The Temporal Mechanisms Guiding Interneuron Differentiation in the Spinal Cord
Abstract
1. Introduction
2. Lessons from the Mouse Spinal Cord
2.1. Early Temporal Mechanisms Guide Molecular Diversity in the Mouse Spinal Cord
2.2. Interneuron Subpopulations Emerge from Temporally Separated Progenitors
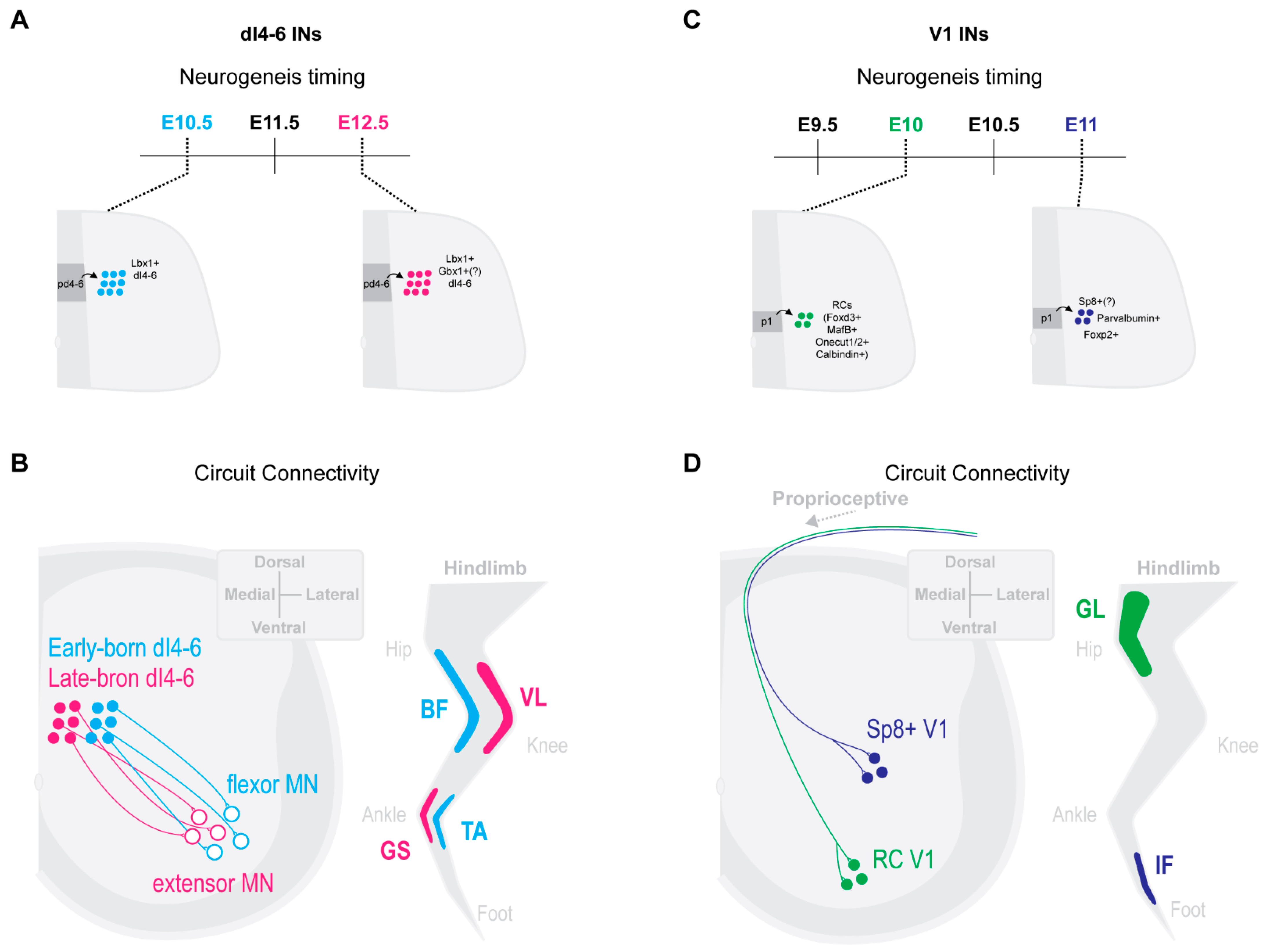
2.3. Select Dorsal IN Populations Emerge from Temporally Separated Progenitors
2.4. Neurogenesis Timing Can Restrict in Specific Circuit Wirings
3. Lessons from the Zebrafish: Sequential Waves of Neurogenesis form ‘Layered’ Locomotor Circuits in the Zebrafish Spinal Cord and Brainstem
3.1. Early Maturation of Swimming Behaviours Is Underscored by Sequential Waves of Neurogenesis
3.2. Spinal Neurons Separate along Neurogenesis Time- and Speed-Matched Axes
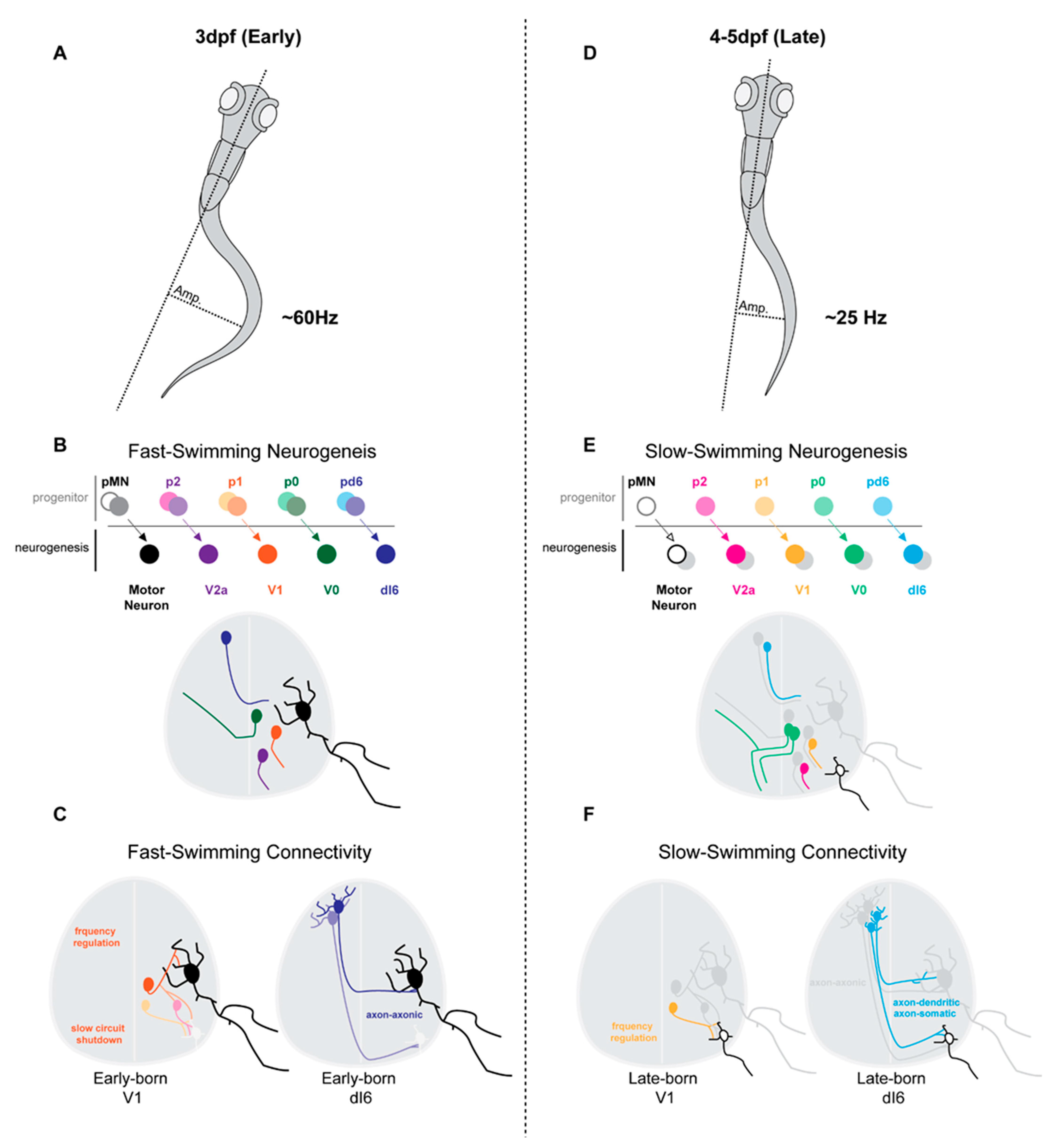
3.3. Neurogenesis and Differentiation Timing Matches Pre- and Post-Synaptic Targets
3.4. Temporal Layering of Spinal Circuits Extends to the Brainstem
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Jessell, T.M. Neuronal Specification in the Spinal Cord: Inductive Signals and Transcriptional Codes. Nat. Rev. Genet. 2000, 1, 20–29. [Google Scholar] [CrossRef]
- Lu, D.C.; Eniu, T.; Alaynick, W.A. Molecular and Cellular Development of Spinal Cord Locomotor Circuitry. Front. Mol. Neurosci. 2015, 8, 25. [Google Scholar] [CrossRef]
- Deska-Gauthier, D.; Zhang, Y. The Functional Diversity of Spinal Interneurons and Locomotor Control. Curr. Opin. Physiol. 2019, 8, 99–108. [Google Scholar] [CrossRef]
- Goulding, M. Circuits Controlling Vertebrate Locomotion: Moving in a New Direction. Nat. Rev. Neurosci. 2009, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Kiehn, O. Decoding the Organization of Spinal Circuits That Control Locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef]
- Bikoff, J.B.; Gabitto, M.I.; Rivard, A.F.; Drobac, E.; Machado, T.; Miri, A.; Brenner-Morton, S.; Famojure, E.; Diaz, C.; Alvarez, F.J.; et al. Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell 2016, 165, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Gosgnach, S.; Bikoff, J.B.; Dougherty, K.J.; El Manira, A.; Lanuza, G.M.; Zhang, Y. Delineating the Diversity of Spinal In-ter-neurons in Locomotor Circuits. J. Neurosci. 2017, 37, 10835–10841. [Google Scholar] [CrossRef]
- Ziskind-Conhaim, L.; Hochman, S. Diversity of Molecularly Defined Spinal Interneurons Engaged in Mammalian Locomotor Pattern Generation. J. Neurophysiol. 2017, 118, 2956–2974. [Google Scholar] [CrossRef]
- Holguera, I.; Desplan, C. Neuronal Specification in Space and Time. Sci. 2018, 362, 176–180. [Google Scholar] [CrossRef]
- Oberst, P.; Agirman, G.; Jabaudon, D. Principles of Progenitor Temporal Patterning in the Developing Invertebrate and Ver-tebrate Nervous System. Curr. Opin. Neurobiol. 2019, 56, 185–193. [Google Scholar] [CrossRef]
- Sagner, A.; Briscoe, J. Establishing Neuronal Diversity in the Spinal Cord: A Time and a Place. Development 2019, 146. [Google Scholar] [CrossRef]
- Delile, J.; Rayon, T.; Melchionda, M.; Edwards, A.; Briscoe, J.; Sagner, A. Single Cell Transcriptomics Reveals Spatial and Temporal Dynamics of Gene Expression in the Developing Mouse Spinal Cord. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Stam, F.J.; Hendricks, T.J.; Zhang, J.; Geiman, E.J.; Francius, C.; Labosky, P.; Clotman, F.; Goulding, M. Renshaw Cell In-ter-neuron Specialization Is Controlled by a Temporally Restricted Transcription Factor Program. Development 2012, 139, 179–190. [Google Scholar] [CrossRef]
- Harris, A.; Masgutova, G.; Collin, A.; Toch, M.; Hidalgo-Figueroa, M.; Jacob, B.; Corcoran, L.M.; Francius, C.; Clotman, F. Onecut Factors and Pou2f2 Regulate the Distribution of V2 Interneurons in the Mouse Developing Spinal Cord. Front. Cell. Neurosci. 2019, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Kabayiza, K.U.; Masgutova, G.; Harris, A.; Rucchin, V.; Jacob, B.; Clotman, F. The Onecut Transcription Factors Regulate Differentiation and Distribution of Dorsal Interneurons During Spinal Cord Development. Front. Mol. Neurosci. 2017, 10, 157. [Google Scholar] [CrossRef]
- Masgutova, G.; Harris, A.; Jacob, B.; Corcoran, L.M.; Clotman, F. Pou2f2 Regulates the Distribution of Dorsal Interneurons in the Mouse Developing Spinal Cord. Front. Mol. Neurosci. 2019, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Hinckley, C.A.; Driscoll, S.P.; Moore, N.J.; Levine, A.; Hilde, K.L.; Sharma, K.; Pfaff, S.L. Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control. Neuron 2018, 97, 869–884.e5. [Google Scholar] [CrossRef]
- Falgairolle, M.; O’Donovan, M.J. V1 interneurons regulate the pattern and frequency of locomotor-like activity in the neonatal mouse spinal cord. PLoS Biol. 2019, 17, e3000447. [Google Scholar] [CrossRef]
- Zhang, J.; Lanuza, G.M.; Britz, O.; Wang, Z.; Siembab, V.C.; Zhang, Y.; Velasquez, T.; Alvarez, F.J.; Frank, E.; Goulding, M. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 2014, 82, 138–150. [Google Scholar] [CrossRef]
- Britz, O.; Zhang, J.; Grossmann, K.S.; Dyck, J.; Kim, J.C.; Dymecki, S.; Gosgnach, S.; Goulding, M. A genetically defined asymmetry underlies the inhibitory control of flexor-extensor locomotor movements. Elife 2015, 4, e04718. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Jonas, P.C.; Sapir, T.; Hartley, R.; Berrocal, M.C.; Geiman, E.J.; Todd, A.J.; Goulding, M. Postnatal Phenotype and Localization of Spinal Cord V1 Derived Interneurons. J. Comp. Neurol. 2005, 493, 177–192. [Google Scholar] [CrossRef]
- Bikoff, J.B. Interneuron Diversity and Function in the Spinal Motor System. Curr. Opin. Physiol. 2019, 8, 36–43. [Google Scholar] [CrossRef]
- Gabitto, M.I.; Pakman, A.; Bikoff, J.B.; Abbott, L.; Jessell, T.M.; Paninski, L. Bayesian Sparse Regression Analysis Documents the Diversity of Spinal Inhibitory Interneurons. Cell 2016, 165, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.B.; Bikoff, J.B.; Gabitto, M.I.; Brenner-Morton, S.; Baek, M.; Yang, J.H.; Tabak, E.G.; Dasen, J.S.; Kintner, C.R.; Jessell, T.M. Origin and Segmental Diversity of Spinal Inhibitory Interneurons. Neuron 2018, 97, 341–355.e3. [Google Scholar] [CrossRef]
- Benito-Gonzalez, A.; Alvarez, F.J. Renshaw Cells and Ia Inhibitory Interneurons Are Generated at Different Times from P1 Progenitors and Differentiate Shortly After Exiting the Cell Cycle. J. Neurosci. 2012, 32, 1156–1170. [Google Scholar] [CrossRef]
- Doe, C.Q. Temporal Patterning in the Drosophila CNS. Annu. Rev. Cell Dev. Biol. 2017, 33, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.M.; Burroughs-Garcia, J.; Kriks, S.; Lewandoski, M.; Waters, S.T. Gbx1 and Gbx2 Are Essential for Normal Pat-terning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord. J. Dev. Biol. 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Boeri, J.; Le Corronc, H.; Lejeune, F.-X.; Le Bras, B.; Mouffle, C.; Angelim, M.K.S.; Mangin, J.-M.; Branchereau, P.; Legendre, P.; Czarnecki, A. Persistent Sodium Current Drives Excitability of Immature Renshaw Cells in Early Embryonic Spinal Networks. J. Neurosci. 2018, 38, 7667–7682. [Google Scholar] [CrossRef]
- Hoang, P.; Chalif, J.I.; Bikoff, J.B.; Jessell, T.M.; Mentis, G.Z.; Wichterle, H. Subtype Diversification and Synaptic Specificity of Stem Cell-Derived Spinal Interneurons. Neuron 2018, 100, 135–149.e7. [Google Scholar] [CrossRef]
- Allan, D.W.; Thor, S. Transcriptional Selectors, Masters, and Combinatorial Codes: Regulatory Principles of Neural SubtypeSpecification. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 505–528. [Google Scholar] [CrossRef]
- Blacklaws, J.; Deska-Gauthier, D.; Jones, C.T.; Petracca, Y.L.; Liu, M.; Zhang, H.; Fawcett, J.P.; Glover, J.C.; Lanuza, G.M.; Zhang, Y. Sim1is Required for the Migration and Axonal Projections of V3 Interneurons in the Developing Mouse Spinal Cord. Dev. Neurobiol. 2015, 75, 1003–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Narayan, S.; Geiman, E.; Lanuza, G.; Velasquez, T.; Shanks, B.; Akay, T.; Dyck, J.; Pearson, K.; Gosgnach, S.; et al. V3 Spinal Neurons Establish a Robust and Balanced Locomotor Rhythm During Walking. Neuron 2008, 60, 84–96. [Google Scholar] [CrossRef]
- Danner, S.M.; Zhang, H.; Shevtsova, N.; Borowska-Fielding, J.; Deska-Gauthier, D.; Rybak, I.A.; Zhang, Y. Spinal V3 In-ter-neurons and Left–Right Coordination in Mammalian Locomotion. Front. Cell. Neurosci. 2019, 13, 516. [Google Scholar] [CrossRef]
- Deska-Gauthier, D.; Borowska-Fielding, J.; Jones, C.T.; Zhang, Y. The Temporal Neurogenesis Patterning of Spinal p3–V3 Interneurons into Divergent Subpopulation Assemblies. J. Neurosci. 2020, 40, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Brohmann, H.; Pierani, A.; Heppenstall, P.A.; Lewin, G.; Jessell, T.M.; Birchmeier, C. The Homeodomain Factor Lbx1 Distinguishes Two Major Programs of Neuronal Differentiation in the Dorsal Spinal Cord. Neuron 2002, 34, 551–562. [Google Scholar] [CrossRef]
- John, A.; Wildner, H.; Britsch, S. The Homeodomain Transcription Factor Gbx1 Identifies a Subpopulation of Late-Born GABAergic Interneurons in the Developing Dorsal Spinal Cord. Dev. Dyn. 2005, 234, 767–771. [Google Scholar] [CrossRef]
- Lai, H.C.; Seal, R.P.; Johnson, J.E. Making Sense Out of Spinal Cord Somatosensory Development. Development 2016, 143, 3434–3448. [Google Scholar] [CrossRef]
- Buckley, D.M.; Burroughs-Garcia, J.; Lewandoski, M.; Waters, S.T. Characterization of the Gbx1−/− Mouse Mutant: A Re-quirement for Gbx1 in Normal Locomotion and Sensorimotor Circuit Development. PLoS ONE 2013, 8, e56214. [Google Scholar] [CrossRef] [PubMed]
- Meziane, H.; Fraulob, V.; Riet, F.; Krężel, W.; Selloum, M.; Geffarth, M.; Acampora, D.; Herault, Y.; Simeone, A.; Brand, M.; et al. The Homeodomain factorGbx1is Required for Locomotion and Cell Specification in the Dorsal Spinal Cord. PeerJ 2013, 1, e142. [Google Scholar] [CrossRef]
- Petracca, Y.L.; Sartoretti, M.M.; Di Bella, D.J.; Marin-Burgin, A.; Carcagno, A.L.; Schinder, A.F.; Lanuza, G.M. The Late and Dual Origin of Cerebrospinal Fluid-Contacting Neurons in the Mouse Spinal Cord. Development 2016, 143, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Böhm, U.L.; Prendergast, A.; Djenoune, L.; Figueiredo, S.N.; Gomez, J.; Stokes, C.; Kaiser, S.; Suster, M.; Kawakami, K.; Charpentier, M.; et al. CSF-Contacting Neurons Regulate Locomotion by Relaying Mechanical Stimuli to Spinal Circuits. Nat. Commun. 2016, 7, 10866. [Google Scholar] [CrossRef]
- Fidelin, K.; Djenoune, L.; Stokes, C.; Prendergast, A.; Gomez, J.; Baradel, A.; del Bene, F.; Wyart, C. State-Dependent Modu-lation of Locomotion by GABAergic Spinal Sensory Neurons. Curr. Biol. 2015, 25, 3035–3047. [Google Scholar] [CrossRef]
- Hubbard, J.; Böhm, U.L.; Prendergast, A.; Tseng, P.-E.B.; Newman, M.; Stokes, C.; Wyart, C. Intraspinal Sensory Neurons Provide Powerful Inhibition to Motor Circuits Ensuring Postural Control During Locomotion. Curr. Biol. 2016, 26, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, M.; Stepien, A.E.; Arber, S. Motor Antagonism Exposed by Spatial Segregation and Timing of Neurogenesis. Nat. Cell Biol. 2011, 479, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Knogler, L.D.; Ryan, J.; Saint-Amant, L.; Drapeau, P. A Hybrid Electrical/Chemical Circuit in the Spinal Cord Generates a Transient Embryonic Motor Behavior. J. Neurosci. 2014, 34, 9644–9655. [Google Scholar] [CrossRef]
- Berg, E.M.; Björnfors, E.R.; Pallucchi, I.; Picton, L.D.; El Manira, A. Principles Governing Locomotion in Vertebrates: Lessons from Zebrafish. Front. Neural Circuits 2018, 12, 73. [Google Scholar] [CrossRef]
- Saint-Amant, L. Development of Motor Rhythms in Zebrafish Embryos. Prog. Brain Res. 2010, 187, 47–61. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.L.; Fan, J.; Higashijima, S.-I.; Hale, M.E.; Fetcho, J.R. A Topographic Map of Recruitment in Spinal Cord. Nat. Cell Biol. 2007, 446, 71–75. [Google Scholar] [CrossRef]
- McLean, D.L.; A Masino, M.; Koh, I.Y.Y.; Lindquist, W.B.; Fetcho, J.R. Continuous Shifts in the Active Set of Spinal Interneurons During Changes in Locomotor Speed. Nat. Neurosci. 2008, 11, 1419–1429. [Google Scholar] [CrossRef]
- Roussel, Y.; Paradis, M.; Gaudreau, S.F.; Lindsey, B.W.; Bui, T.V. Spatiotemporal Transition in the Role of Synaptic Inhibition to the Tail Beat Rhythm of Developing Larval Zebrafish. Eneuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Buss, R.R.; Drapeau, P. Synaptic Drive to Motoneurons During Fictive Swimming in the Developing Zebrafish. J. Neuro Physiol. 2001, 86, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okamura, Y.; Higashijima, S.-I. Alx, a Zebrafish Homolog of Chx10, Marks Ipsilateral Descending Excitatory Interneurons That Participate in the Regulation of Spinal Locomotor Circuits. J. Neurosci. 2006, 26, 5684–5697. [Google Scholar] [CrossRef]
- Kimura, Y.; Higashijima, S.-I. Regulation of Locomotor Speed and Selection of Active Sets of Neurons by V1 Neurons. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Kishore, S.; Fetcho, J.R. Homeostatic Regulation of Dendritic Dynamics in a Motor Map in Vivo. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satou, C.; Kimura, Y.; Higashijima, S.-I. Generation of Multiple Classes of V0 Neurons in Zebrafish Spinal Cord: Progenitor Heterogeneity and Temporal Control of Neuronal Diversity. J. Neurosci. 2012, 32, 1771–1783. [Google Scholar] [CrossRef]
- Ampatzis, K.; Song, J.; Ausborn, J.; El Manira, A. Separate Microcircuit Modules of Distinct V2a Interneurons and Motoneurons Control the Speed of Locomotion. Neuron 2014, 83, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Ausborn, J.; Mahmood, R.; El Manira, A. Decoding the Rules of Recruitment of Excitatory Interneurons in the Adult Zebrafish Locomotor Network. Proc. Natl. Acad. Sci. USA 2012, 109, E3631–E3639. [Google Scholar] [CrossRef]
- Björnfors, E.R.; El Manira, A. Functional Diversity of Excitatory Commissural Interneurons in Adult Zebrafish. Elife 2016, 5, e18579. [Google Scholar] [CrossRef]
- McLean, D.L.; Fetcho, J.R. Spinal Interneurons Differentiate Sequentially from Those Driving the Fastest Swimming Move-ments in Larval Zebrafish to Those Driving the Slowest Ones. J. Neurosci. 2009, 29, 13566–13577. [Google Scholar] [CrossRef]
- Myers, P.; Eisen, J.; Westerfield, M. Development and Axonal Outgrowth of Identified Motoneurons in the Zebrafish. J. Neurosci. 1986, 6, 2278–2289. [Google Scholar] [CrossRef]
- Myers, P.Z. Spinal Motoneurons of the Larval Zebrafish. J. Comp. Neurol. 1985, 236, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Eckenstein, K.; Weihrauch, V.; Stigloher, C.; Brehm, P. Primary and Secondary Motoneurons Use Different Calcium Channel Types to Control Escape and Swimming Behaviors in Zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 26429–26437. [Google Scholar] [CrossRef]
- Kishore, S.; Cadoff, E.B.; Agha, M.A.; McLean, D.L. Orderly Compartmental Mapping of Premotor Inhibition in the Developing Zebrafish Spinal Cord. Science 2020, 370, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Pujala, A.; Koyama, M. Chronology-Based Architecture of Descending Circuits That Underlie the Development of Locomotor Repertoire After Birth. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deska-Gauthier, D.; Zhang, Y. The Temporal Mechanisms Guiding Interneuron Differentiation in the Spinal Cord. Int. J. Mol. Sci. 2021, 22, 8025. https://doi.org/10.3390/ijms22158025
Deska-Gauthier D, Zhang Y. The Temporal Mechanisms Guiding Interneuron Differentiation in the Spinal Cord. International Journal of Molecular Sciences. 2021; 22(15):8025. https://doi.org/10.3390/ijms22158025
Chicago/Turabian StyleDeska-Gauthier, Dylan, and Ying Zhang. 2021. "The Temporal Mechanisms Guiding Interneuron Differentiation in the Spinal Cord" International Journal of Molecular Sciences 22, no. 15: 8025. https://doi.org/10.3390/ijms22158025
APA StyleDeska-Gauthier, D., & Zhang, Y. (2021). The Temporal Mechanisms Guiding Interneuron Differentiation in the Spinal Cord. International Journal of Molecular Sciences, 22(15), 8025. https://doi.org/10.3390/ijms22158025




