Autoimmune Responses in Oncology: Causes and Significance
Abstract
1. Introduction
2. Immune Tolerance and Anti-Tumoral Immunity
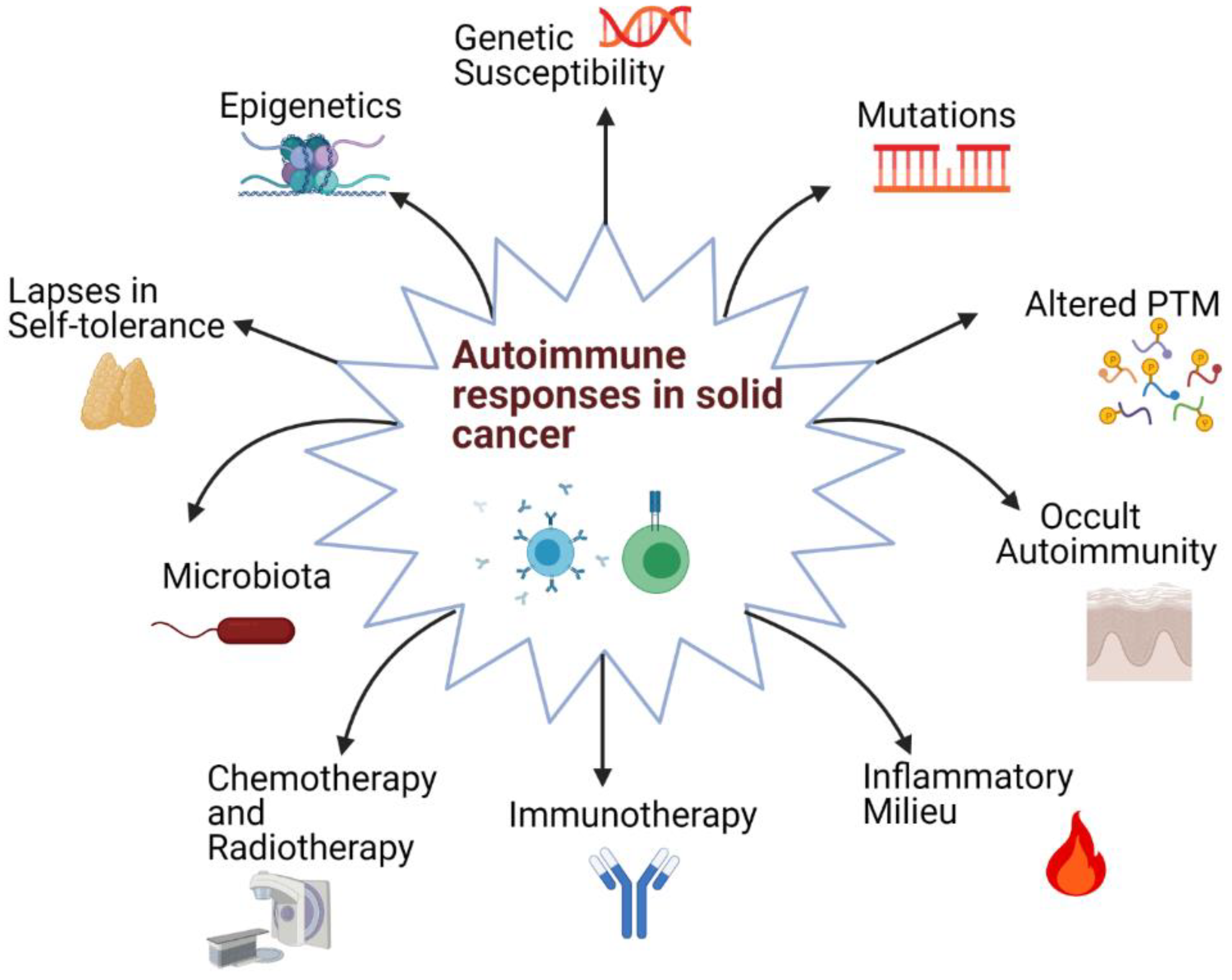
3. Origins of Autoimmune Responses in Oncology
3.1. Shared Genetic Factors
3.2. Microbiota
3.3. Current Onco-Immunotherapies Associated with Autoimmune Responses
3.3.1. ICI-Induced Autoimmune Responses
3.3.2. Autoimmunity in Other Onco-Immunotherapy Approaches
3.4. Conventional Onco-Therapies
3.5. Changes in Self-Antigens
- aAbs against mutated proteins: aAbs against a commonly mutated gene product, p53, have been observed in several solid cancer types including colorectal, ovarian, lung, and breast cancer [82,83,84,85]. aAbs against some other frequently mutated proteins in cancer that have a role in cell cycle, such as c-myc and cyclin B1, are also found in some patients with solid cancers, including ovarian and lung cancer [82,86]. It is of note that these aAbs can also be observed in SLE, which is an auto-immune pathology [86]. Given the importance of apoptosis in both cancer and SLE, these aAbs might have pathophysiological importance [87].
- aAbs against proteins with aberrant PTM: Aberrant or modified PTMs could also induce an autoimmune response by increasing the amount of and the affinity for the presented self-peptides [88]. PTMs are a diverse set of modifications, including phosphorylation, acetylation, SUMOylation, and O-glycosylation. Glycosylation is particularly important in cell recognition, adhesion, and motility. Mucin-1 (Muc-1) is a common TAA in epithelial cancers due to its aberrant glycosylation [89]. Muc-1 aAbs have been detected with prognostic significance in lung cancer, among others [90]. In terms of other PTMs, amino acids such as aspartic acid residues can be converted to isoaspartyl residues that create neo-epitopes [91]. In oncoproteomics, state-of-the-art, high-throughput, high-content, highly reproducible, and robust screening approaches such as nucleic acid programmable protein arrays (NAPPA) and reverse phase protein arrays (RPPA), as well as mass spectroscopy techniques, are being employed to further define the aberrant PTM landscape of cancers (cancer PTMome) and its associated antibodies in cancer [88,92].
- aAbs against cancer testis antigens and oncofetal proteins: These proteins could also be immunogenic because of their aberrant expression in terms of location and stage of life. These antigens are normally only expressed during embryonic life and are not found in adult somatic cells. They can be re-expressed in tumor cells via processes such as DNA methylation, histone modification, or mi-RNA regulation [93]. Several examples include important TAAs such as MAGE-A1 and NY-ESO-1, which can induce immune responses in melanoma and lung cancer, respectively [94]. NY-ESO-1 expression is normally restricted to germline and embryological cells; however, it gets re-expressed in a wide range of tumors including esophageal squamous cell carcinoma, breast, lung, and prostate cancers [94]. Additionally, the presence of NY-ESO-1 aAbs was found to be a good biomarker for a better response to anti-PD-1 therapy in NSCLC [95]. Of clinical application importance, in a study where anti-NY-ESO-1 aAbs were analyzed by enzyme-linked immunosorbent assay (ELISA), aAbs were present in 7–31% of cases of esophageal cancer, lung cancer, hepatocellular carcinoma, gastric cancer, colorectal cancer, prostate cancer, and breast cancer; however, none of the healthy controls had these aAbs, making aAbs against this antigen a highly specific potential cancer biomarker [96]. A recent study that used protein microarrays to screen for the presence of 30 aAbs in nasopharyngeal cancer patients also showed that NY-ESO-1 (along with cyclin B1, survivin, and IMP3) could serve as a biomarker for the detection of this type of cancer [97]. A commercial product using NY-ESO-1, among others, is being used for early diagnosis of lung cancer in high-risk individuals. Furthermore, in protein assays using lung cancer analytes (PAULA’s test), this aAb is being assayed together with three tumor antigens for early detection of NSCLC.
- aAbs against proteins with altered expression levels: Examples of aAbs against the proteins that are expressed at aberrant levels are survivin as well as heat shock proteins. Survivin is a protein that inhibits apoptosis via caspase inhibition through its interacting partners [98]. This molecule also inhibits autophagy, another process that has emerged as pivotal both in cancer and autoimmune disorders. Autophagy has important physiological roles such as fine-tuning protein levels and preventing the accumulation of damaged cellular components [99]. Therefore, faults in autophagy can lead to the accumulation of damaged and/or altered proteins, which can induce autoimmune responses. Autophagy can also promote genetic instability, which provides the leeway for cancer cells to counteract treatment or immune attacks. As a protein that helps in both the evasion of apoptosis as well as autophagy, survivin is overexpressed in many solid cancers including lung and breast cancer. It is also an important molecule for the T cell receptor formation of thymocytes and differentiation into effector and memory T cells [100]. aAbs against survivin are found in both chronic hepatitis and liver carcinoma patients, pointing at a shared target molecule for both types of diseases [101].
- Heat shock proteins (HSPs) are expressed in cellular stress situations to help the cell in coping with the demand imposed by the stressor. HSPs are usually chaperones that aid in increased protein translation and/or correct folding of misfolded proteins. HSPs have anti-apoptotic properties and can help cancer cells evade apoptosis. Accordingly, they are overexpressed in a wide range of cancers, and this overexpression is a bad prognostic marker for some cancers [102,103]. Moreover, these molecules are shown to be involved in ICD. In ICD, damage-associated molecular patterns (DAMPs) are released, which ‘notify’ the immune system of the presence of harm and the need for immune responses. HSPs that are released into the extracellular environment can act as DAMPs and can induce immune responses, mainly through the activation of dendritic cells [102]. Hence, HSP 70 and 90 are being tested as cancer vaccines in breast cancer, renal carcinoma, etc. [102]. aAbs against various HSPs including HSP 70–90 have been consistently detected in cancer patients [86]. These aAbs are also observed in a wide range of autoimmune diseases including SLE and RA [86]. Anti-HSP90 aAbs in breast cancer patients are associated with a bad prognosis [103]. It was shown that an aAb against a single HSP was present in between 8%–40% of cancer patients as opposed to 1.6–25% of healthy subjects [103]. Anti-HSP aAbs are also found in some aAb panels that are being investigated for the diagnosis of various cancers including NSCLC, hepatocellular carcinoma, and prostate cancer [103].
4. The Significance of Autoimmune Responses in Solid Cancers
4.1. The Significance of Autoantibodies
4.2. The Significance of irAEs
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Min, B.; Legge, K.L.; Pack, C.; Zaghouani, H. Neonatal Exposure to a Self-Peptide–Immunoglobulin Chimera Circumvents the Use of Adjuvant and Confers Resistance to Autoimmune Disease by a Novel Mechanism Involving Interleukin 4 Lymph Node Deviation and Interferon γ–Mediated Splenic Anergy. J. Exp. Med. 1998, 188, 2007–2017. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.-Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.-Q.; Gao, C.-Y.; Wang, B.-L.; Zhang, Y.-M.; et al. Tumor-Induced Perturbations of Cytokines and Immune Cell Networks. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. CMC 2019, 26, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, D.; Cui, X.; Zhang, L. Meta-analysis of Immune-related Adverse Events of Immune Checkpoint Inhibitor Therapy in Cancer Patients. Thorac. Cancer 2020, 11, 2406–2430. [Google Scholar] [CrossRef]
- Andersen, M.H. Cancer and Autoimmunity. Semin. Immunopathol. 2017, 39, 241–243. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, A.A.; Lebeau, P.; Majeed, F.; Polena, E.; Lhotak, Š.; Collins, C.A.F.; Pinthus, J.H.; Gonzalez-Gronow, M.; Hoogenes, J.; Pizzo, S.V.; et al. Autoantibodies against the Cell Surface–Associated Chaperone GRP78 Stimulate Tumor Growth via Tissue Factor. J. Biol. Chem. 2017, 292, 21180–21192. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Shoenfeld, Y. Harnessing Autoimmunity (Vitiligo) to Treat Melanoma: A Myth or Reality? Ann. N. Y. Acad. Sci. 2007, 1110, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Pelosof, L.C.; Gerber, D.E. Paraneoplastic Syndromes: An Approach to Diagnosis and Treatment. Mayo Clin. Proc. 2010, 85, 838–854. [Google Scholar] [CrossRef]
- González-González, M.; Sayagués, J.M.; Muñoz-Bellvís, L.; Pedreira, C.E.; de Campos, M.L.R.; García, J.; Alcázar, J.A.; Braz, P.F.; Galves, B.L.; González, L.M.; et al. Tracking the Antibody Immunome in Sporadic Colorectal Cancer by Using Antigen Self-Assembled Protein Arrays. Cancers 2021, 13, 2718. [Google Scholar] [CrossRef] [PubMed]
- Kyewski, B.; Klein, L. A central role for central tolerance. Annu. Rev. Immunol. 2006, 24, 571–606. [Google Scholar] [CrossRef]
- Nemazee, D. Mechanisms of Central Tolerance for B Cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Andersen, M.H. Anti-Regulatory T Cells. Semin Immunopathol. 2017, 39, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Arce-Sillas, A.; Álvarez-Luquín, D.D.; Tamaya-Domínguez, B.; Gomez-Fuentes, S.; Trejo-García, A.; Melo-Salas, M.; Cárdenas, G.; Rodríguez-Ramírez, J.; Adalid-Peralta, L. Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation. J. Immunol. Res. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Boros, P.; Ochando, J.; Zeher, M. Myeloid Derived Suppressor Cells and Autoimmunity. Hum. Immunol. 2016, 77, 631–636. [Google Scholar] [CrossRef]
- Ai, L.; Mu, S.; Wang, Y.; Wang, H.; Cai, L.; Li, W.; Hu, Y. Prognostic Role of Myeloid-Derived Suppressor Cells in Cancers: A Systematic Review and Meta-Analysis. BMC Cancer 2018, 18, 1220. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef]
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 2016, 76, 5597–5601. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Zayakin, P.; Buisseret, L.; Rulle, U.; Silina, K.; de Wind, A.; Van den Eyden, G.; Larsimont, D.; Willard-Gallo, K.; Linē, A. Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in Situ by Tumor-Infiltrating B Cells in Breast Cancer. Front. Immunol. 2018, 9, 2660. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational Landscape and Significance across 12 Major Cancer Types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Pellegrino, M. The Double Role of P53 in Cancer and Autoimmunity and Its Potential as Therapeutic Target. IJMS 2016, 17, 1975. [Google Scholar] [CrossRef]
- Leech, M.; Xue, J.R.; Dacumos, A.; Hall, P.; Santos, L.; Yang, Y.; Li, M.; Kitching, A.R.; Morand, E.F. The Tumour Suppressor Gene P53 Modulates the Severity of Antigen-Induced Arthritis and the Systemic Immune Response: P53 and the Immune Response. Clin. Exp. Immunol. 2008, 152, 345–353. [Google Scholar] [CrossRef]
- Okuda, Y.; Okuda, M.; Bernard, C.C.A. Regulatory Role of P53 in Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2003, 135, 29–37. [Google Scholar] [CrossRef]
- Park, J.-S.; Lim, M.-A.; Cho, M.-L.; Ryu, J.-G.; Moon, Y.-M.; Jhun, J.-Y.; Byun, J.-K.; Kim, E.-K.; Hwang, S.-Y.; Ju, J.H.; et al. P53 Controls Autoimmune Arthritis via STAT-Mediated Regulation of the Th17 Cell/Treg Cell Balance in Mice: P53 Induces Treg Cells and Controls Autoimmune Arthritis. Arthritis Rheum. 2013, 65, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Volodko, N. TP53 Codon 72 Arg/Arg Polymorphism Is Associated with a Higher Risk for Inflammatory Bowel Disease Development. WJG 2015, 21, 10358. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Traversi, G.; Arena, A.; Cappa, M.; Rosado, M.M.; Andreani, M.; Delfino, D.V.; Moretti, F.; Fierabracci, A. Effect of P53 Activation through Targeting MDM2/MDM4 Heterodimer on T Regulatory and Effector Cells in the Peripheral Blood of Type 1 Diabetes Patients. PLoS ONE 2020, 15, e0228296. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, L.; Liu, W.; Shi, G.; Zhang, J. Autoantibody to MDM2: A Potential Serological Marker of Systemic Lupus Erythematosus. J. Immunol. Res. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, X.; Wang, Y.; Chen, S.; Sun, Y.; Lin, Q.; Shi, G. Autoantibody to MDM2: A Potential Serological Marker of Primary Sjogren’s Syndrome. Oncotarget 2017, 8, 14306–14313. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Shi, J.-X.; Dai, L.-P.; Chai, Y.-R.; Zhang, H.-F.; Kankonde, M.; Kankonde, P.; Yu, B.-F.; Zhang, J.-Y. Serum Anti-MDM2 and Anti-c-Myc Autoantibodies as Biomarkers in the Early Detection of Lung Cancer. OncoImmunology 2016, 5, e1138200. [Google Scholar] [CrossRef]
- Himoto, T.; Yoneyama, H.; Kurokohchi, K.; Inukai, M.; Masugata, H.; Goda, F.; Haba, R.; Watanabe, S.; Senda, S.; Masaki, T. Clinical Significance of Autoantibodies to P53 Protein in Patients with Autoimmune Liver Diseases. Can. J. Gastroenterol. 2012, 26, 125–129. [Google Scholar] [CrossRef]
- Li, Y.; Karjalainen, A.; Koskinen, H.; Hemminki, K.; Vainio, H.; Shnaidman, M.; Ying, Z.; Pukkala, E.; Brandt-Rauf, P.W. P53 Autoantibodies Predict Subsequent Development of Cancer. Int. J. Cancer 2005, 114, 157–160. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in Cancer: Mediator and More. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Kwok-Shing Ng, P.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; de Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A.; et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/MTOR Pathway Alterations. Cancer Cell 2017, 31, 820–832.e3. [Google Scholar] [CrossRef]
- Parsons, M.J.; Jones, R.G.; Tsao, M.-S.; Odermatt, B.; Ohashi, P.S.; Woodgett, J.R. Expression of Active Protein Kinase B in T Cells Perturbs Both T and B Cell Homeostasis and Promotes Inflammation. J. Immunol. 2001, 167, 42–48. [Google Scholar] [CrossRef]
- Sauer, S.; Bruno, L.; Hertweck, A.; Finlay, D.; Leleu, M.; Spivakov, M.; Knight, Z.A.; Cobb, B.S.; Cantrell, D.; O’Connor, E.; et al. T Cell Receptor Signaling Controls Foxp3 Expression via PI3K, Akt, and MTOR. Proc. Natl. Acad. Sci. USA 2008, 105, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Huynh, A.; DuPage, M.; Priyadharshini, B.; Sage, P.T.; Quiros, J.; Borges, C.M.; Townamchai, N.; Gerriets, V.A.; Rathmell, J.C.; Sharpe, A.H.; et al. Control of PI(3) Kinase in Treg Cells Maintains Homeostasis and Lineage Stability. Nat. Immunol. 2015, 16, 188–196. [Google Scholar] [CrossRef]
- Lai, K.; Zhang, W.; Li, S.; Zhang, Z.; Xie, S.; Xu, M.; Li, C.; Zeng, K. MTOR Pathway Regulates the Differentiation of Peripheral Blood Th2/Treg Cell Subsets in Patients with Pemphigus Vulgaris. Acta Biochim. Biophys. Sin. 2021, 53, 438–445. [Google Scholar] [CrossRef]
- Tischner, D.; Woess, C.; Ottina, E.; Villunger, A. Bcl-2-Regulated Cell Death Signalling in the Prevention of Autoimmunity. Cell Death Dis. 2010, 1, e48. [Google Scholar] [CrossRef] [PubMed]
- The AACR Project GENIE Consortium AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [CrossRef]
- Wong, R.S. Apoptosis in Cancer: From Pathogenesis to Treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Mehrian, R.; Quismorio, F.P.; Strassmann, G.; Stimmler, M.M.; Horwitz, D.A.; Kitridou, R.C.; Gauderman, W.J.; Morrison, J.; Brautbar, C.; Jacob, C.O. Synergistic Effect between IL-10 and Bcl-2 Genotypes in Determining Susceptibility to Systemic Lupus Erythematosus. Arthritis Rheum 1998, 41, 596–602. [Google Scholar] [CrossRef]
- Davey, G.M.; Kurts, C.; Miller, J.F.A.P.; Bouillet, P.; Strasser, A.; Brooks, A.G.; Carbone, F.R.; Heath, W.R. Peripheral Deletion of Autoreactive CD8 T Cells by Cross Presentation of Self-Antigen Occurs by a Bcl-2–Inhibitable Pathway Mediated by Bim. J. Exp. Med. 2002, 196, 947–955. [Google Scholar] [CrossRef]
- Fabbri, M.; Calin, G.A. Epigenetics and miRNAs in Human Cancer. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2010; Volume 70, pp. 87–99. ISBN 978-0-12-380866-0. [Google Scholar]
- Park, S.; Kim, G.W.; Kwon, S.H.; Lee, J. Broad Domains of Histone H3 Lysine 4 Trimethylation in Transcriptional Regulation and Disease. FEBS J. 2020, 287, 2891–2902. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic Modifications and Human Disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Ligthart, S.; Marzi, C.; Aslibekyan, S.; Mendelson, M.M.; Conneely, K.N.; Tanaka, T.; Colicino, E.; Waite, L.L.; Joehanes, R.; Guan, W.; et al. DNA Methylation Signatures of Chronic Low-Grade Inflammation Are Associated with Complex Diseases. Genome Biol. 2016, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, Y.; Uehara, T.; Sano, K.; Matsuda, K.; Maruyama, Y.; Kobayashi, Y.; Nakajima, T.; Hamano, H.; Kawa, S.; Higuchi, K.; et al. Methylation of Tumor Suppressor Genes in Autoimmune Pancreatitis. Pancreas 2017, 46, 614–618. [Google Scholar] [CrossRef]
- Pradhan, V.; Patwardhan, M.; Ghosh, K. Anti-Nucleosome Antibodies as a Disease Marker in Systemic Lupus Erythematosus and Its Correlation with Disease Activity and Other Autoantibodies. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 145. [Google Scholar] [CrossRef]
- Matzaraki, V.; Kumar, V.; Wijmenga, C.; Zhernakova, A. The MHC Locus and Genetic Susceptibility to Autoimmune and Infectious Diseases. Genome Biol. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–Microbiota Interactions in Immune-Mediated Diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and Maintenance of Intestinal Regulatory T Cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Itzhaki, O.; Levy, D.; Zikich, D.; Treves, A.J.; Markel, G.; Schachter, J.; Besser, M.J. Adoptive T-Cell Transfer in Melanoma. Immunotherapy 2013, 5, 79–90. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The Role of the Lung Microbiota and the Gut-Lung Axis in Respiratory Infectious Diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef]
- Young, A.; Quandt, Z.; Bluestone, J.A. The Balancing Act between Cancer Immunity and Autoimmunity in Response to Immunotherapy. Cancer Immunol. Res. 2018, 6, 1445–1452. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal Microbiota Transplant Overcomes Resistance to Anti-PD-1 Therapy in Melanoma Patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Khan, S.; Gerber, D.E. Autoimmunity, Checkpoint Inhibitor Therapy and Immune-Related Adverse Events: A Review. Semin. Cancer Biol. 2020, 64, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Brevi, A.; Cogrossi, L.L.; Grazia, G.; Masciovecchio, D.; Impellizzieri, D.; Lacanfora, L.; Grioni, M.; Bellone, M. Much More Than IL-17A: Cytokines of the IL-17 Family Between Microbiota and Cancer. Front. Immunol. 2020, 11, 565470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, B.; Zhao, L.; Li, H. The Gut Microbiota: Emerging Evidence in Autoimmune Diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L.I.; Bogdanos, D.P. Multiple Hit Infection and Autoimmunity: The Dysbiotic Microbiota–ACPA Connection in Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2018, 30, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Regen, T.; Isaac, S.; Amorim, A.; Núñez, N.G.; Hauptmann, J.; Shanmugavadivu, A.; Klein, M.; Sankowski, R.; Mufazalov, I.A.; Yogev, N.; et al. IL-17 Controls Central Nervous System Autoimmunity through the Intestinal Microbiome. Sci. Immunol. 2021, 6, eaaz6563. [Google Scholar] [CrossRef]
- Dhodapkar, K.M. Autoimmune Complications of Cancer Immunotherapy. Curr. Opin. Immunol. 2019, 61, 54–59. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Shah, A.A. The Relationships between Cancer and Autoimmune Rheumatic Diseases. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101472. [Google Scholar] [CrossRef]
- Ladak, K.; Bass, A.R. Checkpoint Inhibitor-Associated Autoimmunity. Best Pract. Res. Clin. Rheumatol. 2018, 32, 781–802. [Google Scholar] [CrossRef]
- Cubas, R.; Khan, Z.; Gong, Q.; Moskalenko, M.; Xiong, H.; Ou, Q.; Pai, C.; Rodriguez, R.; Cheung, J.; Chan, A.C. Autoimmunity Linked Protein Phosphatase PTPN22 as a Target for Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e001439. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.M.; Duong, C.P.M.; Westwood, J.A.; Ritchie, D.S.; Junghans, R.P.; Darcy, P.K.; Kershaw, M.H. Autoimmunity Associated with Immunotherapy of Cancer. Blood 2011, 118, 499–509. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Chiche, L.; Lazaro, E.; Halfon, P.; Charpin, C.; Arniaud, D.; Retornaz, F.; Blanco, P.; Jourde-Chiche, N.; Richez, C.; et al. Opportunistic Autoimmunity Secondary to Cancer Immunotherapy (OASI): An Emerging Challenge. La Rev. De Médecine Interne 2017, 38, 513–525. [Google Scholar] [CrossRef]
- Lamers, C.H.J.; Sleijfer, S.; Vulto, A.G.; Kruit, W.H.J.; Kliffen, M.; Debets, R.; Gratama, J.W.; Stoter, G.; Oosterwijk, E. Treatment of Metastatic Renal Cell Carcinoma With Autologous T-Lymphocytes Genetically Retargeted Against Carbonic Anhydrase IX: First Clinical Experience. JCO 2006, 24, e20–e22. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human Chimeric Antigen Receptor Macrophages for Cancer Immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, S.; Johnston, B. Concepts and Mechanisms Underlying Chemotherapy Induced Immunogenic Cell Death: Impact on Clinical Studies and Considerations for Combined Therapies. Oncotarget 2015, 6, 41600–41619. [Google Scholar] [CrossRef]
- Egiziano, G.; Bernatsky, S.; Shah, A.A. Cancer and Autoimmunity: Harnessing Longitudinal Cohorts to Probe the Link. Best Pract. Res. Clin. Rheumatol. 2016, 30, 53–62. [Google Scholar] [CrossRef]
- Valencia, J.C.; Egbukichi, N.; Erwin-Cohen, R.A. Autoimmunity and Cancer, the Paradox Comorbidities Challenging Therapy in the Context of Preexisting Autoimmunity. J. Interferon Cytokine Res. 2019, 39, 72–84. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Farhood, B.; Eleojo Musa, A.; Taeb, S.; Rezaeyan, A.; Najafi, M. Abscopal Effect in Radioimmunotherapy. Int. Immunopharmacol. 2020, 85, 106663. [Google Scholar] [CrossRef] [PubMed]
- Fortner, R.T.; Damms-Machado, A.; Kaaks, R. Systematic Review: Tumor-Associated Antigen Autoantibodies and Ovarian Cancer Early Detection. Gynecol. Oncol. 2017, 147, 465–480. [Google Scholar] [CrossRef]
- Mu, Y.; Xie, F.; Sun, T. Clinical Value of Seven Autoantibodies Combined Detection in the Diagnosis of Lung Cancer. J. Clin. Lab. Anal. 2020, 34. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhou, D.; Huang, J. Autoantibodies as Biomarkers for Colorectal Cancer: A Systematic Review, Meta-Analysis, and Bioinformatics Analysis. Int. J. Biol. Markers 2019, 34, 334–347. [Google Scholar] [CrossRef]
- Pagaza-Straffon, C.; Marchat, L.A.; Herrera, L.; Díaz-Chávez, J.; Avante, M.G.; Rodríguez, Y.P.; Arreola, M.C.; López-Camarillo, C. Evaluation of a Panel of Tumor-Associated Antigens in Breast Cancer. CBM 2020, 27, 207–211. [Google Scholar] [CrossRef]
- Bei, R. The Crossroads between Cancer Immunity and Autoimmunity Antibodies to Self Antigens. Front. Biosci. 2017, 22, 1289–1329. [Google Scholar] [CrossRef]
- Herkel, J.; Mimran, A.; Erez, N.; Kam, N.; Lohse, A.W.; Märker-Hermann, E.; Rotter, V.; Cohen, I.R. Autoimmunity to the P53 Protein Is a Feature of Systemic Lupus Erythematosus (SLE) Related to Anti-DNA Antibodies. J. Autoimmun. 2001, 17, 63–69. [Google Scholar] [CrossRef]
- Atak, A.; Mukherjee, S.; Jain, R.; Gupta, S.; Singh, V.A.; Gahoi, N.K.P.M.; Srivastava, S. Protein Microarray Applications: Autoantibody Detection and Posttranslational Modification. Proteomics 2016, 16, 2557–2569. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Kohno, N.; Yokoyama, A.; Kondo, K.; Hiwada, K.; Miyake, M. Natural Autoantibody to MUC1 Is a Prognostic Indicator for Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2000, 161, 589–594. [Google Scholar] [CrossRef]
- Bei, R.; Masuelli, L.; Palumbo, C.; Modesti, M.; Modesti, A. A Common Repertoire of Autoantibodies Is Shared by Cancer and Autoimmune Disease Patients: Inflammation in Their Induction and Impact on Tumor Growth. Cancer Lett. 2009, 281, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.C.; Dun, M.D.; Verrills, N.M. Harnessing the Power of Proteomics for Identification of Oncogenic, Druggable Signalling Pathways in Cancer. Expert Opin. Drug Discov. 2017, 12, 431–447. [Google Scholar] [CrossRef]
- Macdonald, I.K.; Parsy-Kowalska, C.B.; Chapman, C.J. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer 2017, 3, 198–213. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef]
- Ohue, Y.; Kurose, K.; Karasaki, T.; Isobe, M.; Yamaoka, T.; Futami, J.; Irei, I.; Masuda, T.; Fukuda, M.; Kinoshita, A.; et al. Serum Antibody Against NY-ESO-1 and XAGE1 Antigens Potentially Predicts Clinical Responses to Anti–Programmed Cell Death-1 Therapy in NSCLC. J. Thorac. Oncol. 2019, 14, 2071–2083. [Google Scholar] [CrossRef]
- Oshima, Y.; Shimada, H.; Yajima, S.; Nanami, T.; Matsushita, K.; Nomura, F.; Kainuma, O.; Takiguchi, N.; Soda, H.; Ueda, T.; et al. NY-ESO-1 Autoantibody as a Tumor-Specific Biomarker for Esophageal Cancer: Screening in 1969 Patients with Various Cancers. J. Gastroenterol. 2016, 51, 30–34. [Google Scholar] [CrossRef]
- Shi, Q.-P.; Wang, X.; Liu, Z.-X.; Zhang, J.-J.; Wang, Z.-Y. Autoantibody Signatures as a Biomarker Panel for the Detection of Nasopharyngeal Carcinoma. Arch. Med. Res. 2021, S0188440921000424. [Google Scholar] [CrossRef]
- Wheatley, S.P.; Altieri, D.C. Survivin at a Glance. J. Cell Sci. 2019, 132, jcs223826. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef]
- Gravina, G.; Wasén, C.; Garcia-Bonete, M.J.; Turkkila, M.; Erlandsson, M.C.; Töyrä Silfverswärd, S.; Brisslert, M.; Pullerits, R.; Andersson, K.M.; Katona, G.; et al. Survivin in Autoimmune Diseases. Autoimmun. Rev. 2017, 16, 845–855. [Google Scholar] [CrossRef]
- Pan, X.; Gao, Y.; Liu, J.; Liu, C.; Xia, Y. Progress in Studies on Autoantibodies against Tumor-Associated Antigens in Hepatocellular Carcinoma. Transl. Cancer Res. 2016, 5, 845–859. [Google Scholar] [CrossRef]
- Das, J.K.; Xiong, X.; Ren, X.; Yang, J.-M.; Song, J. Heat Shock Proteins in Cancer Immunotherapy. J. Oncol. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Shi, L.; Chevolot, Y.; Souteyrand, E.; Laurenceau, E. Autoantibodies against Heat Shock Proteins as Biomarkers for the Diagnosis and Prognosis of Cancer. CBM 2017, 18, 105–116. [Google Scholar] [CrossRef]
- Chapman, C.J.; Healey, G.F.; Murray, A.; Boyle, P.; Robertson, C.; Peek, L.J.; Allen, J.; Thorpe, A.J.; Hamilton-Fairley, G.; Parsy-Kowalska, C.B.; et al. EarlyCDT®-Lung Test: Improved Clinical Utility through Additional Autoantibody Assays. Tumor Biol. 2012, 33, 1319–1326. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Qiu, C.; Qin, J.; Wang, K.; Sun, G.; Jiang, D.; Li, J.; Wang, L.; Shi, J.; et al. Using Protein Microarray to Identify and Evaluate Autoantibodies to Tumor-associated Antigens in Ovarian Cancer. Cancer Sci. 2021, 112, 537–549. [Google Scholar] [CrossRef]
- Anderson, K.S.; Cramer, D.W.; Sibani, S.; Wallstrom, G.; Wong, J.; Park, J.; Qiu, J.; Vitonis, A.; LaBaer, J. Autoantibody Signature for the Serologic Detection of Ovarian Cancer. J. Proteome Res. 2015, 14, 578–586. [Google Scholar] [CrossRef]
- Garranzo-Asensio, M.; Guzmán-Aránguez, A.; Povedano, E.; Ruiz-Valdepeñas Montiel, V.; Poves, C.; Fernandez-Aceñero, M.J.; Montero-Calle, A.; Solís-Fernández, G.; Fernandez-Diez, S.; Camps, J.; et al. Multiplexed Monitoring of a Novel Autoantibody Diagnostic Signature of Colorectal Cancer Using HaloTag Technology-Based Electrochemical Immunosensing Platform. Theranostics 2020, 10, 3022–3034. [Google Scholar] [CrossRef]
- Zaenker, P.; Lo, J.; Pearce, R.; Cantwell, P.; Cowell, L.; Lee, M.; Quirk, C.; Law, H.; Gray, E.; Ziman, M. A Diagnostic Autoantibody Signature for Primary Cutaneous Melanoma. Oncotarget 2018, 9, 30539–30551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortona, E.; Pierdominici, M.; Berstein, L. Autoantibodies to Estrogen Receptors and Their Involvement in Autoimmune Diseases and Cancer. J. Steroid Biochem. Mol. Biol. 2014, 144, 260–267. [Google Scholar] [CrossRef]
- Wu, W.; Yie, S.; Ye, S.; Xie, K.; Zhang, J.; Cao, M.; Chen, J.; He, X.; Ma, X.; Zhang, J. An Autoantibody Against Human DNA-Topoisomerase I Is a Novel Biomarker for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2018, 105, 1664–1670. [Google Scholar] [CrossRef]
- He, X.; Jiang, X.; Yie, K.Y.-X.; Chen, J.; Zhang, J.; Yie, S. An Autoantibody against a 48-Kd Fragment of Human DNA-Topoiomerase I in Breast Cancer: Implication for Diagnosis and Prognosis, and Antibody-Dependent Cellular Cytotoxicity in Vitro. Cell. Immunol. 2020, 347, 104007. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, Z.; He, X.; Zhang, J.; Xie, K.; Chen, J.; Cao, M.; Zhang, J.; Yie, S. Clinical Significance of Plasma Anti-TOPO48 Autoantibody and Blood Survivin-Expressing Circulating Cancer Cells in Patients with Early Stage Endometrial Carcinoma. Arch. Gynecol. Obstet 2019, 299, 229–237. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Kakimi, K.; Karasaki, T.; Matsushita, H.; Sugie, T. Advances in Personalized Cancer Immunotherapy. Breast Cancer 2017, 24, 16–24. [Google Scholar] [CrossRef]
- Joseph, C.G.; Darrah, E.; Shah, A.A.; Skora, A.D.; Casciola-Rosen, L.A.; Wigley, F.M.; Boin, F.; Fava, A.; Thoburn, C.; Kinde, I.; et al. Association of the Autoimmune Disease Scleroderma with an Immunologic Response to Cancer. Science 2014, 343, 152–157. [Google Scholar] [CrossRef]
- Best, M.; Molinari, N.; Chasset, F.; Vincent, T.; Cordel, N.; Bessis, D. Use of Anti-Transcriptional Intermediary Factor-1 Gamma Autoantibody in Identifying Adult Dermatomyositis Patients with Cancer: A Systematic Review and Meta-Analysis. Acta Derm Venerol. 2019, 99, 256–262. [Google Scholar] [CrossRef]
- Rosenfeld, M.R.; Dalmau, J. Paraneoplastic Neurologic Syndromes. Neurologic. Clin. 2018, 36, 675–685. [Google Scholar] [CrossRef]
- Zekeridou, A.; Majed, M.; Heliopoulos, I.; Lennon, V.A. Paraneoplastic Autoimmunity and Small-cell Lung Cancer: Neurological and Serological Accompaniments. Thorac. Cancer 2019, 10, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Gahoi, N.; Syed, P.; Choudhary, S.; Epari, S.; Moiyadi, A.; Varma, S.G.; Gandhi, M.N.; Srivastava, S. A Protein Microarray-Based Investigation of Cerebrospinal Fluid Reveals Distinct Autoantibody Signature in Low and High-Grade Gliomas. Front. Oncol. 2020, 10, 543947. [Google Scholar] [CrossRef] [PubMed]
- Ruth, J.H.; Gurrea-Rubio, M.; Athukorala, K.S.; Rasmussen, S.M.; Weber, D.P.; Randon, P.M.; Gedert, R.J.; Lind, M.E.; Amin, M.A.; Campbell, P.L.; et al. CD6 Is a Target for Cancer Immunotherapy. JCI Insight 2021, 6, e145662. [Google Scholar] [CrossRef]

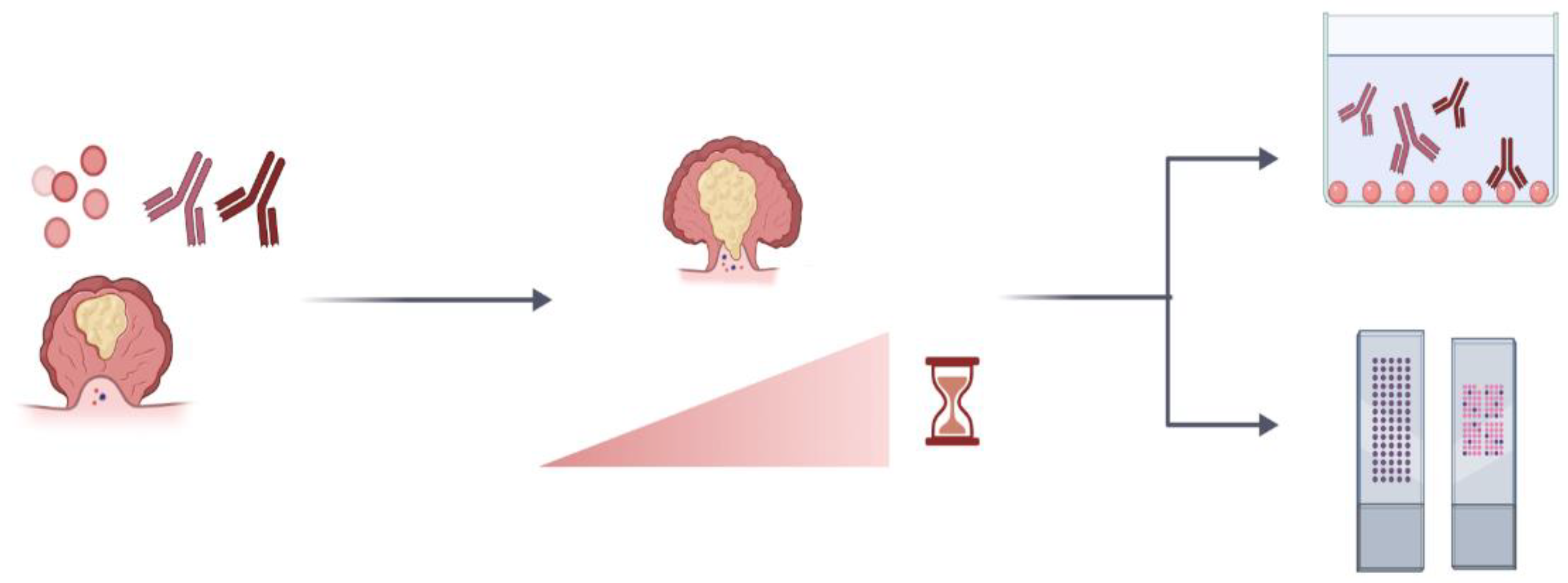
| Type of Cancer | Comparison Group | Antibody Panel | Method Used | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | Breast cancer patients vs. healthy donors | p53/PRDX6/c-Myc/Hsp70/Nm23 | ELISA | 34 | 100 | [85] |
| Lung cancer | Patients with recent diagnosis of lung cancer vs. healthy controls | p53, NY-ESO-1, CAGE, GBU4–5, MAGE A4, SOX2, and Hu-D | ELISA (Early CDT-Lung) | 41 | 93 | [104] |
| Lung cancer | Lung cancer patients vs. healthy controls and lung benign disease group | p53,PGP9.5, SOX2, GAGE7, GBU4–5, MAGE A1, and CAGE | ELISA | 25.4 | 91.7 | [83] |
| Ovarian cancer | Early (stage I-II) stage ovarian cancer patients vs. healthy controls | p53, GNAS, and NPM1 | ELISA | 57 | 86 | [105] |
| Late-stage (stage III–IV) ovarian cancer patients vs. healthy controls | 49 | 86 | ||||
| Ovarian cancer | Ovarian cancer patients vs. healthy controls | p53, PTPRA, and PTGFR | Luminex bead assay | 23.3 | 98.3 | [106] |
| Colorectal cancer | Colorectal cancer patients vs. healthy individuals and breast and lung cancer patients | p53, GTF2B, MAPKAPK3, PIM1, PKN1, SRC, STK4, and SULF1 | Luminex bead assay and electrochemical immunosensing by HaloTag fusion | 76.0 | 98.6 | [107] |
| Melanoma | Early stage melanoma patients vs. healthy controls | p53, ZBTB7B, PRKCH, PCTK1, PQBP1, UBE2V1, IRF4, MAPK8_tv2, MSN, and TPM1 | Protein microarray | 79 | 84 | [108] |
| Nasopharyngeal carcinoma | Nasopharyngeal cancer patients vs. healthy individuals | cyclin B1, NY-ESO-1, survivin and IMP3 | Protein microarray | 54 | 86 | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bareke, H.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Hernandez, A.-P.; Cruz, J.J.; Bellido, L.; Fonseca, E.; Niebla-Cárdenas, A.; Montalvillo, E.; Góngora, R.; et al. Autoimmune Responses in Oncology: Causes and Significance. Int. J. Mol. Sci. 2021, 22, 8030. https://doi.org/10.3390/ijms22158030
Bareke H, Juanes-Velasco P, Landeira-Viñuela A, Hernandez A-P, Cruz JJ, Bellido L, Fonseca E, Niebla-Cárdenas A, Montalvillo E, Góngora R, et al. Autoimmune Responses in Oncology: Causes and Significance. International Journal of Molecular Sciences. 2021; 22(15):8030. https://doi.org/10.3390/ijms22158030
Chicago/Turabian StyleBareke, Halin, Pablo Juanes-Velasco, Alicia Landeira-Viñuela, Angela-Patricia Hernandez, Juan Jesús Cruz, Lorena Bellido, Emilio Fonseca, Alfonssina Niebla-Cárdenas, Enrique Montalvillo, Rafael Góngora, and et al. 2021. "Autoimmune Responses in Oncology: Causes and Significance" International Journal of Molecular Sciences 22, no. 15: 8030. https://doi.org/10.3390/ijms22158030
APA StyleBareke, H., Juanes-Velasco, P., Landeira-Viñuela, A., Hernandez, A.-P., Cruz, J. J., Bellido, L., Fonseca, E., Niebla-Cárdenas, A., Montalvillo, E., Góngora, R., & Fuentes, M. (2021). Autoimmune Responses in Oncology: Causes and Significance. International Journal of Molecular Sciences, 22(15), 8030. https://doi.org/10.3390/ijms22158030








