Copresence of High-Risk Human Papillomaviruses and Epstein–Barr Virus in Colorectal Cancer: A Tissue Microarray and Molecular Study from Lebanon
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Characteristics of the Cohort
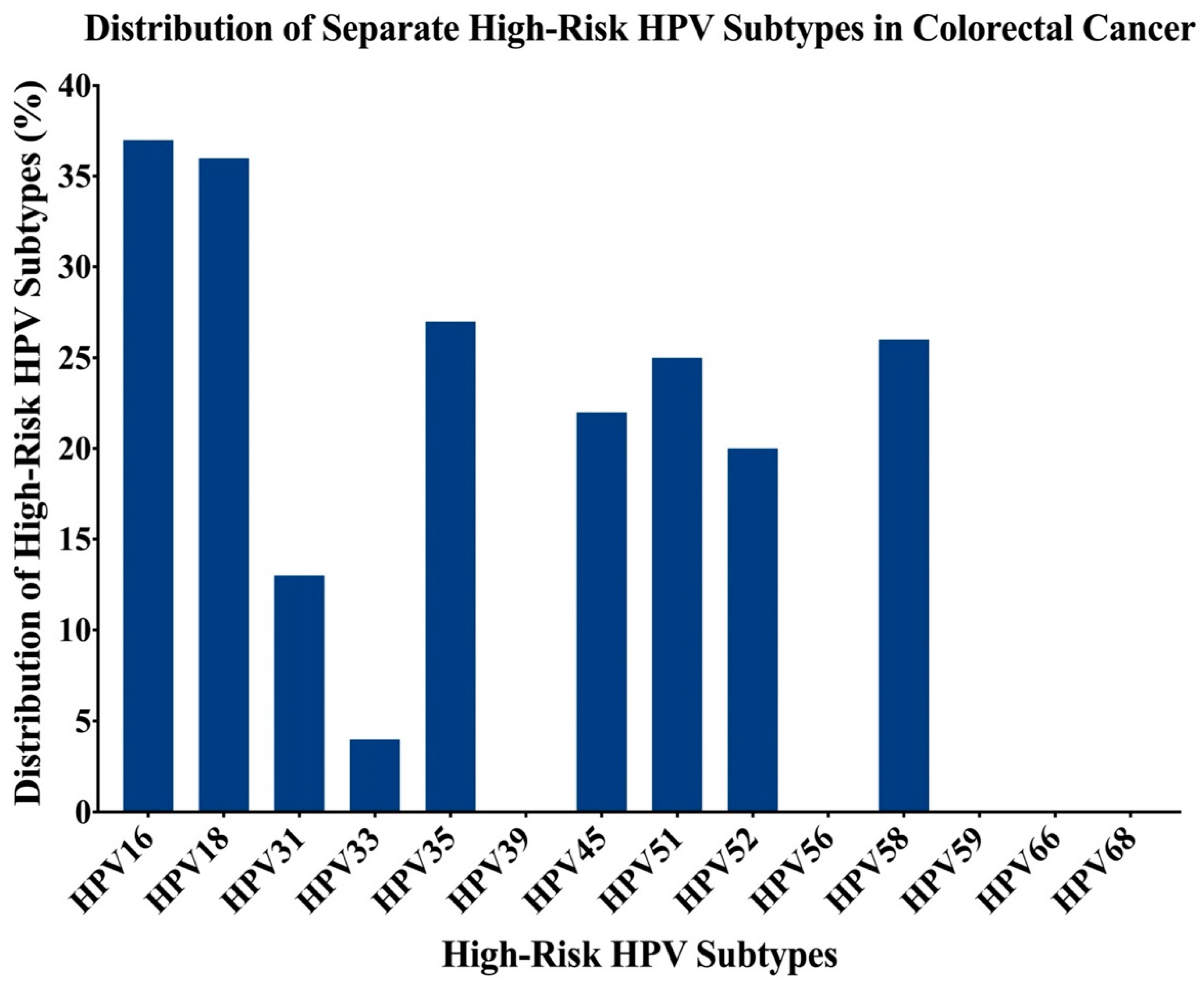
2.2. Presence of High-Risk HPV Subtypes and EBV by PCR
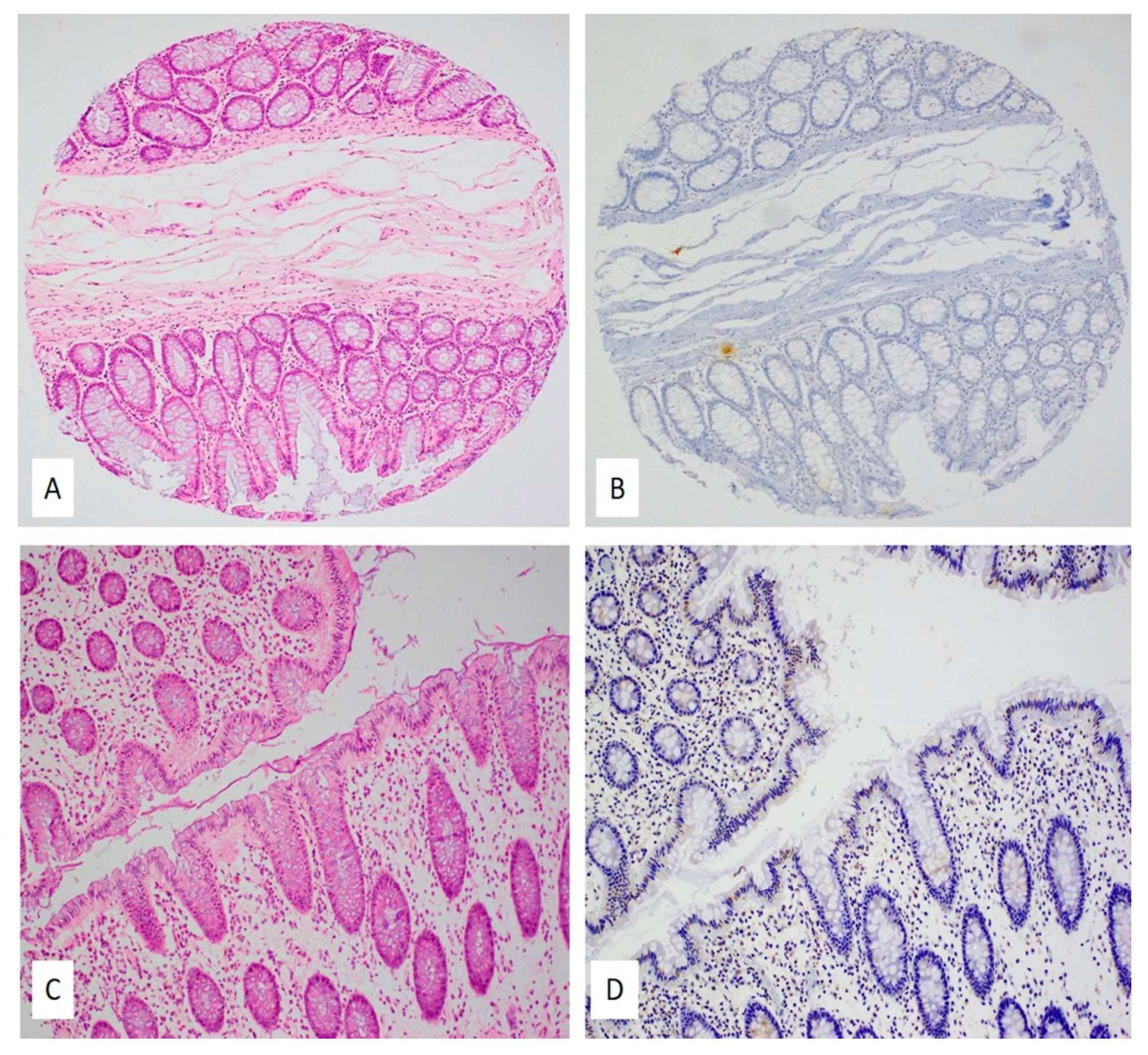
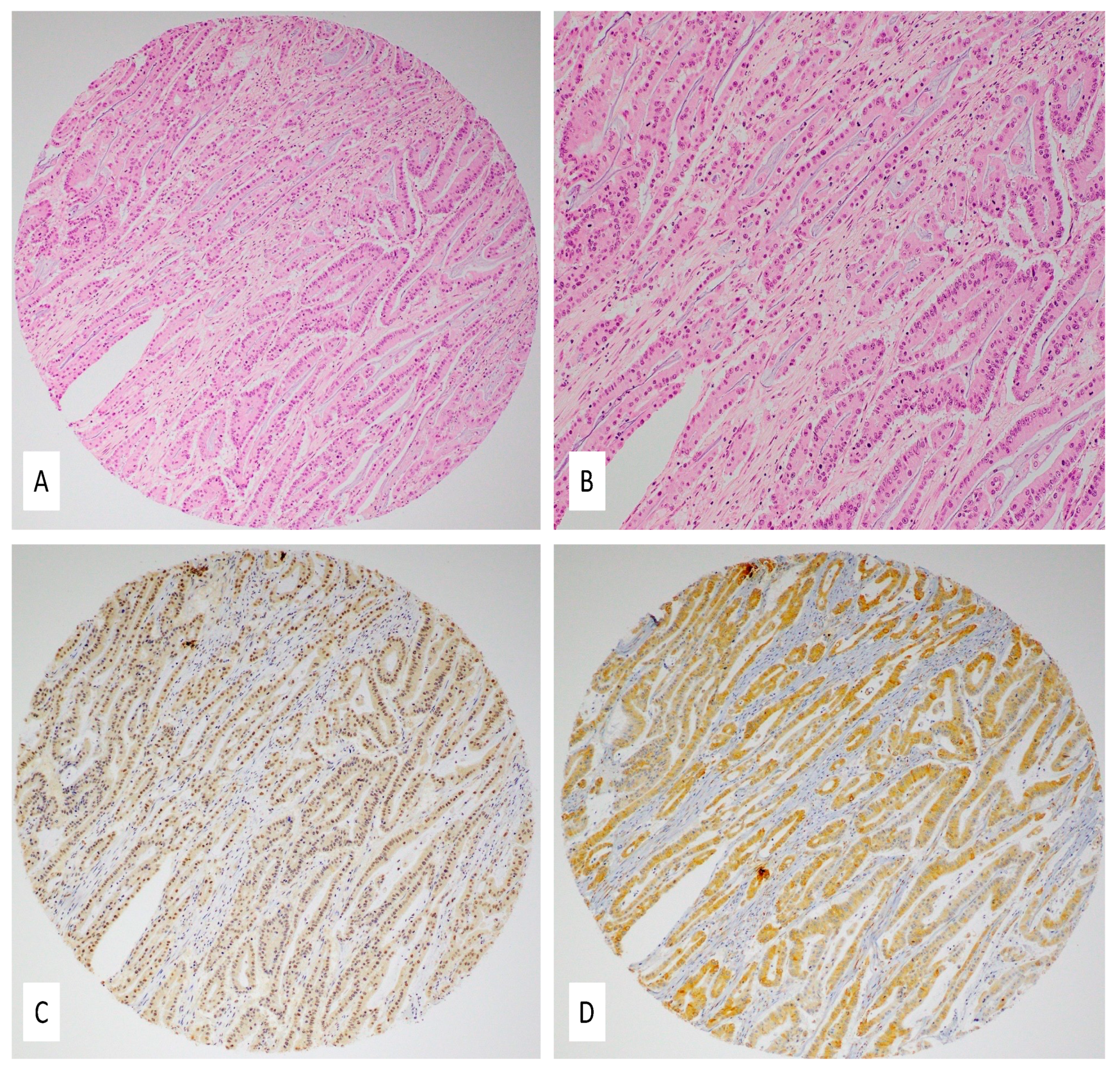
2.3. Expression Patterns of E6 and LMP1 Oncoproteins of High-Risk HPVs and EBV
2.4. Correlation of Clinicopathological Characteristics with HPV/EBV Positivity
3. Discussion
4. Materials and Methods
4.1. Sample Collection and DNA Extraction
4.2. HPV and EBV Detection by PCR
4.3. Tissue Microarray (TMA)
4.4. Immunohistochemistry (IHC)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- De Rosa, M.; Pace, U.; Rega, D.; Costabile, V.; Duraturo, F.; Izzo, P.; Delrio, P. Genetics, diagnosis and management of colorectal cancer (Review). Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef]
- Thrift, A.P.; Gong, J.; Peters, U.; Chang-Claude, J.; Rudolph, A.; Slattery, M.L.; Chan, A.T.; Locke, A.E.; Kahali, B.; Justice, A.E.; et al. Mendelian Randomization Study of Body Mass Index and Colorectal Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1024–1031. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef]
- Antonic, V.; Stojadinovic, A.; Kester, K.E.; Weina, P.J.; Brücher, B.L.; Protic, M.; Avital, I.; Izadjoo, M. Significance of Infectious Agents in Colorectal Cancer Development. J. Cancer 2013, 4, 227–240. [Google Scholar] [CrossRef]
- Venuti, A.B.G.; Rizzo, C.; Mafera, B.; Rahimi, S.; Vigili, M. Presence of HPV in head and neck tumours: High prevalence in tonsillar localization. J. Exp. Clin. Cancer Res. 2004, 23, 561–566. [Google Scholar] [PubMed]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004, 101, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ragin, C.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Peng, S.-L.; Yang, L.-F.; Chen, X.; Tao, Y.-G.; Cao, Y. Co-infection of Epstein-Barr virus and human papillomavirus in human tumorigenesis. Chin. J. Cancer 2016, 35, 16. [Google Scholar] [CrossRef]
- Motoyama, S.; Ladines-Llave, C.A.; Luis Villanueva, S.; Maruo, T. The role of human papilloma virus in the molecular biology of cervical carcinogenesis. Kobe J. Med. Sci. 2004, 50, 9–19. [Google Scholar]
- Heck, D.V.; Yee, C.L.; Howley, P.M.; Münger, K. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. USA 1992, 89, 4442–4446. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Díaz, M.; de Sanjosé, S.; Muñoz, N.; Herrero, R.; Franceschi, S.; Peeling, R.W.; Ashley, R.; Smith, J.S.; Snijders, P.J.; et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J. Natl. Cancer Inst. 2006, 98, 303–315. [Google Scholar] [CrossRef]
- Poletti, P.A.; Halfon, A.; Marti, M.C. Papillomavirus and anal carcinoma. Int. J. Colorectal Dis. 1998, 13, 108–111. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Wang, Y.-H.; Yang, X.-C.; Zhao, Y.; Wang, M.-L.; Liang, Y.; Niu, H.-T. The relationship between human papillomavirus and penile cancer over the past decade: A systematic review and meta-analysis. Asian J. Androl. 2019, 21, 375–380. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Stephens, S.J.; Palta, M.; Willett, C.G.; Czito, B.G. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J. Gastrointest. Oncol. 2015, 6, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Liaw, K.-L.; Johnson, L.G.; Madeleine, M.M. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1611–1622. [Google Scholar] [CrossRef]
- Kado, S.; Kawamata, Y.; Shino, Y.; Kasai, T.; Kubota, K.; Iwasaki, H.; Fukazawa, I.; Takano, H.; Nunoyama, T.; Mitsuhashi, A.; et al. Detection of Human Papillomaviruses in Cervical Neoplasias Using Multiple Sets of Generic Polymerase Chain Reaction Primers. Gynecol. Oncol. 2001, 81, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Al-Awadhi, R.; Missaoui, N.; Adam, I.; Durusoy, R.; Ghabreau, L.; Akil, N.; Ahmed, H.G.; Yasmeen, A.; Alsbeih, G. Human papillomaviruses-related cancers.Presence and Prevention Strategies in the Middle East and North African Regions. Hum. Vaccines Immunother. 2014, 10, 1812–1821. [Google Scholar] [CrossRef]
- Huibregtse, J.; Scheffner, M.; Howley, P.M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell. Biol. 1993, 13, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Münger, K.; Huibregtse, J.M.; Howley, P.M. Targeted degradation of the retinoblastoma protein by human papillomavirus E7-E6 fusion proteins. EMBO J. 1992, 11, 2425–2431. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E. E5 and E6/E7 of high-risk HPVs cooperate to enhance cancer progression through EMT initiation. Cell Adh. Migr. 2015, 9, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, A.; Bismar, T.A.; Kandouz, M.; Foulkes, W.D.; Desprez, P.-Y.; Al Moustafa, A.-E. E6/E7 of HPV Type 16 Promotes Cell Invasion and Metastasis of Human Breast Cancer Cells. Cell Cycle 2007, 6, 2038–2042. [Google Scholar] [CrossRef]
- Ghabreau, L.; Segal, E.; Yasmeen, A.; Kassab, A.; Akil, N.; Al Moustafa, A.-E. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: A tissue microarray study. Clin. Cancer Investig. J. 2012, 1, 26–30. [Google Scholar] [CrossRef]
- Yavuzer, D.; Karadayi, N.; Salepci, T.; Baloglu, H.; Dabak, R.; Bayramicli, O.U. Investigation of human papillomavirus DNA in colorectal carcinomas and adenomas. Med. Oncol. 2011, 28, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Buyru, N.; Tezol, A.; Dalay, N. Coexistence of K-ras mutations and HPV infection in colon cancer. BMC Cancer 2006, 6, 115. [Google Scholar] [CrossRef]
- Salepci, T.; Yazici, H.; Dane, F.; Topuz, E.; Dalay, N.; Onat, H.; Aykan, F.; Seker, M.; Aydiner, A. Detection of human papillomavirus DNA by polymerase chain reaction and southern blot hybridization in colorectal cancer patients. Off. J. Balk. Union Oncol. 2009, 14, 495–499. [Google Scholar]
- Young, L.S.; Rickinson, A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef]
- Middeldorp, J.M.; Brink, A.A.; van den Brule, A.J.; Meijer, C.J. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit. Rev. Oncol./Hematol. 2003, 45, 1–36. [Google Scholar] [CrossRef]
- Murata, T.; Tsurumi, T. Switching of EBV cycles between latent and lytic states. Rev. Med. Virol. 2014, 24, 142–153. [Google Scholar] [CrossRef]
- Thompson, M.P.; Kurzrock, R. Epstein-Barr Virus and Cancer. Clin. Cancer Res. 2004, 10, 803–821. [Google Scholar] [CrossRef]
- Münz, C.; Moormann, A. Immune escape by Epstein-Barr virus associated malignancies. Semin. Cancer Biol. 2008, 18, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Shimakage, M.; Horii, K.; Tempaku, A.; Kakudo, K.; Shirasaka, T.; Sasagawa, T. Association of Epstein-Barr virus with oral cancers. Hum. Pathol. 2002, 33, 608–614. [Google Scholar] [CrossRef]
- Horiuchi, K.; Mishima, K.; Ichijima, K.; Sugimura, M.; Ishida, T.; Kirita, T. Epstein-Barr virus in the proliferative diseases of squamous epithelium in the oral cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1995, 79, 57–63. [Google Scholar] [CrossRef]
- Al-Antary, N.; Farghaly, H.; Aboulkassim, T.; Yasmeen, A.; Akil, N.; Al Moustafa, A.-E. Epstein-Barr virus and its association with Fascin expression in colorectal cancers in the Syrian population: A tissue microarray study. Hum. Vaccines Immunother. 2017, 13, 1573–1578. [Google Scholar] [CrossRef]
- Bedri, S.; Sultan, A.A.; Alkhalaf, M.; Al Moustafa, A.-E.; Vranic, S. Epstein-Barr virus (EBV) status in colorectal cancer: A mini review. Hum. Vaccines Immunother. 2019, 15, 603–610. [Google Scholar] [CrossRef]
- Habib, M.A. The possible role of EBV in carcinogenesis of colorectal carcinoma. Fac. Med. Baghdad 2010, 52, 172–174. [Google Scholar]
- Liu, H.; Ding, Y.Q.; Li, X.; Yao, K.T. Investigation of Epstein-barr virus in Chinese colorectal tumors. World J. Gastroenterol. 2003, 9, 2464–2468. [Google Scholar] [CrossRef]
- Mehrabani-Khasraghi, S.; Ghane, M.; Ameli, M. Detection of Epstein-Barr virus in colorectal cancer and Polyp by using PCR technique. J. Paramed. Sci. 2014, 5, 96–101. [Google Scholar]
- Song, L.-B.; Zhang, X.; Zhang, C.-Q.; Zhang, Y.; Pan, Z.-Z.; Liao, W.-T.; Li, M.-Z.; Zeng, M.-S. Infection of Epstein-Barr virus in colorectal cancer in Chinese. Chin. J. Cancer 2006, 25, 1356–1360. [Google Scholar]
- Tafvizi, F.; Fard, Z.T.; Assareh, R. Epstein-Barr virus DNA in colorectal carcinoma in Iranian patients. Pol. J. Pathol. 2015, 66, 154–160. [Google Scholar] [CrossRef]
- Yuen, S.; Chung, L.P.; Leung, S.Y.; Luk, I.S.; Chan, S.Y.; Ho, J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am. J. Surg. Pathol. 1994, 18, 1158–1163. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E.; Al-Antary, N.; Aboulkassim, T.; Akil, N.; Batist, G.; Yasmeen, A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum. Vaccines Immunother. 2016, 12, 1936–1939. [Google Scholar] [CrossRef]
- Al-Thawadi, H.; Gupta, I.; Jabeen, A.; Skenderi, F.; Aboulkassim, T.; Yasmeen, A.; Malki, M.I.; Batist, G.; Vranic, S.; Al Moustafa, A.-E. Co-Presence of Human Papillomaviruses and Epstein–Barr Virus is Linked with Advanced Tumor Stage: A Tissue Microarray Study in Head and Neck Cancer Patients. Cancer Cell Int. 2020, 20, 361. [Google Scholar] [CrossRef]
- Al-Thawadi, H.; Ghabreau, L.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Batist, G.; Al Moustafa, A.-E. Co-Incidence of Epstein-Barr Virus and High-Risk Human Papillomaviruses in Cervical Cancer of Syrian Women. Front. Oncol. 2018, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Jabeen, A.; Al-Sarraf, R.; Farghaly, H.; Vranic, S.; Sulta, A.A.; Al Moustafa, A.-E.; Al-Thawadi, H. The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Hum. Vaccines Immunother. 2020, 17, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Malki, M.I.; Gupta, I.; Fernandes, Q.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.E.; Al-Thawadi, H.A. Co-presence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian colorectal cancer samples. Hum. Vaccines Immunother. 2020, 16, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Al Farsi, H.; Jabeen, A.; Skenderi, F.; Al-Thawadi, H.; AlAhmad, Y.M.; Abdelhafez, I.; Al Moustafa, A.-E.; Vranic, S. High-Risk Human Papillomaviruses and Epstein–Barr Virus in Colorectal Cancer and Their Association with Clinicopathological Status. Pathogens 2020, 9, 452. [Google Scholar] [CrossRef]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.; Henson, D.E.; Hutter, R.V.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.S.; Irfan, B.; Zahid, I.; Mirza, Y.; Ali, S.A. Comparison of Polymerase Chain Reaction and Immunohistochemistry Assays for Analysing Human Papillomavirus Infection in Oral Squamous Cell Carcinoma. J. Clin. Diagn. Res. 2017, 11, XC10–XC13. [Google Scholar] [CrossRef]
- Chan, K.H.; Ng, M.H.; Seto, W.H.; Peiris, J.S.M. Epstein-Barr Virus (EBV) DNA in Sera of Patients with Primary EBV Infection. J. Clin. Microbiol. 2001, 39, 4152–4154. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-L.; Han, X.-Q.; Hu, J.; Wang, G.-H.; Gao, J.-W.; Wang, X.; Liang, D.-Y. Comparison of three methods for the detection of Epstein-Barr virus in Hodgkin’s lymphoma in paraffin-embedded tissues. Mol. Med. Rep. 2013, 7, 89–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ala-Eddin, A.M.; Noor, A.-A.; Amber, Y. High-Risk Human Papillomavirus and Colorectal Carcinogenesis, Human Papillomavirus-Research in a Global Perspective. In High-Risk Human Papillomavirus and Colorectal Carcinogenesis; Rajkumar, R., Ed.; Intech Open: London, UK, 2016. [Google Scholar] [CrossRef]
- Bernabe-Dones, R.D.; Gonzalez-Pons, M.; Villar-Prados, A.; Lacourt-Ventura, M.; Rodríguez-Arroyo, H.; Fonseca-Williams, S.; Velazquez, F.E.; Diaz-Algorri, Y.; Lopez-Diaz, S.M.; Rodríguez, N.; et al. High Prevalence of Human Papillomavirus in Colorectal Cancer in Hispanics: A Case-Control Study. Gastroenterol. Res. Pract. 2016, 2016, 7896716. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, S.; Yamanegi, K.; Xiao, S.-Y.; Da Costa, M.; Palefsky, J.M.; Zheng, Z.-M. Colorectal papillomavirus infection in patients with colorectal cancer. Clin. Cancer Res. 2005, 11, 2862–2867. [Google Scholar] [CrossRef]
- Damin, D.C.; Caetano, M.B.; Rosito, M.A.; Schwartsmann, G.; Damin, A.S.; Frazzon, A.P.; Ruppenthal, R.D.; Alexandre, C.O.P. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur. J. Surg. Oncol. 2007, 33, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Huang, C.-C.; Yeh, K.-T.; Chang, S.-H.; Chang, S.-W.; Sung, W.-W.; Cheng, Y.-W.; Lee, H. Human papilloma virus 16 E6 oncoprotein associated with p53 inactivation in colorectal cancer. World J. Gastroenterol. 2012, 18, 4051–4058. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mou, X.; Zhao, N.; Lin, J.; Teng, L.; Xiang, C. Prevalence of human papillomavirus in Chinese patients with colorectal cancer. Colorectal Dis. 2011, 13, 865–871. [Google Scholar] [CrossRef]
- Mahmoudvand, S.; Safaei, A.; Erfani, N.; Sarvari, J. Presence of Human Papillomavirus DNA in Colorectal Cancer Tissues in Shiraz, Southwest Iran. Asian Pac. J. Cancer Prev. 2015, 16, 7883–7887. [Google Scholar] [CrossRef]
- Malekpour Afshar, R.; Deldar, Z.; Mollaei, H.R.; Arabzadeh, S.A.; Iranpour, M. Evaluation of HPV DNA positivity in colorectal cancer patients in Kerman, Southeast Iran. Asian Pac. J. Cancer Prev. 2018, 19, 193–198. [Google Scholar] [CrossRef]
- Meshkat, M.; Tayyebi Meibodi, N.; Sepahi, S.; Fadaee, N.; Salehpour, M.; Meshkat, Z. The frequency of human papillomaviruses in colorectal cancer samples in Mashhad, northeastern Iran. Turk. J. Med. Sci. 2014, 44, 501–503. [Google Scholar] [CrossRef]
- Gazzaz, F.; Mosli, M.H.; Jawa, H.; Sibiany, A. Detection of human papillomavirus infection by molecular tests and its relation to colonic polyps and colorectal cancer. Saudi Med. J. 2016, 37, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Maroun, C.A.; Al Feghali, K.; Traboulsi, H.; Dabbous, H.; Abbas, F.; Dunya, G.; Ziade, G.; Mahfouz, R.; Youssef, B.; Tamim, H.; et al. HPV-related oropharyngeal cancer prevalence in a middle eastern population using E6/E7 PCR. Infect. Agent Cancer 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Mroueh, A.M.; Seoud, M.A.; Kaspar, H.G.; Zalloua, P.A. Prevalence of genital human papillomavirus among Lebanese women. Eur. J. Gynaecol. Oncol. 2002, 23, 429–432. [Google Scholar]
- Motlagh, A.; Azadeh, P.; Hashemi, M.; Molaei, M.; MajidSheibani, K.; Alidoosti, A.; Fazlalizadeh, A.; Shafaghi, B.; Fudazi, M.; Valaei, N.; et al. Human Papillomavirus Infection, p53 Overexpression and Histopathologic Characteristics in Colorectal Cancer. Govaresh 2012, 12, 8. [Google Scholar]
- Tanzi, E.; Bianchi, S.; Frati, E.R.; Amicizia, D.; Martinelli, M.; Bragazzi, N.L.; Brisigotti, M.P.; Colzani, D.; Fasoli, E.; Zehender, G.; et al. Human papillomavirus detection in paraffin-embedded colorectal cancer tissues. J. Gen. Virol. 2015, 96, 206–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karbalaie Niya, M.H.; Keyvani, H.; Safarnezhad Tameshkel, F.; Salehi-Vaziri, M.; Teaghinezhad-S, S.; Bokharaei Salim, F.; Monavari, S.H.R.; Javanmard, D. Human Papillomavirus Type 16 Integration Analysis by Real-time PCR Assay in Associated Cancers. Transl. Oncol. 2018, 11, 593–598. [Google Scholar] [CrossRef]
- Ricciardi, R.; Ghabreau, L.; Yasmeen, A.; Darnel, A.D.; Akil, N.; Al Moustafa, A.E. Role of E6/E7 onco-proteins of high-risk human papillomaviruses in human colorectal carcinogenesis. Cell Cycle 2009, 8, 1964–1965. [Google Scholar] [CrossRef]
- Soto, Y.; Limia, C.M.; González, L.; Grá, B.; Hano, O.M.; Martínez, P.A.; Kourí, V. Molecular evidence of high-risk human papillomavirus infection in colorectal tumours from Cuban patients. Memórias Inst. Oswaldo Cruz 2016, 111, 731–736. [Google Scholar] [CrossRef]
- Fiorina, L.; Ricotti, M.; Vanoli, A.; Luinetti, O.; Dallera, E.; Riboni, R.; Paolucci, S.; Brugnatelli, S.; Paulli, M.; Pedrazzoli, P.; et al. Systematic analysis of human oncogenic viruses in colon cancer revealed EBV latency in lymphoid infiltrates. Infect. Agent Cancer 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, P.; Myszka, A.; Ramsey, D.; Kielan, W.; Sasiadek, M.M. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour. Biol. 2011, 32, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani-Khasraghi, S.; Ameli, M.; Khalily, F. Demonstration of Herpes Simplex Virus, Cytomegalovirus, and Epstein-Barr Virus in Colorectal Cancer. Iran. Biomed. J. 2016, 20, 302–306. [Google Scholar] [CrossRef]
- Militello, V.; Trevisan, M.; Squarzon, L.; Biasolo, M.A.; Rugge, M.; Militello, C.; Palù, G.; Barzon, L. Investigation on the presence of polyomavirus, herpesvirus, and papillomavirus sequences in colorectal neoplasms and their association with cancer. Int. J. Cancer 2009, 124, 2501–2503. [Google Scholar] [CrossRef]
- Park, J.M.; Choi, M.-G.; Kim, S.W.; Chung, I.-S.; Yang, C.W.; Kim, Y.S.; Jung, C.K.; Lee, K.Y.; Kang, J.-H. Increased Incidence of Colorectal Malignancies in Renal Transplant Recipients: A Case Control Study. Am. J. Transplant. 2010, 10, 2043–2050. [Google Scholar] [CrossRef]
- Salyakina, D.; Tsinoremas, N.F. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum. Genom. 2013, 7, 23. [Google Scholar] [CrossRef]
- Kijima, Y.; Hokita, S.; Takao, S.; Baba, M.; Natsugoe, S.; Yoshinaka, H.; Aridome, K.; Otsuji, T.; Itoh, T.; Tokunaga, M.; et al. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J. Med. Virol. 2001, 64, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Boguszakova, L.; Hirsch, I.; Brichacek, B.; Faltyn, J.; Fric, P.; Dvorakova, H.; Vonka, V. Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol. 1988, 32, 303–308. [Google Scholar] [PubMed]
- Cho, Y.J.; Chang, M.S.; Park, S.H.; Kim, H.S.; Kim, W.H. In situ hybridization of Epstein-Barr virus in tumor cells and tumor-infiltrating lymphocytes of the gastrointestinal tract. Hum. Pathol. 2001, 32, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.V.; Daniel, R.W.; Simons, J.W.; Vogelstein, B. Investigation of colon cancers for human papillomavirus genomic sequences by polymerase chain reaction. J. Surg. Oncol. 1992, 51, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.O.; Abba, M.C.; Laguens, R.M.; Golijow, C.D. Analysis of adenocarcinoma of the colon and rectum: Detection of human papillomavirus (HPV) DNA by polymerase chain reaction. Colorectal Dis. 2005, 7, 492–495. [Google Scholar] [CrossRef]
- Grinstein, S.; Preciado, M.V.; Gattuso, P.; Chabay, P.A.; Warren, W.H.; De Matteo, E.; Gould, V.E. Demonstration of Epstein-Barr Virus in Carcinomas of Various Sites. Cancer Res. 2002, 62, 4876–4878. [Google Scholar]
- Morewaya, J.; Koriyama, C.; Akiba, S.; Shan, D.; Itoh, T.; Eizuru, Y. Epstein-Barr virus-associated gastric carcinoma in Papua New Guinea. Oncol. Rep. 2004, 12, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Nishigami, T.; Kataoka, T.R.; Torii, I.; Sato, A.; Tamura, K.; Hirano, H.; Hida, N.; Ikeuchi, H.; Tsujimura, T. Concomitant adenocarcinoma and colonic non-Hodgkin’s lymphoma in a patient with ulcerative colitis: A case report and molecular analysis. Pathol. Res. Pract. 2010, 206, 846–850. [Google Scholar] [CrossRef]
- Wong, N.A.; Herbst, H.; Herrmann, K.; Kirchner, T.; Krajewski, A.S.; Moorghen, M.; Niedobitek, F.; Rooney, N.; Shepherd, N.A.; Niedobitek, G. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: Detection of the virus in lymphomas but not in adenocarcinomas. J. Pathol. 2003, 201, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.-A.; Sand, L. Human Papilloma Virus, Herpes Simplex Virus and Epstein Barr Virus in Oral Squamous Cell Carcinoma from Eight Different Countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein–Barr virus coinfection and oral carcinogenesis. J. Oral Pathol. Med. 2015, 44, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Polz-Gruszka, D.; Stec, A.; Dworzański, J.; Polz-Dacewicz, M. EBV, HSV, CMV and HPV in Laryngeal and Oropharyngeal Carcinoma in Polish Patients. Anticancer Res. 2015, 35, 1657–1661. [Google Scholar]
- Whitaker, N.J.; Glenn, W.K.; Sahrudin, A.; Orde, M.M.; Delprado, W.; Lawson, J.S. Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate 2013, 73, 236–241. [Google Scholar] [CrossRef]
- Epstein, M.A.; Henle, G.; Achong, B.G.; Barr, Y.M. Morphological and biological studies on a virus in cultured lymphoblasts from burkitt’s lymphoma. J. Exp. Med. 1965, 121, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Cyprian, F.S.; Al-Antary, N.; Yasmeen, A. High-Risk Human Papillomaviruses and Epstein-Barr Virus Presence and Crosstalk in Human Oral Carcinogenesis. In Development of Oral Cancer: Risk Factors and Prevention Strategies; Al Moustafa, A.-E., Ed.; Springer International Publishing: Cham, Germany, 2017; pp. 83–94. [Google Scholar] [CrossRef]
- Gupta, I.; Ghabreau, L.; Al-Thawadi, H.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.-E.; Malki, M. Co-Incidence of Human Papillomaviruses and Epstein-Barr Virus is Associated with High to Intermediate Tumor grade in Human Head and Neck Cancer in Syria. Front. Oncol. 2020, 10, 1016. [Google Scholar] [CrossRef]
- Akhtar, S.; Vranic, S.; Cyprian, F.S.; Al Moustafa, A.E. Epstein-Barr Virus in Gliomas: Cause, Association, or Artifact? Front. Oncol. 2018, 8, 123. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E.; Chen, D.; Ghabreau, L.; Akil, N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med. Hypotheses 2009, 73, 184–186. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Vranic, S.; Cyprian, F.S.; Akhtar, S.; Al Moustafa, A.-E. The Role of Epstein-Barr Virus in Cervical Cancer: A Brief Update. Front. Oncol. 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Gupta, I.; Vranic, S.; Al Moustafa, A.E. Human Papillomaviruses and Epstein-Barr Virus Interactions in Colorectal Cancer: A Brief Review. Pathogens 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.T.; Scott, R.S. The interaction between human papillomavirus and other viruses. Virus Res. 2017, 231, 139–147. [Google Scholar] [CrossRef]
- Cyprian, F.S.; Al-Farsi, H.F.; Vranic, S.; Akhtar, S.; Al Moustafa, A.-E. Epstein-Barr Virus and Human Papillomaviruses Interactions and Their Roles in the Initiation of Epithelial-Mesenchymal Transition and Cancer Progression. Front. Oncol. 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Ramalho, A.C.; Marques, M.; Ribeiro, D. The Interplay between Antiviral Signalling and Carcinogenesis in Human Papillomavirus Infections. Cancers 2020, 12, 646. [Google Scholar] [CrossRef]
- Akil, N.; Yasmeen, A.; Kassab, A.; Ghabreau, L.; Darnel, A.D.; Al Moustafa, A.E. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: A tissue microarray study. Br. J. Cancer 2008, 99, 404–407. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K. Multiple oncogenic viruses are present in human breast tissues before development of virus associated breast cancer. Infect. Agent Cancer 2017, 12, 55. [Google Scholar] [CrossRef]
- Gupta, I.; Jabeen, A.; Vranic, S.; Al Moustafa, A.-E.; Al-Thawadi, H. Oncoproteins of high-risk HPV and EBV cooperate to enhance cell motility and invasion of human breast cancer cells via Erk1/Erk2 and beta-catenin signaling pathways. Front. Oncol. 2021. In Press. [Google Scholar] [CrossRef]
- Darnel, A.D.; Wang, D.; Ghabreau, L.; Yasmeen, A.; Sami, S.; Akil, N.; Al Moustafa, A.E. Correlation between the presence of high-risk human papillomaviruses and Id gene expression in Syrian women with cervical cancer. Clin. Microbiol. Infect. 2010, 16, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Aboulkassim, T.; Yasmeen, A.; Akil, N.; Batist, G.; Al Moustafa, A.-E. Incidence of Epstein-Barr virus in Syrian women with breast cancer: A tissue microarray study. Hum. Vaccines Immunother. 2015, 11, 951–955. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Categories | Number (%) |
|---|---|---|
| Gender | Male | 37 (34.6) |
| Female | 70 (65.4) | |
| Age | ≤50 | 32 (29.9) |
| >50 | 75 (70.1) | |
| Tumor Location | Recto-sigmoid colon | 46 (49) |
| Other parts of colon | 48 (51) | |
| Tumor Grade φ | Grade 1 (low-grade) | 72 (76.6) |
| Grade 2 (high-grade) | 22 (23.4) | |
| Tumor Stage (pT) * | pT1 | 6 (6.4) |
| pT2 | 5 (5.3) | |
| pT3 | 79 (84) | |
| pT4 | 4 (4.3) | |
| Lymph Node Involvement (pN) | pN0 | 52 (55.3) |
| pN1 | 20 (21.3) | |
| pN2 | 22 (23.4) | |
| pN3 | 0 (0) |
| Samples | No. of Cases | High-Risk HPV Types | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 18 | 31 | 33 | 35 | 45 | 51 | 52 | 58 | ||
| EBV (+) | 27 | 15 | 24 | 7 | 4 | 17 | 9 | 11 | 11 | 20 |
| EBV (−) | 67 | 22 | 12 | 6 | 0 | 10 | 13 | 14 | 9 | 6 |
| Total | 94 | 37 | 36 | 13 | 4 | 27 | 22 | 25 | 20 | 26 |
| p-value | 0.07 | <0.0001 *** | 0.07 | 0.006 ** | <0.0001 *** | 0.24 | 0.09 | <0.0001 *** | <0.0001 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagi, K.; Gupta, I.; Jurdi, N.; Yasmeen, A.; Vranic, S.; Batist, G.; Moustafa, A.-E.A. Copresence of High-Risk Human Papillomaviruses and Epstein–Barr Virus in Colorectal Cancer: A Tissue Microarray and Molecular Study from Lebanon. Int. J. Mol. Sci. 2021, 22, 8118. https://doi.org/10.3390/ijms22158118
Nagi K, Gupta I, Jurdi N, Yasmeen A, Vranic S, Batist G, Moustafa A-EA. Copresence of High-Risk Human Papillomaviruses and Epstein–Barr Virus in Colorectal Cancer: A Tissue Microarray and Molecular Study from Lebanon. International Journal of Molecular Sciences. 2021; 22(15):8118. https://doi.org/10.3390/ijms22158118
Chicago/Turabian StyleNagi, Karim, Ishita Gupta, Nawaf Jurdi, Amber Yasmeen, Semir Vranic, Gerald Batist, and Ala-Eddin Al Moustafa. 2021. "Copresence of High-Risk Human Papillomaviruses and Epstein–Barr Virus in Colorectal Cancer: A Tissue Microarray and Molecular Study from Lebanon" International Journal of Molecular Sciences 22, no. 15: 8118. https://doi.org/10.3390/ijms22158118
APA StyleNagi, K., Gupta, I., Jurdi, N., Yasmeen, A., Vranic, S., Batist, G., & Moustafa, A.-E. A. (2021). Copresence of High-Risk Human Papillomaviruses and Epstein–Barr Virus in Colorectal Cancer: A Tissue Microarray and Molecular Study from Lebanon. International Journal of Molecular Sciences, 22(15), 8118. https://doi.org/10.3390/ijms22158118










