Protective Effect of Memantine on Bergmann Glia and Purkinje Cells Morphology in Optogenetic Model of Neurodegeneration in Mice
Abstract
1. Introduction
2. Results
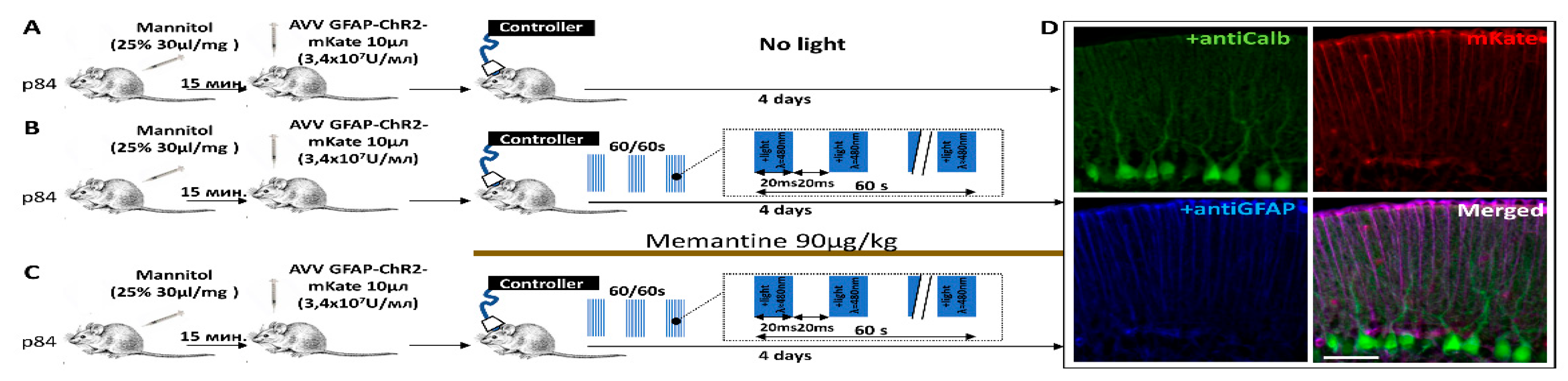
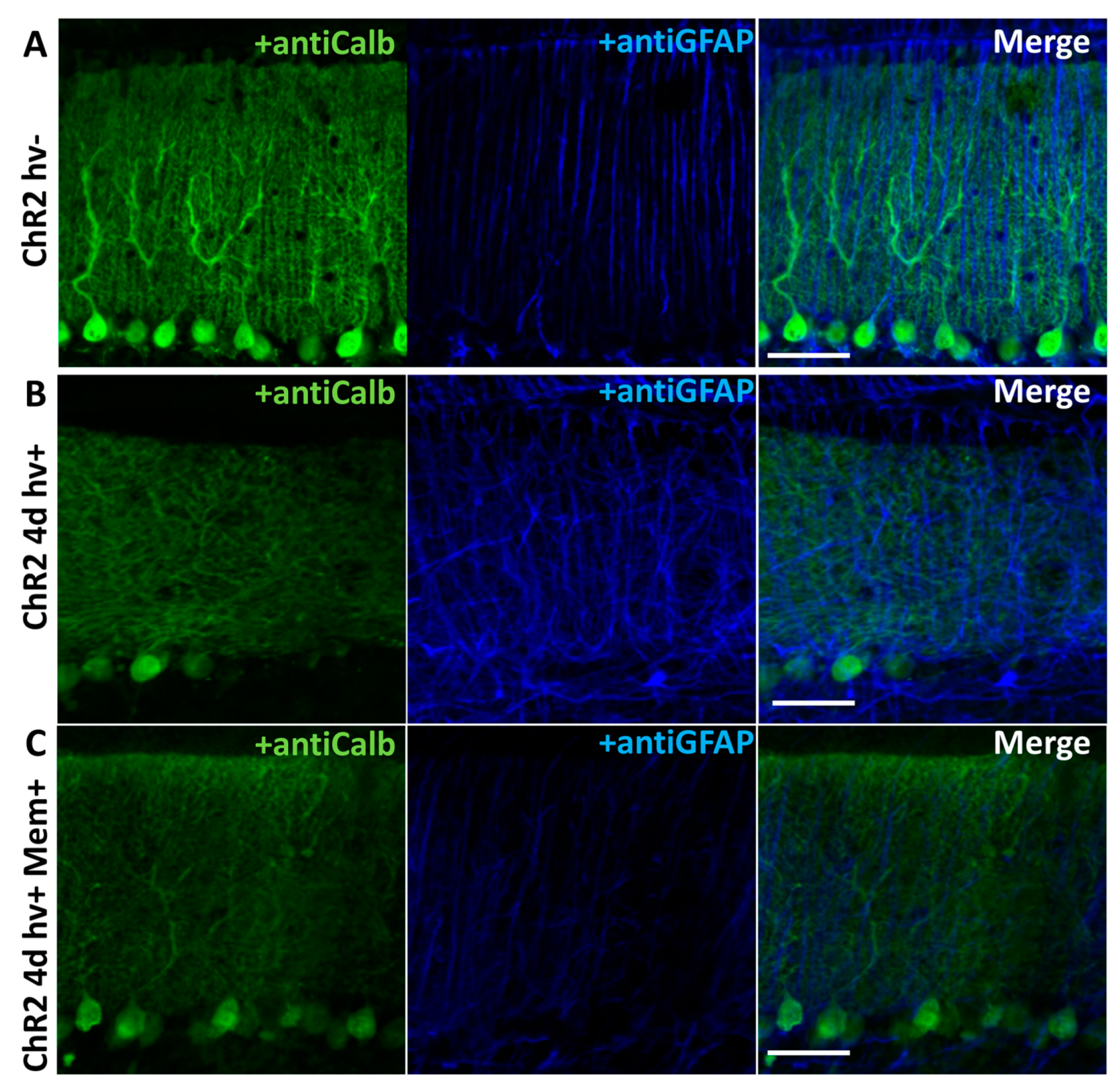
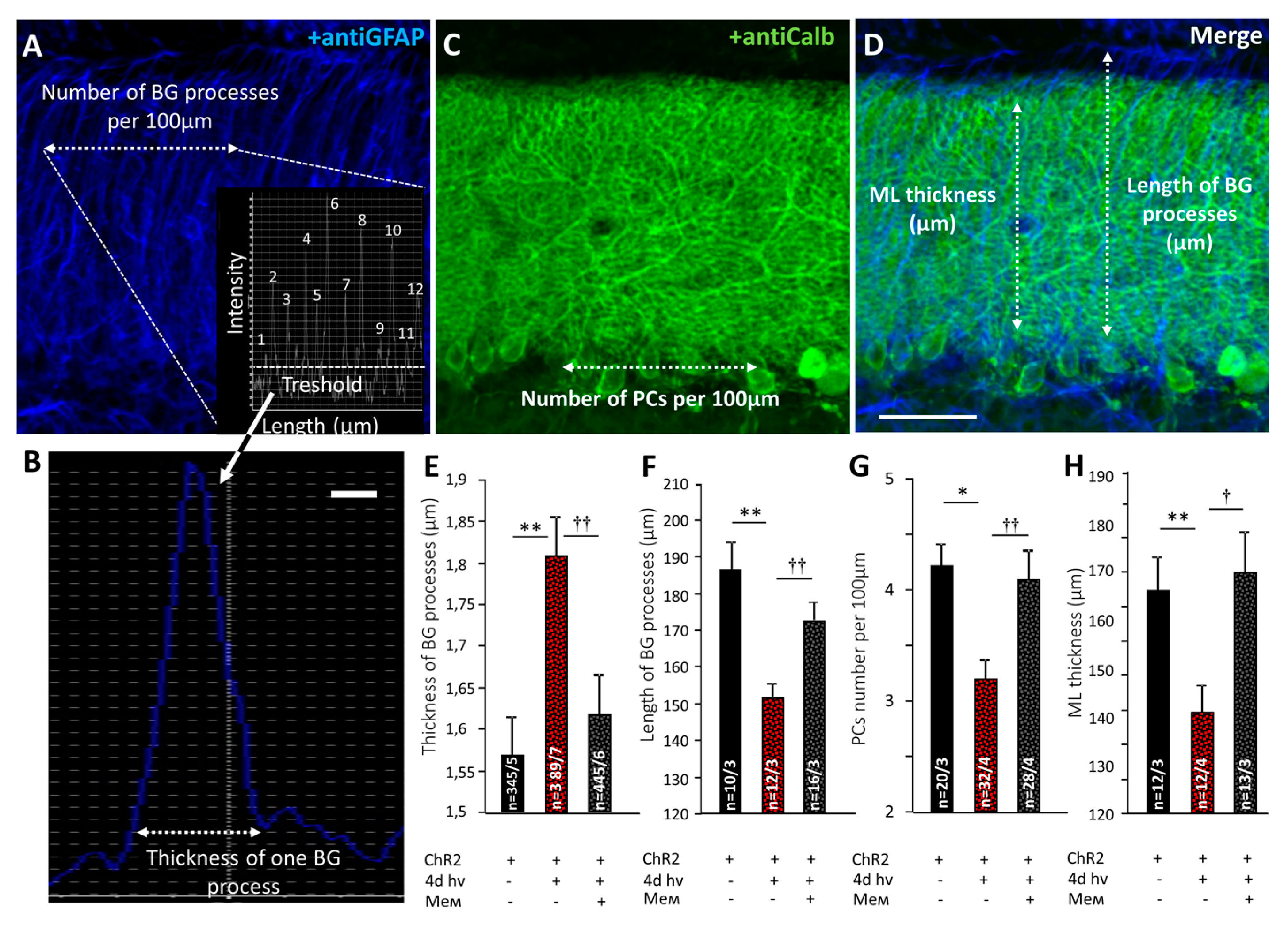
2.1. Memantine Prevents Pathological Changes in BG Morphology after Chronic Optogenetic Activation
2.2. Memantine Rescued PC Morphology and Synaptic Transmission in PF-PC Synapses after Chronic Photostimulation of BG
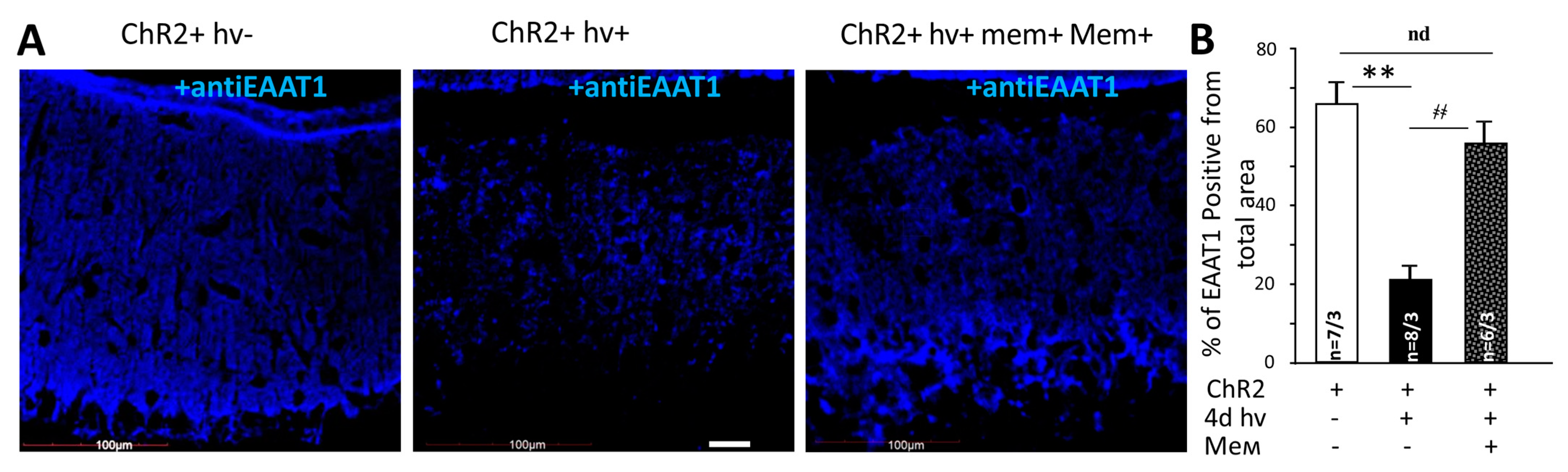
2.3. Memantine Prevents the Loss EAAT1 after Chronic Optogenetic Stimulation of BG
3. Discussion
4. Materials and Methods
4.1. Adenoviral Vector (AVV)
4.2. Modeling Neurodegeneration
4.3. Patch Clamp Recordings
4.4. Immunohistochemistry
4.5. Confocal Microscopy and Morphometric Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.; Anderson, A.R.; Beasley, S.J.; Barnett, N.L.; Poronnik, P.; Pow, D.V. A new splice variant of the glutamate-aspartate transporter: Cloning and immunolocalization of GLAST1c in rat, pig and human brains. J. Chem. Neuroanat. 2012, 43, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Power, E.M.; Empson, R.M. Functional contributions of glutamate transporters at the parallel fiber to Purkinje neuron synapse–relevance for the progression of cerebellar ataxia. Cerebellum Ataxias 2014, 1, 3. [Google Scholar] [CrossRef][Green Version]
- Cerrato, V. Cerebellar Astrocytes: Much More Than Passive Bystanders in Ataxia Pathophysiology. J. Clin. Med. 2020, 9, 757. [Google Scholar] [CrossRef]
- Valtcheva, S.; Venance, L. Control of Long-Term Plasticity by Glutamate Transporters. Front. Synaptic Neurosci. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, A.N.; Belozor, O.S.; Mozhei, O.; Yakovleva, D.A.; Potapenko, I.V.; Shuvaev, A.N.; Smolnikova, M.V.; Salmin, V.V.; Salmina, A.B.; Hirai, H.; et al. Chronic optogenetic stimulation of Bergman glia leads to dysfunction of EAAT1 and Purkinje cell death, mimicking the events caused by expression of pathogenic ataxin-1. Neurobiol. Dis. 2021, 154, 105340. [Google Scholar] [CrossRef] [PubMed]
- Slemmer, J.E.; De Zeeuw, C.I.; Weber, J.T. Don’t get too excited: Mechanisms of glutamate-mediated Purkinje cell death. Prog. Brain Res. 2005, 148, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Van Marum, R.J. Update on the use of memantine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2009, 5, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Céline, B.; Guy, B.; Annick, A.; Germán, S.; Mariano, C. Properties and molecular identity of NMDA receptors at synaptic and non-synaptic inputs in cerebellar molecular layer interneurons. Front. Synaptic Neurosci. 2015, 7, 1. [Google Scholar] [CrossRef]
- Lehre, K.P.; Danbolt, N.C. The number of glutamate transporter subtype molecules at glutamatergic synapses: Chemical and stereological quantification in young adult rat brain. J. Neurosci. 1998, 18, 8751–8757. [Google Scholar] [CrossRef]
- Kacerovsky, B.J.; Murai, K.K. Stargazing: Monitoring subcellular dynamics of brain astrocytes. Neuroscience 2016, 323, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Paton, J.F.; Kasparov, S. Viral vectors based on bidirectional cell-specific mammalian promoters and transcriptional amplification strategy for use in vitro and in vivo. BMC Biotechnol. 2008, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science 2010, 329, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Illarionova, N.B.; Brismar, H.; Aperia, A.; Gunnarson, E. Role of Na, K-ATPase α1 and α2 isoforms in the support of astrocyte glutamate uptake. PLoS ONE 2014, 9, e98469. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Kirchhoff, F. NMDA Receptors in glia. Neuroscientist 2007, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- López, T.; López-Colomé, A.M.; Ortega, A. NMDA receptors in cultured radial glia. FEBS Lett. 1997, 405, 245–248. [Google Scholar] [CrossRef]
- Müller, T.; Grosche, J.; Ohlemeyer, C.; Kettenmann, H. NMDA-activated currents in Bergmann glial cells. Neuroreport 1993, 4, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Palygin, O.; Lalo, U.; Pankratov, Y. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br. J. Pharmacol. 2011, 163, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Cvetanovic, M. Decreased expression of glutamate transporter GLAST in Bergmann glia is associated with the loss of Purkinje neurons in the spinocerebellar ataxia type 1. Cerebellum 2015, 14, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, A.; Nakamura, K.; Hirai, H. Long-term oral administration of the NMDA receptor antagonist memantine extends life span in spinocerebellar ataxia type 1 knock-in mice. Neurosci. Lett. 2015, 592, 37–41. [Google Scholar] [CrossRef]
- Da Re, F.; Rucci, F.; Isella, V. Retrospective study on agitation provoked by memantine in dementia. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e10–e13. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Lane, S.; Stout, R.F., Jr.; Liu, B.; Parpura, V.; Teschemacher, A.G.; Kasparov, S. Comparative analysis of optogenetic actuators in cultured astrocytes. Cell Calcium. 2014, 56, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.S.; Hwang, D.H.; Seok, J.I.; Shin, S.K.; Kim, S.U. Oral administration of memantine prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis. J. Clin. Neurol. 2007, 3, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, A.N.; Hosoi, N.; Sato, Y.; Yanagihara, D.; Hirai, H. Progressive impairment of cerebellar mGluR signalling and its therapeutic potential for cerebellar ataxia in spinocerebellar ataxia type 1 model mice. J. Physiol. 2017, 595, 141–164. [Google Scholar] [CrossRef] [PubMed]
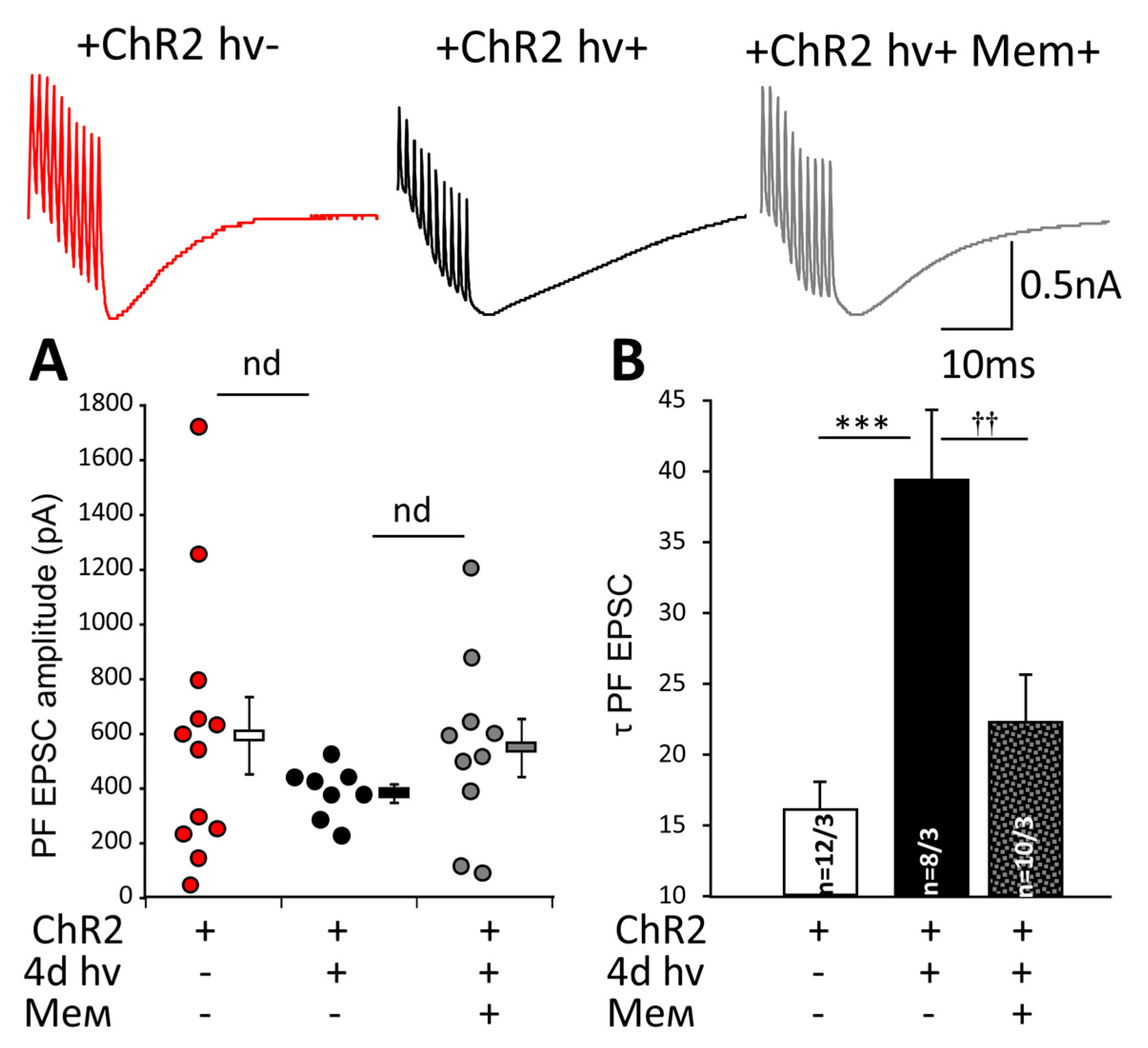
| Group | Capacity (pF) | Ra (MΩ) | Rm (MΩ) | Amplitude of PF EPSC (pA) | PPF | Rise Time (ms) | Decay Time (τ) |
|---|---|---|---|---|---|---|---|
| +GFAP-ChR2-mKate (hv−) (n = 8/3) | 725.1 ± 39.5 | 12.4 ± 0.6 | 186.3 ± 28.2 | 298.1 ± 64.6 | 1.8 ± 0.1 | 2.2 ± 0.3 | 16.0 ± 2.1 |
| +GFAP-ChR2-mKate (+ 4 d hv) (n = 10/3) | 494.2 ± 63.2 ** | 13.8 ± 1.4 | 211.5 ± 34.5 | 141.0 ± 23.1 * | 1.8 ± 0.1 | 2.4 ± 0.4 | 21.0 ± 3.0 |
| +GFAP-ChR2-mKate (+ 4 d hv) + Mem (n = 7/3) | 682.2 ± 24.5 † | 14.3 ± 0.9 | 173.4 ± 33.6 | 195.0 ± 33.7 | 1.8 ± 0.1 | 2.2 ± 0.2 | 18.8 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuvaev, A.N.; Belozor, O.S.; Mozhei, O.I.; Khilazheva, E.D.; Shuvaev, A.N.; Fritsler, Y.V.; Kasparov, S. Protective Effect of Memantine on Bergmann Glia and Purkinje Cells Morphology in Optogenetic Model of Neurodegeneration in Mice. Int. J. Mol. Sci. 2021, 22, 7822. https://doi.org/10.3390/ijms22157822
Shuvaev AN, Belozor OS, Mozhei OI, Khilazheva ED, Shuvaev AN, Fritsler YV, Kasparov S. Protective Effect of Memantine on Bergmann Glia and Purkinje Cells Morphology in Optogenetic Model of Neurodegeneration in Mice. International Journal of Molecular Sciences. 2021; 22(15):7822. https://doi.org/10.3390/ijms22157822
Chicago/Turabian StyleShuvaev, Anton N., Olga S. Belozor, Oleg I. Mozhei, Elena D. Khilazheva, Andrey N. Shuvaev, Yana V. Fritsler, and S. Kasparov. 2021. "Protective Effect of Memantine on Bergmann Glia and Purkinje Cells Morphology in Optogenetic Model of Neurodegeneration in Mice" International Journal of Molecular Sciences 22, no. 15: 7822. https://doi.org/10.3390/ijms22157822
APA StyleShuvaev, A. N., Belozor, O. S., Mozhei, O. I., Khilazheva, E. D., Shuvaev, A. N., Fritsler, Y. V., & Kasparov, S. (2021). Protective Effect of Memantine on Bergmann Glia and Purkinje Cells Morphology in Optogenetic Model of Neurodegeneration in Mice. International Journal of Molecular Sciences, 22(15), 7822. https://doi.org/10.3390/ijms22157822







