Chronic Myeloid Leukemia in Children and Adolescents: The Achilles Heel of Oncogenesis and Tyrosine Kinase Inhibitors
1. Introduction
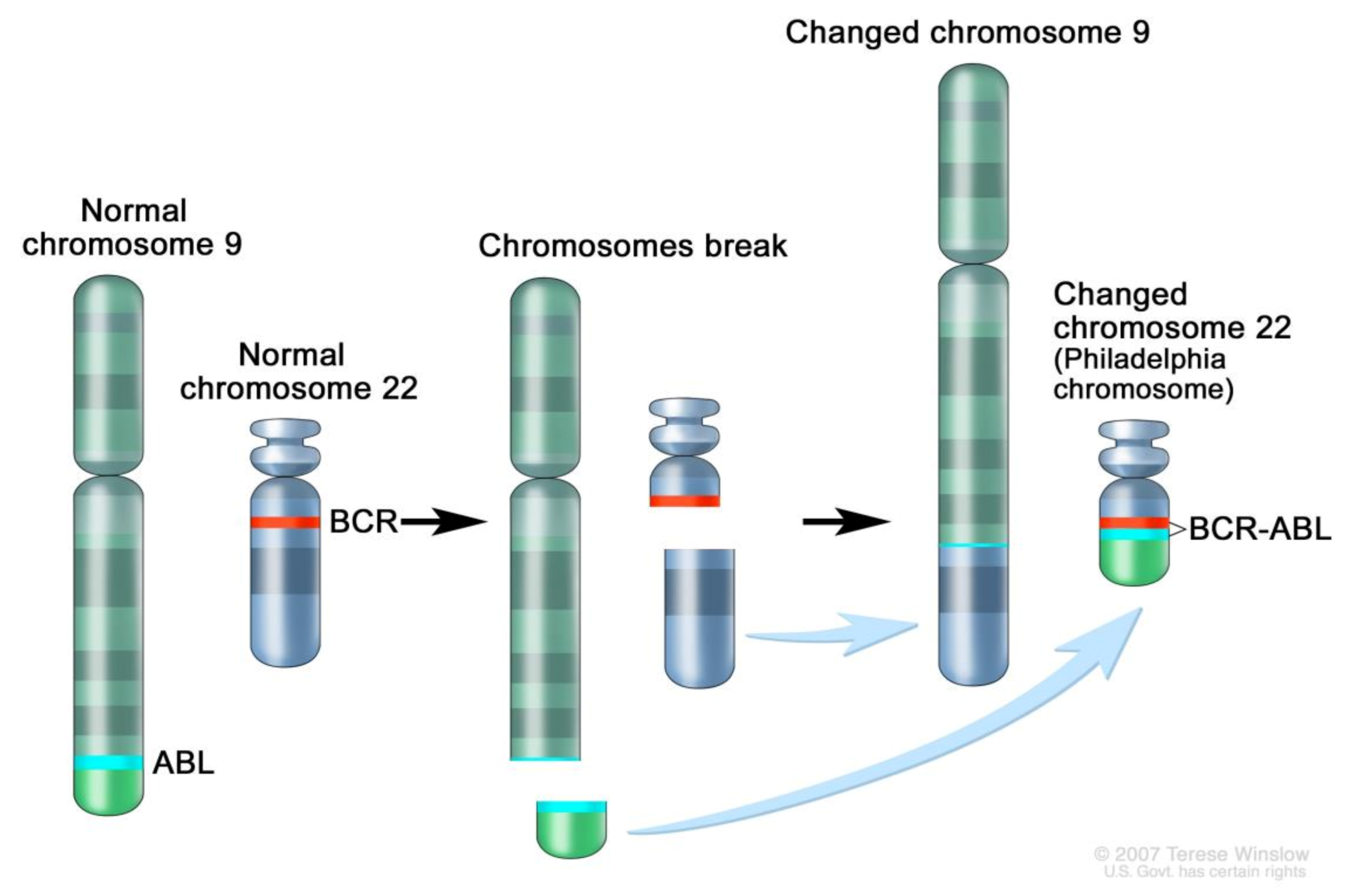
2. Genomics
3. Treatment with Imatinib and the Tyrosine Kinase Inhibitors Era
Prognosis and Side Effects
4. Special Populations
4.1. Infants
4.2. Adolescents and Young Adults (AYAs)
4.3. Vaccination
4.4. Reproductive Capacity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chronic Myelogenous Leukemia Treatment (PDQ®): Patient Version; National Cancer Institute: Bethesda, MD, USA, 2002. Available online: https://www.cancer.gov/types/leukemia/patient/cml-treatment-pdq (accessed on 31 January 2021).
- Crisà, E.; Nicolosi, M.; Ferri, V.; Favini, C.; Gaidano, G.; Patriarca, A. Atypical Chronic Myeloid Leukemia: Where Are We Now? Int. J. Mol. Sci. 2020, 21, 6862. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, L.; Martelli, M.; Cavo, M.; Soverini, S. Mechanisms of Disease Progression and Resistance to Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukemia: An Update. Int. J. Mol. Sci. 2019, 20, 6141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carofiglio, F.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Nicolotti, O.; Denora, N.; Stefanachi, A.; Leonetti, F. Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML. Int. J. Mol. Sci. 2020, 21, 4469. [Google Scholar] [CrossRef] [PubMed]
- Polillo, M.; Galimberti, S.; Baratè, C.; Petrini, M.; Danesi, R.; Di Paolo, A. Pharmacogenetics of BCR/ABL Inhibitors in Chronic Myeloid Leukemia. Int. J. Mol. Sci. 2015, 16, 22811–22829. [Google Scholar] [CrossRef]
- Stella, S.; Zammit, V.; Vitale, S.R.; Pennisi, M.S.; Massimino, M.; Tirrò, E.; Forte, S.; Spitaleri, A.; Antolino, A.; Siracusa, S.; et al. Clinical Implications of Discordant Early Molecular Responses in CML Patients Treated with Imatinib. Int. J. Mol. Sci. 2019, 20, 2226. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschovi, M.; Kelaidi, C. Chronic Myeloid Leukemia in Children and Adolescents: The Achilles Heel of Oncogenesis and Tyrosine Kinase Inhibitors. Int. J. Mol. Sci. 2021, 22, 7806. https://doi.org/10.3390/ijms22157806
Moschovi M, Kelaidi C. Chronic Myeloid Leukemia in Children and Adolescents: The Achilles Heel of Oncogenesis and Tyrosine Kinase Inhibitors. International Journal of Molecular Sciences. 2021; 22(15):7806. https://doi.org/10.3390/ijms22157806
Chicago/Turabian StyleMoschovi, Maria, and Charikleia Kelaidi. 2021. "Chronic Myeloid Leukemia in Children and Adolescents: The Achilles Heel of Oncogenesis and Tyrosine Kinase Inhibitors" International Journal of Molecular Sciences 22, no. 15: 7806. https://doi.org/10.3390/ijms22157806
APA StyleMoschovi, M., & Kelaidi, C. (2021). Chronic Myeloid Leukemia in Children and Adolescents: The Achilles Heel of Oncogenesis and Tyrosine Kinase Inhibitors. International Journal of Molecular Sciences, 22(15), 7806. https://doi.org/10.3390/ijms22157806





