International Journal of Molecular Sciences. 2021; 22(15):8245. https://doi.org/10.3390/ijms22158245
Chicago/Turabian StyleChoi, So-Young, Soo Hyun Kang, Su Young Oh, Kah Young Lee, Heon-Jin Lee, Sangil Gum, Tae-Geon Kwon, Jin-Wook Kim, Sung-Tak Lee, Yoo Jin Hong, and et al. 2021. "Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft" International Journal of Molecular Sciences 22, no. 15: 8245. https://doi.org/10.3390/ijms22158245
APA StyleChoi, S.-Y., Kang, S. H., Oh, S. Y., Lee, K. Y., Lee, H.-J., Gum, S., Kwon, T.-G., Kim, J.-W., Lee, S.-T., Hong, Y. J., Kim, D.-G., & Hong, S.-H. (2021). Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. International Journal of Molecular Sciences, 22(15), 8245. https://doi.org/10.3390/ijms22158245








