Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner
Abstract
1. Introduction
2. Results
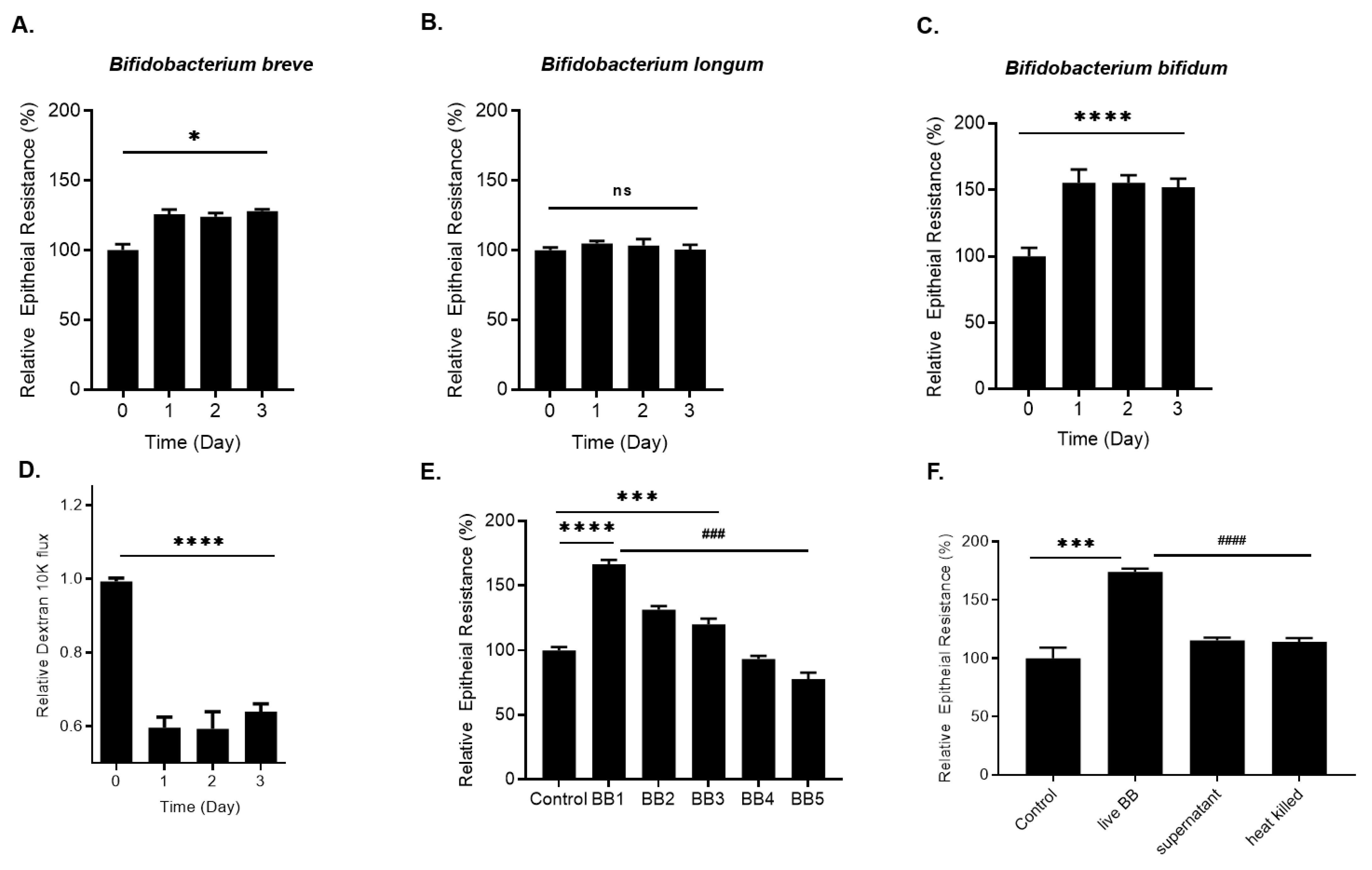
2.1. Effect of Bifidobacterium Species on Caco-2 Intestinal Epithelial TJ Barrier Function
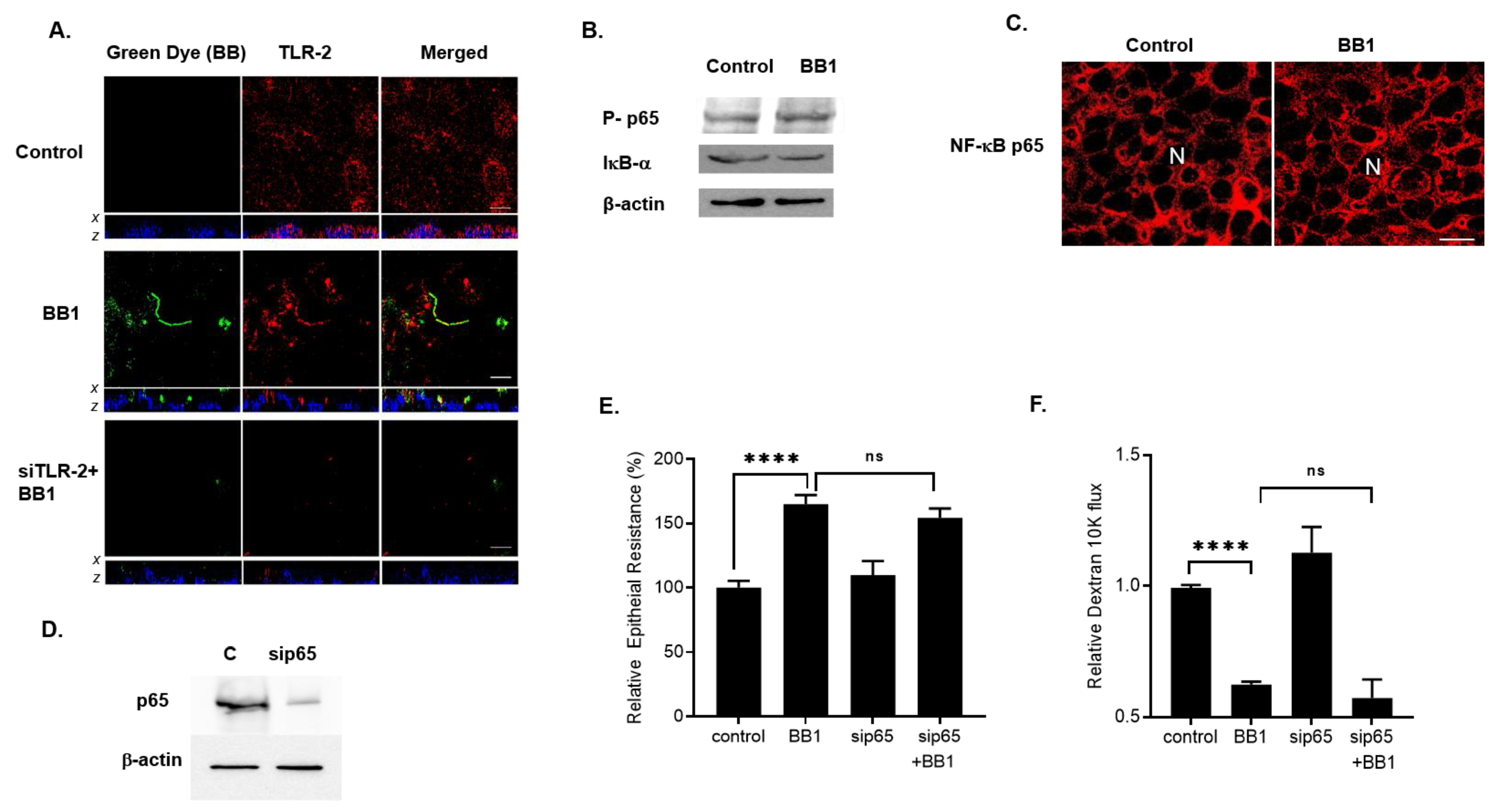
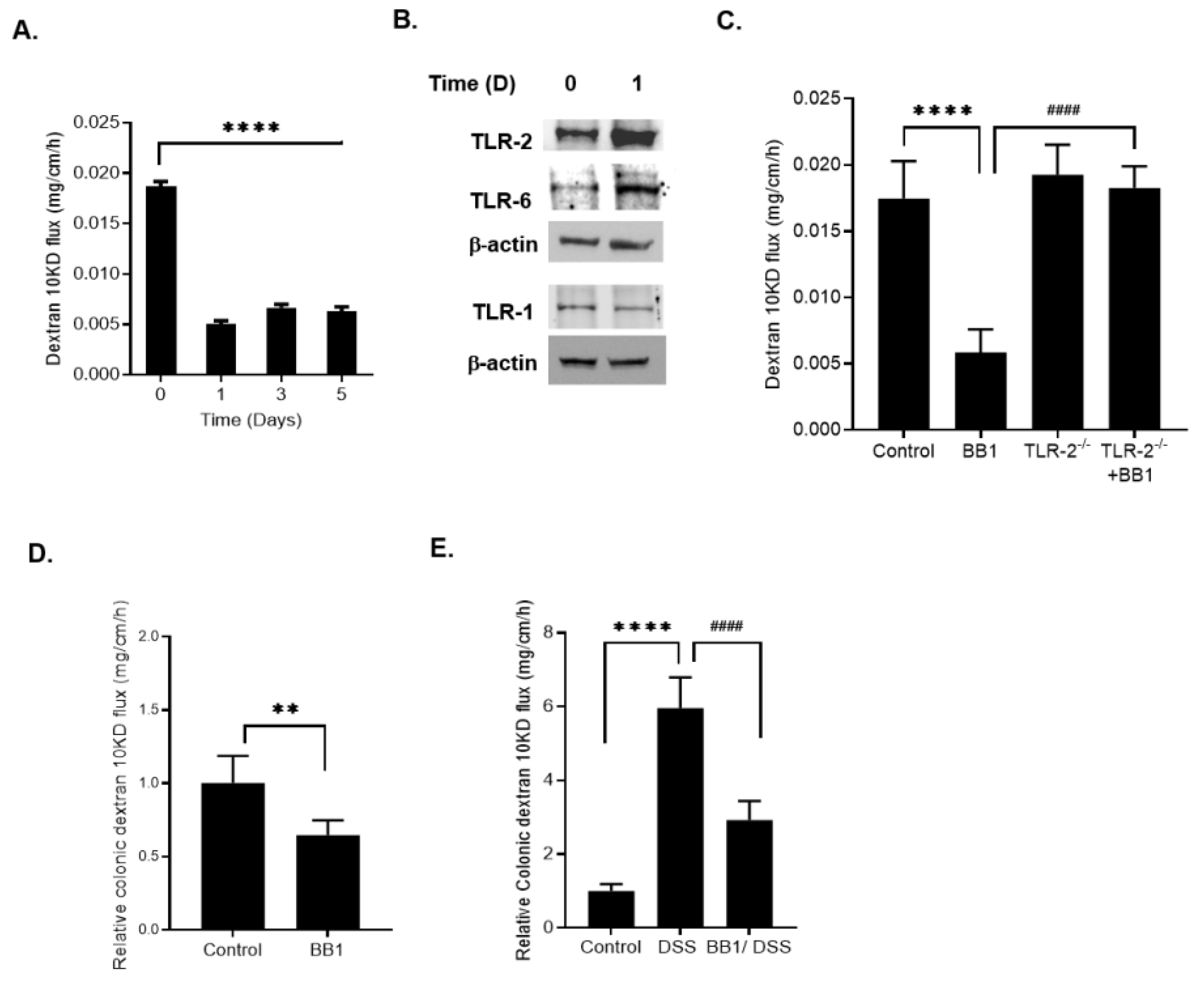
2.2. Involvement of Toll-like Receptors in BB1 Enhancement of Caco-2 TJ Barrier Function
2.3. Mechanism of BB1 Attachment to Caco-2 Apical Membrane Surface and the Signaling Pathway Mediating the BB Enhancement of Intestinal TJ Barrier
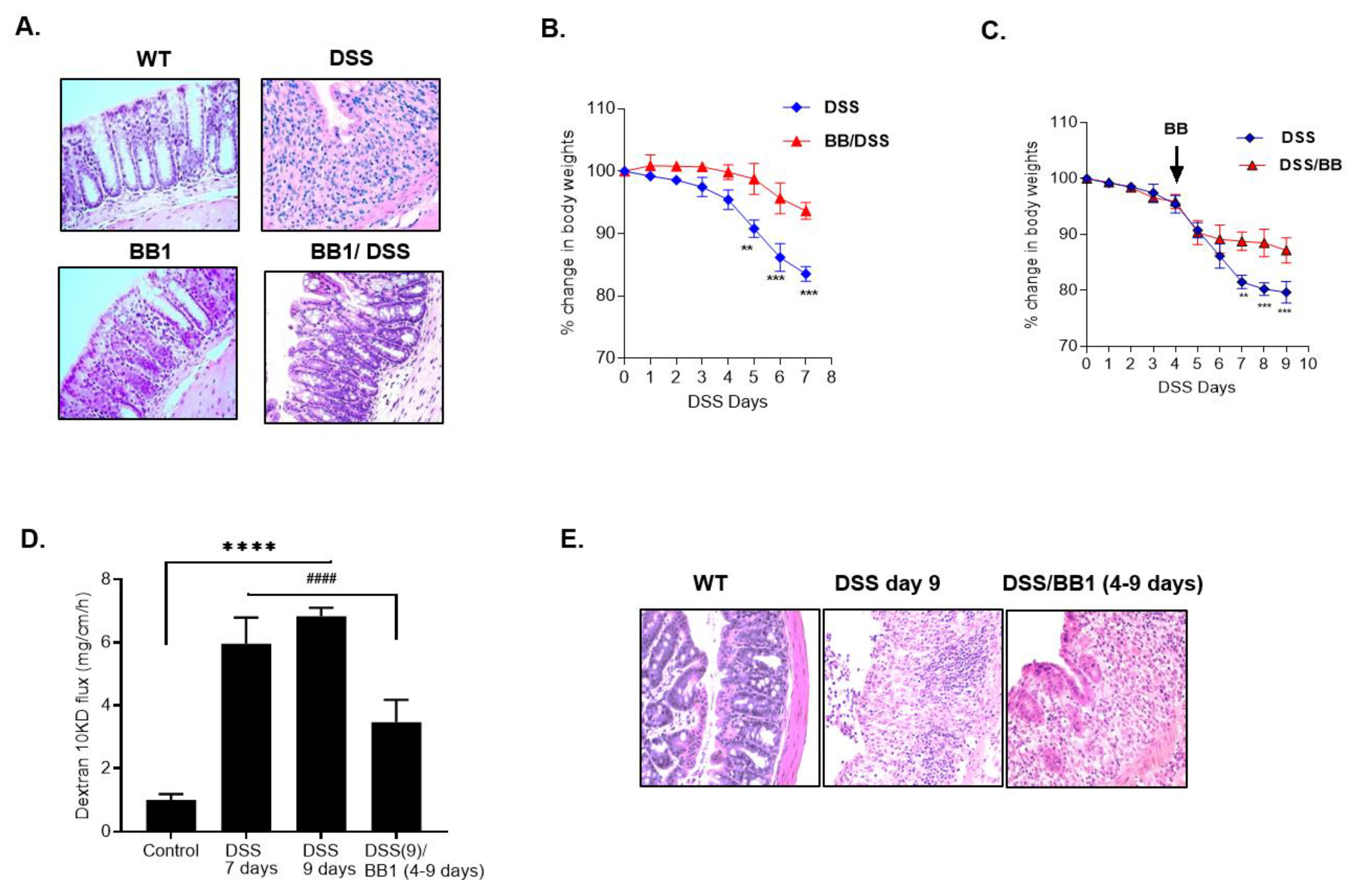
2.4. Effect of BB1 on Mouse Intestinal Permeability In-Vivo and Protection against DSS-Induced Increase in Colonic Permeability and Colitis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Determination of Epithelial Monolayer Resistance and Paracellular Permeability
4.3. Preparation of Bacterial Culture and Cell-Free Culture Supernatant
4.4. Determination of Mouse Small Intestinal Permeability In-Vivo and Measurement of Trans-Epithelial Electrical Resistance
4.5. Induction of DSS-Colitis and Determination of Mouse Colonic Permeability In Vivo
4.6. Assessment of Protein Expression by Western Blot Analysis
4.7. Immunostaining of NF-κB p65
4.8. Confocal Immunofluorescence
4.9. Transfection of Targeted siRNA
4.10. ELISA-Based In Vitro p38 Kinase Activity
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Sadi, R.; Nighot, P.; Nighot, M.; Haque, M.; Rawat, M.; Ma, T.Y. Lactobacillus acidophilus induces a strain-specific and toll-like receptor-2 dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 2021, 191, 872–884. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am. J. Physiol Gastrointest. Liver Physiol. 2019, 316, G278–G290. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D. The Intestinal Permeability Barrier—A Hypothesis as to Its Regulation and Involvement in Crohns-Disease. Scand. J. Gastroenterol. 1992, 27, 721–726. [Google Scholar] [CrossRef]
- Buonpane, C.; Yuan, C.; Wood, D.; Ares, G.; Klonoski, S.C.; Hunter, C.J. ROCK1 inhibitor stabilizes E-cadherin and improves barrier function in experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G781–G792. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Travis, S.P. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef]
- Kiesslich, R.; Duckworth, C.A.; Moussata, D.; Gloeckner, A.; Lim, L.G.; Goetz, M.; Pritchard, D.M.; Galle, P.R.; Neurath, M.F.; Watson, A.J. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012, 61, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- DiBona, D.R. Functional analysis of tight junction organization. Pflüg. Arch. 1985, 405 (Suppl. 1), S59–S66. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.L.; Greenstein, A.J.; Geller, S.A.; Gordon, R.E.; Aufses, A.H., Jr. A freeze fracture study of Crohn’s disease of the terminal ileum: Changes in epithelial tight junction organization. Am. J. Gastroenterol. 1983, 78, 537–547. [Google Scholar]
- Chichlowski, M.; De Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Furrie, E. A molecular revolution in the study of intestinal microflora. Gut 2006, 55, 141–143. [Google Scholar] [CrossRef][Green Version]
- Gallo, A.; Passaro, G.; Gasbarrini, A.; Landolfi, R.; Montalto, M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016, 22, 7186–7202. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernandez, N.; Solis, G.; Hernandez-Barranco, A.; Margolles, A.; de Los Reyes-Gavilan, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Salazar, N.; Solis, G.; Fernandez, N.; Hernandez-Barranco, A.M.; Cuesta, I.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Assessment of intestinal microbiota modulation ability of Bifidobacterium strains in in vitro fecal batch cultures from preterm neonates. Anaerobe 2013, 19, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Nwosu, C.; Ruiz-Moyano, S.; Aldredge, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Endo-beta-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell. Proteom. 2012, 11, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Dallas, D.C.; Mills, D.A. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: Mechanisms and implications. Microbiology 2013, 159 Pt 4, 649–664. [Google Scholar] [CrossRef]
- Soto, A.; Martin, V.; Jimenez, E.; Mader, I.; Rodriguez, J.M.; Fernandez, L. Lactobacilli and bifidobacteria in human breast milk: Influence of antibiotherapy and other host and clinical factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.R.; Liu, S.X.; Tian, R.; Kushnir, A.; Turner, J.R.; Li, H.L.; Chou, P.M.; Weber, C.R.; De Plaen, I.G. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 2013, 182, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Gaiani, F.; Mancabelli, L.; Milani, C.; Grandi, A.; Bolchi, A.; Santoni, A.; Lugli, G.A.; Ferrario, C.; Mangifesta, M.; et al. Elucidating the gut microbiome of ulcerative colitis: Bifidobacteria as novel microbial biomarkers. FEMS Microbiol. Ecol. 2016, 92, fiw191. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef]
- Khailova, L.; Dvorak, K.; Arganbright, K.M.; Halpern, M.D.; Kinouchi, T.; Yajima, M.; Dvorak, B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G940–G949. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Laval, L.; Chain, F.; Miquel, S.; Natividad, J.; Cherbuy, C.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.; Bermudez-Humaran, L.G.; et al. Bifidobacterium animalis ssp. lactis CNCM-I2494 Restores Gut Barrier Permeability in Chronically Low-Grade Inflamed Mice. Front. Microbiol. 2016, 7, 608. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Murphy, C.; O’Neill, L.A.J.; Creagh, E.M. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010, 3, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Pinero, P.; Juanola, O.; Caparros, E.; Zapater, P.; Gimenéz, P.; Gonzaléz-Navajas, J.M.; Such, J.; Francés, R. Toll-like receptor polymorphisms compromise the inflammatory response against bacterial antigen translocation in cirrhosis. Sci. Rep. 2017, 7, 46425. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Rawat, M.; Ma, T.Y. TNF-alpha Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-alpha Axis Activation of the Canonical NF-kappaB Pathway. Am. J. Pathol. 2016, 186, 1151–1165. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. Cellular and molecular mechanism of interleukin-1beta modulation of Caco-2 intestinal epithelial tight junction barrier. J. Cell. Mol. Med. 2011, 15, 970–982. [Google Scholar] [CrossRef]
- Böcker, U.; Yezerskyy, O.; Feick, P.; Manigold, T.; Panja, A.; Kalina, U.; Herweck, F.; Rossol, S.; Singer, M.V. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int. J. Colorectal Dis. 2003, 18, 25–32. [Google Scholar] [CrossRef]
- Dáňová, K.; Klapetková, A.; Kayserová, J.; Šedivá, A.; Špíšek, R.; Jelínková, L.P. NF-kappaB, p38 MAPK, ERK1/2, mTOR, STAT3 and increased glycolysis regulate stability of paricalcitol/dexamethasone-generated tolerogenic dendritic cells in the inflammatory environment. Oncotarget 2015, 6, 14123–14138. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhou, L.; Zhang, J.; Wang, B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int. J. Clin. Exp. Pathol. 2015, 8, 5153–5160. [Google Scholar]
- Sokovic Bajic, S.; Djokic, J.; Dinic, M.; Veljovic, K.; Golic, N.; Mihajlovic, S.; Tolinacki, M. GABA-Producing Natural Dairy Isolate From Artisanal Zlatar Cheese Attenuates Gut Inflammation and Strengthens Gut Epithelial Barrier in vitro. Front. Microbiol. 2019, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar]
- Corridoni, D.; Pastorelli, L.; Mattioli, B.; Locovei, S.; Ishikawa, D.; Arseneau, K.O.; Chieppa, M.; Cominelli, F.; Pizarro, T.T. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS ONE 2012, 7, e42067. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Jimenez-Espinoza, R.; Eom, H.J.; Block, D.E.; Mills, D.A. Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis isolates. Food Microbiol. 2013, 33, 262–270. [Google Scholar] [CrossRef]
- Kolacek, S.; Grguric, J.; Percl, M.; Booth, I.W. Home-made modular diet versus semi-elemental formula in the treatment of chronic diarrhoea of infancy: A prospective randomized trial. Eur. J. Pediatr. 1996, 155, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shoji, H.; Sato, H.; Nagata, S.; Ohtsuka, Y.; Shimizu, T.; Yamashiro, Y. Effects of oral administration of bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 252–257. [Google Scholar] [CrossRef]
- Arunachalam, K.; Gill, H.S.; Chandra, R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr. 2000, 54, 263–267. [Google Scholar] [CrossRef]
- Lopez, P.; Gonzalez-Rodriguez, I.; Gueimonde, M.; Margolles, A.; Suarez, A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS ONE 2011, 6, e24776. [Google Scholar] [CrossRef]
- Satoh, T.; Izumi, H.; Iwabuchi, N.; Odamaki, T.; Namba, K.; Abe, F.; Xiao, J.Z. Bifidobacterium breve prevents necrotising enterocolitis by suppressing inflammatory responses in a preterm rat model. Benef. Microbes 2016, 7, 75–82. [Google Scholar] [CrossRef]
- Costeloe, K.; Bowler, U.; Brocklehurst, P.; Hardy, P.; Heal, P.; Juszczak, E.; King, A.; Panton, N.; Stacey, F.; Whiley, A.; et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: The Probiotics in Preterm infantS (PiPS) trial. Health Technol. Assess. 2016, 20, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef] [PubMed]
- Rozkova, D.; Novotna, L.; Pytlik, R.; Hochova, I.; Kozak, T.; Bartunkova, J.; Spisek, R. Toll-like receptors on B-CLL cells: Expression and functional consequences of their stimulation. Int. J. Cancer 2010, 126, 1132–1143. [Google Scholar] [CrossRef]
- Gilani, S.; Howarth, G.S.; Nattrass, G.; Kitessa, S.M.; Barekatain, R.; Forder, R.E.A.; Tran, C.D.; Hughes, R.J. Gene expression and morphological changes in the intestinal mucosa associated with increased permeability induced by short-term fasting in chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, e653–e661. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Jia, R.; Wang, X.M.; Jia, L.G.; Petito, V.; Goodman, W.A.; Meddings, J.B.; Cominelli, F.; Reuter, B.K.; Pizarro, T.T. Epithelial-specific Toll-like Receptor (TLR)5 Activation Mediates Barrier Dysfunction in Experimental Ileitis. Inflamm. Bowel Dis. 2017, 23, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.G.; Corr, S.C. Toll-Like Receptor Signalling and the Control of Intestinal Barrier Function. Method Mol Biol. 2016, 1390, 287–300. [Google Scholar]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Song, S.K.; Lee, I.K.; Ko, S.; Han, S.E.; Bae, S.; Ji, S.Y.; Park, B.C.; Song, K.D.; Lee, H.K.; et al. Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol. Vet. Res. 2016, 47, 25. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, C.; Lagaraine, C.; Martin, L.; Velge-Roussel, F.; Lebranchu, Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 2006, 117, 696–702. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.N.; Frøkiær, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef]
- Palani, C.D.; Ramanathapuram, L.; Lam-Ubol, A.; Kurago, Z.B. Toll-like receptor 2 induces adenosine receptor A2a and promotes human squamous carcinoma cell growth via extracellular signal regulated kinases (1/2). Oncotarget 2018, 9, 6814–6829. [Google Scholar] [CrossRef]
- Yamada, T.; Ogi, K.; Sakashita, M.; Kanno, M.; Kubo, S.; Ito, Y.; Imoto, Y.; Tokunaga, T.; Okamoto, M.; Narita, N.; et al. Toll-like receptor ligands induce cytokine and chemokine production in human inner ear endolymphatic sac fibroblasts. Auris Nasus Larynx 2017, 44, 398–403. [Google Scholar] [CrossRef]
- Dunne, A.; O’Neill, L.A. The interleukin-1 receptor/Toll-like receptor superfamily: Signal transduction during inflammation and host defense. Sci. STKE 2000, 2003, re3. [Google Scholar] [CrossRef]
- Rocha-Ramirez, L.M.; Perez-Solano, R.A.; Castanon-Alonso, S.L.; Moreno Guerrero, S.S.; Ramirez Pacheco, A.; Garcia Garibay, M.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef]
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef]
- Kaushal, D.; Kansal, V.K. Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum improves phagocytic potential of macrophages in aged mice. J. Food Sci. Technol. 2014, 51, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Ohta, T.; Takahashi, T.; Kushiro, A.; Nomoto, K.; Yokokura, T.; Okada, N.; Danbara, H. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes Infect. 2006, 8, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Perdigon, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Olson, S.; Kalina, W.V.; Ruthel, G.; Demmin, G.L.; Warfield, K.L.; Bavari, S.; Klaenhammer, T.R. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, C.; Martin, L.; Faugaret, D.; Baron, C.; Dauba, A.; Aubert-Jacquin, C.; Velge-Roussel, F.; Lebranchu, Y. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS ONE 2008, 3, e2753. [Google Scholar] [CrossRef]
- Miyauchi, E.; Ogita, T.; Miyamoto, J.; Kawamoto, S.; Morita, H.; Ohno, H.; Suzuki, T.; Tanabe, S. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: Involvement of intestinal epithelial costimulatory molecules. PLoS ONE 2013, 8, e79735. [Google Scholar] [CrossRef]
- Sultana, R.; McBain, A.J.; O’Neill, C.A. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl. Environ. Microbiol. 2013, 79, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.L.; Ihara, E.; Hirota, S.A.; MacDonald, J.A.; Meng, D.; Nanthakumar, N.N.; Podolsky, D.K.; Xavier, R.J. Exploring the interplay of barrier function and leukocyte recruitment in intestinal inflammation by targeting fucosyltransferase VII and trefoil factor 3. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G43–G53. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Souza, E.L.S.; Campos, C.L.V.; Reis, D.C.; Cassali, G.D.; Generoso, S.V.; Cardoso, V.N.; Azevedo, V.; Medeiros, J.D.; Fernandes, G.R.; Nicoli, J.R.; et al. Beneficial effects resulting from oral administration of Escherichia coli Nissle 1917 on a chronic colitis model. Benef. Microbes 2020, 11, 779–790. [Google Scholar] [CrossRef]
- Nighot, P.; Al-Sadi, R.; Rawat, M.; Guo, S.; Watterson, D.M.; Ma, T. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G988–G997. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef]
- Ye, D.; Guo, S.; Al-Sadi, R.; Ma, T.Y. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology 2011, 141, 1323–1333. [Google Scholar] [CrossRef]
- Nighot, P.; Ma, T. Endocytosis of Intestinal Tight Junction Proteins: In Time and Space. Inflamm. Bowel Dis. 2021, 27, 283–290. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Dokladny, K.; Alhmoud, T.; Ereifej, L.; Said, H.M.; Ma, T.Y. Mechanism of IL-1beta modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J. Immunol. 2013, 190, 6596–6606. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070. https://doi.org/10.3390/ijms22158070
Al-Sadi R, Dharmaprakash V, Nighot P, Guo S, Nighot M, Do T, Ma TY. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. International Journal of Molecular Sciences. 2021; 22(15):8070. https://doi.org/10.3390/ijms22158070
Chicago/Turabian StyleAl-Sadi, Rana, Viszwapriya Dharmaprakash, Prashant Nighot, Shuhong Guo, Meghali Nighot, Toan Do, and Thomas Y. Ma. 2021. "Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner" International Journal of Molecular Sciences 22, no. 15: 8070. https://doi.org/10.3390/ijms22158070
APA StyleAl-Sadi, R., Dharmaprakash, V., Nighot, P., Guo, S., Nighot, M., Do, T., & Ma, T. Y. (2021). Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. International Journal of Molecular Sciences, 22(15), 8070. https://doi.org/10.3390/ijms22158070





