Modulation of Rice Leaf Angle and Grain Size by Expressing OsBCL1 and OsBCL2 under the Control of OsBUL1 Promoter
Abstract
:1. Introduction
2. Results
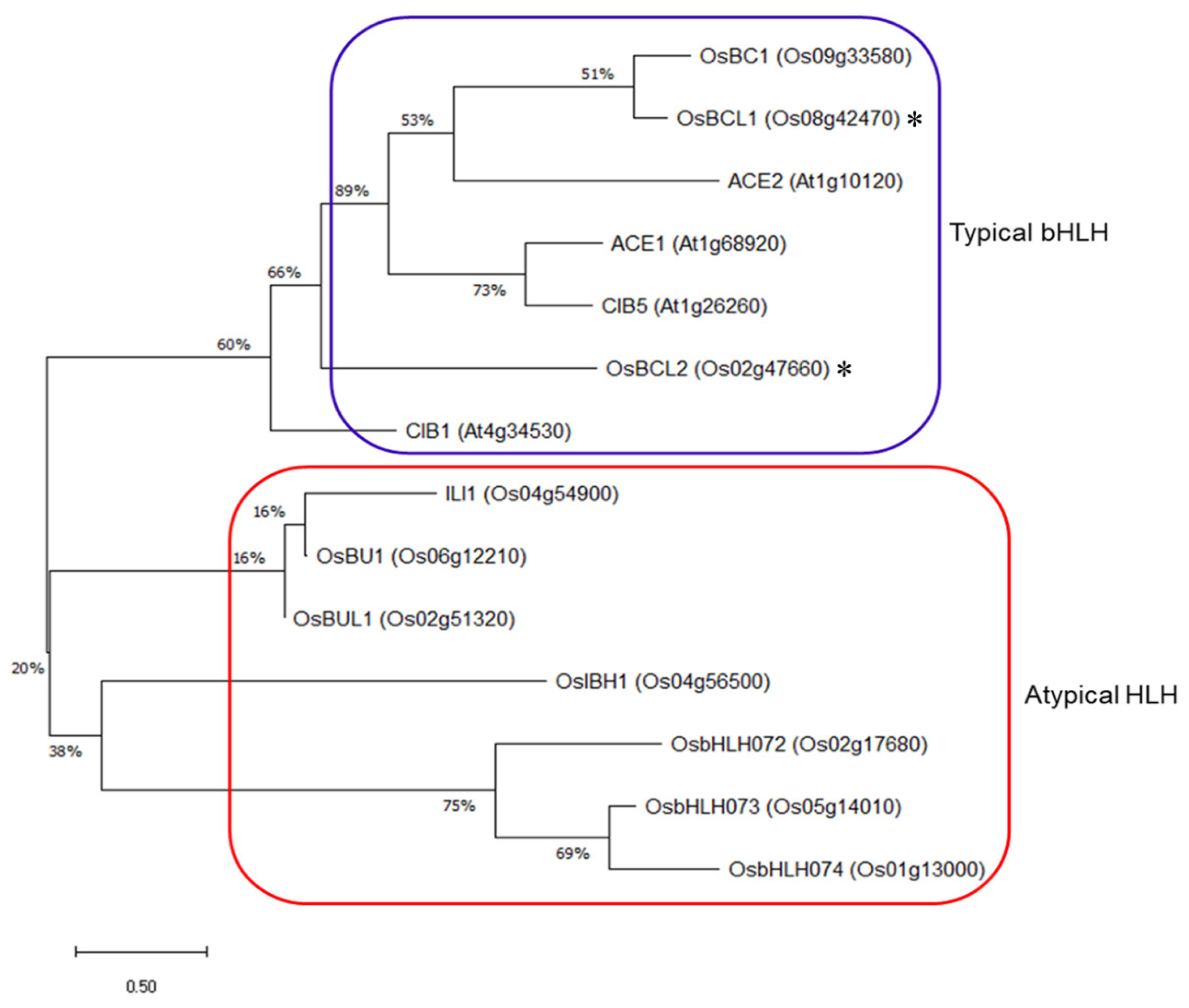
2.1. Isolation of OsBCL1 and OsBCL2
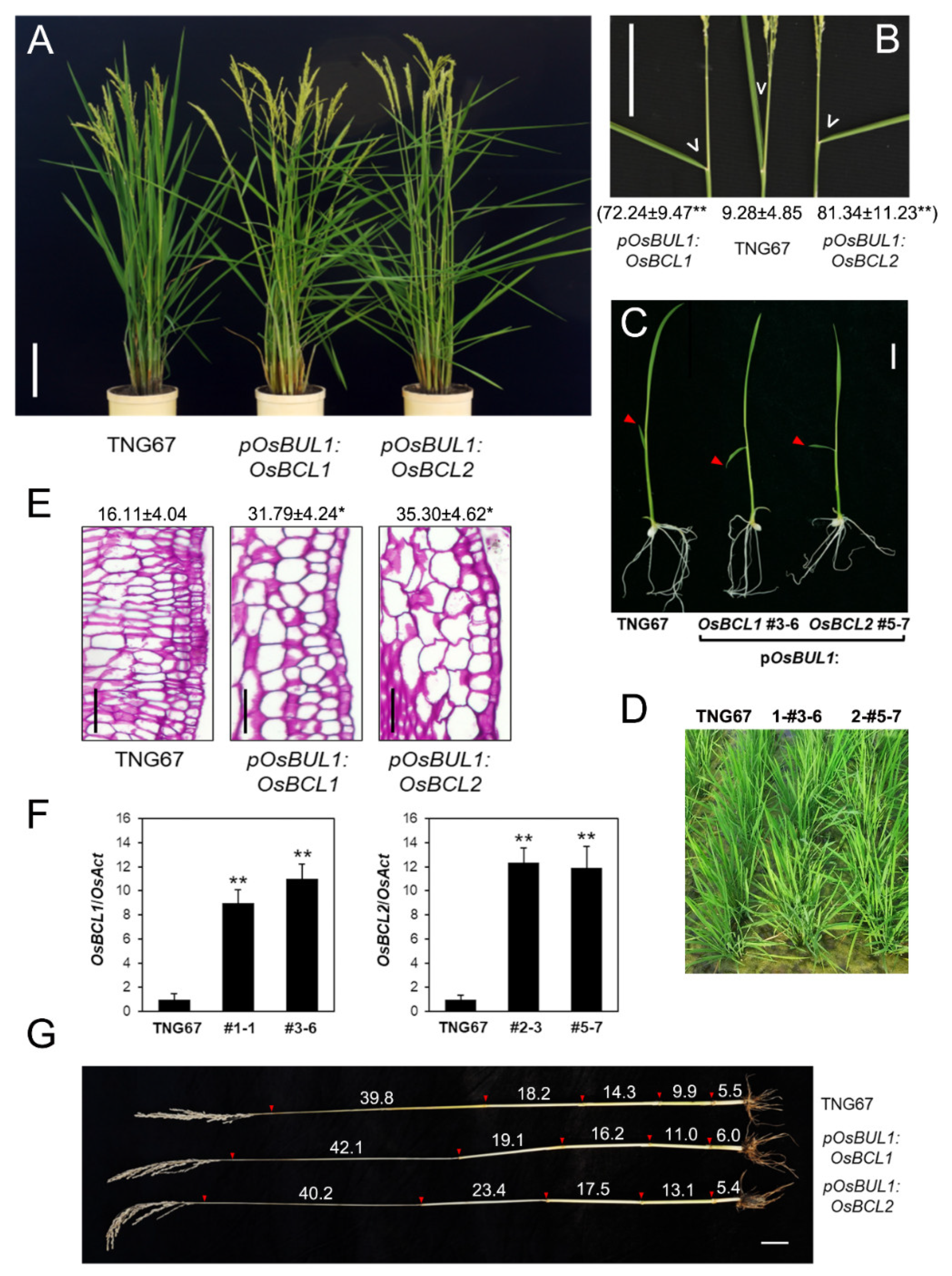
2.2. Increased Inclination Angle of Leaves Was Caused by Higher Expression of OsBCL1 and OsBCL2 in the Lamina Joint
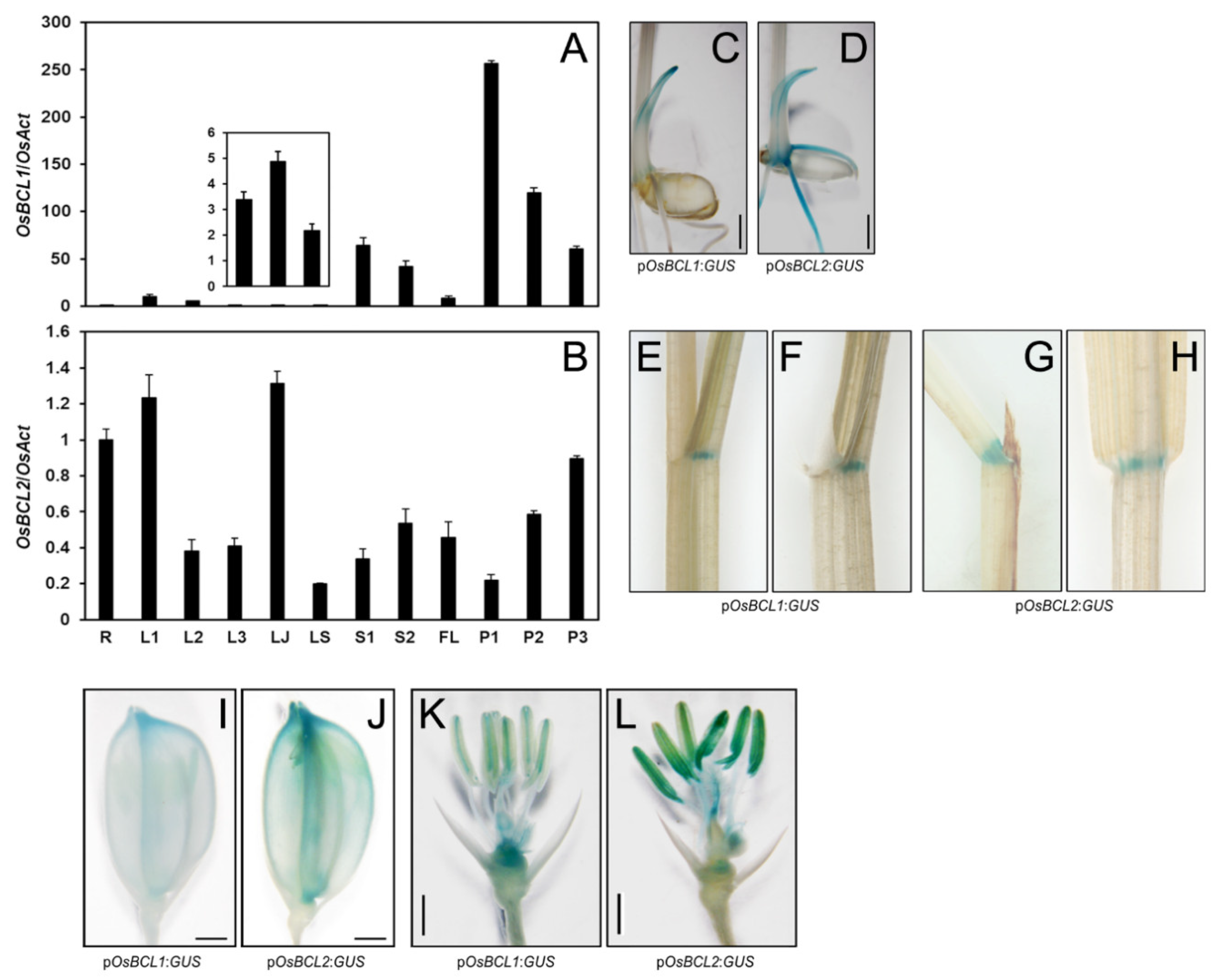
2.3. GUS Expression Driven by OsBCL1 and OsBCL2 Promoters Exhibited Similar but Different Patterns in Rice
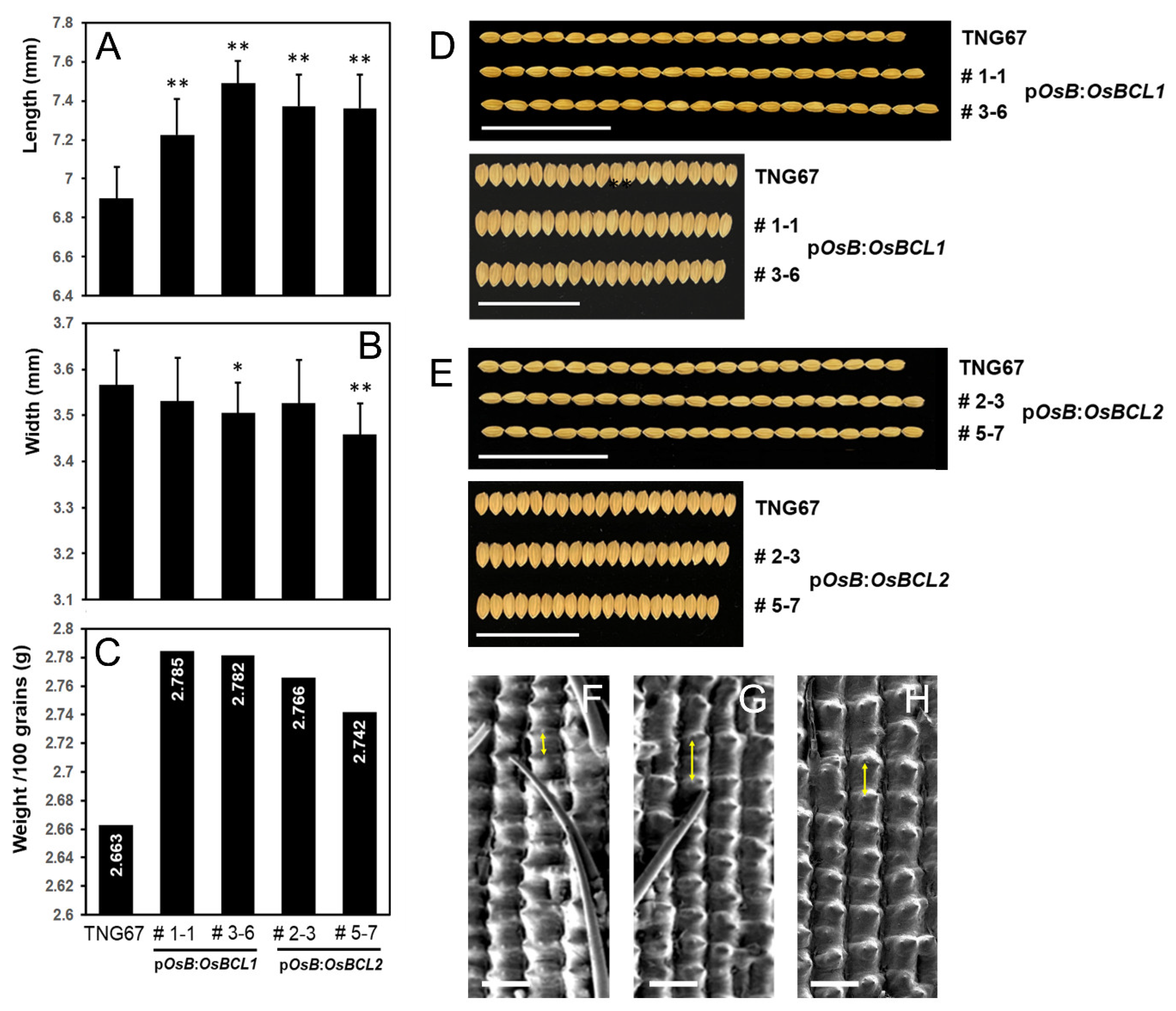
2.4. Elongated Grains Were Produced by the Increased Expression of OsBCL1 and OsBCL2 in Rice Flowers
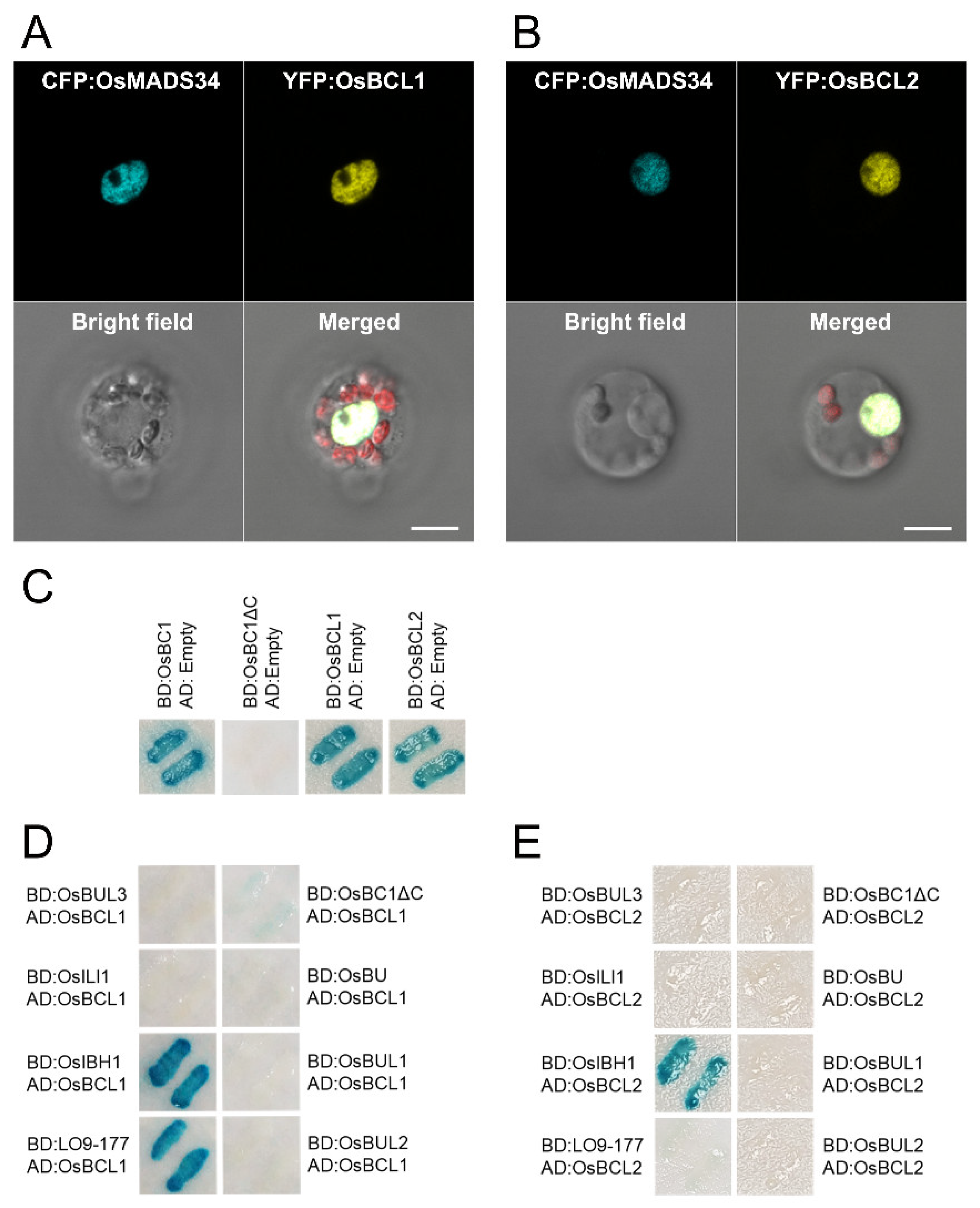
2.5. Both OsBCL1 and OsBCL2 Are Nuclear Proteins and Interact with OsIBH1, a Negative Regulator of Cell Elongation in Rice
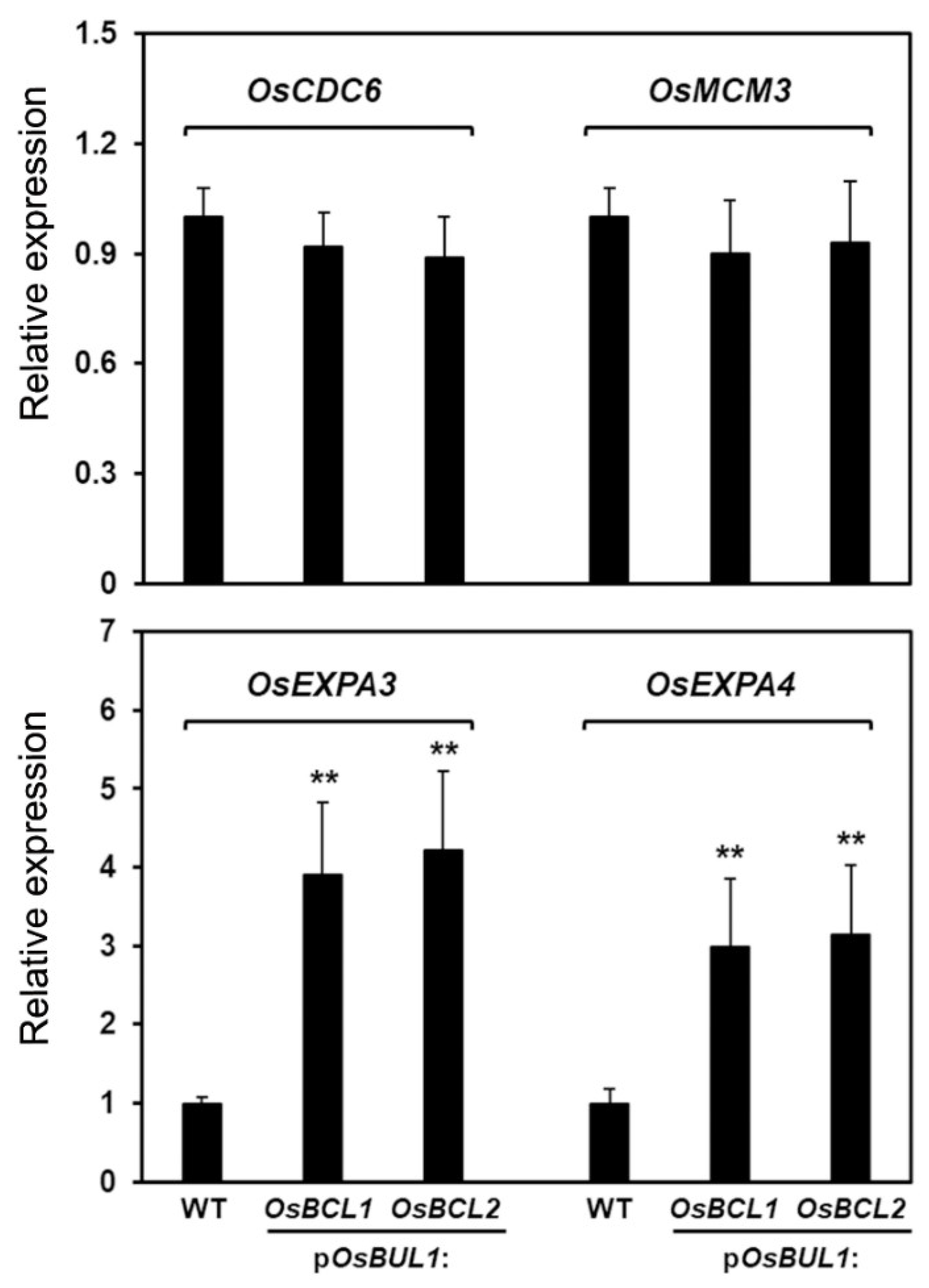
2.6. More GA3 Was Detected in the Transgenic Rice with Higher Expression of OsBCL1 and OsBCL2
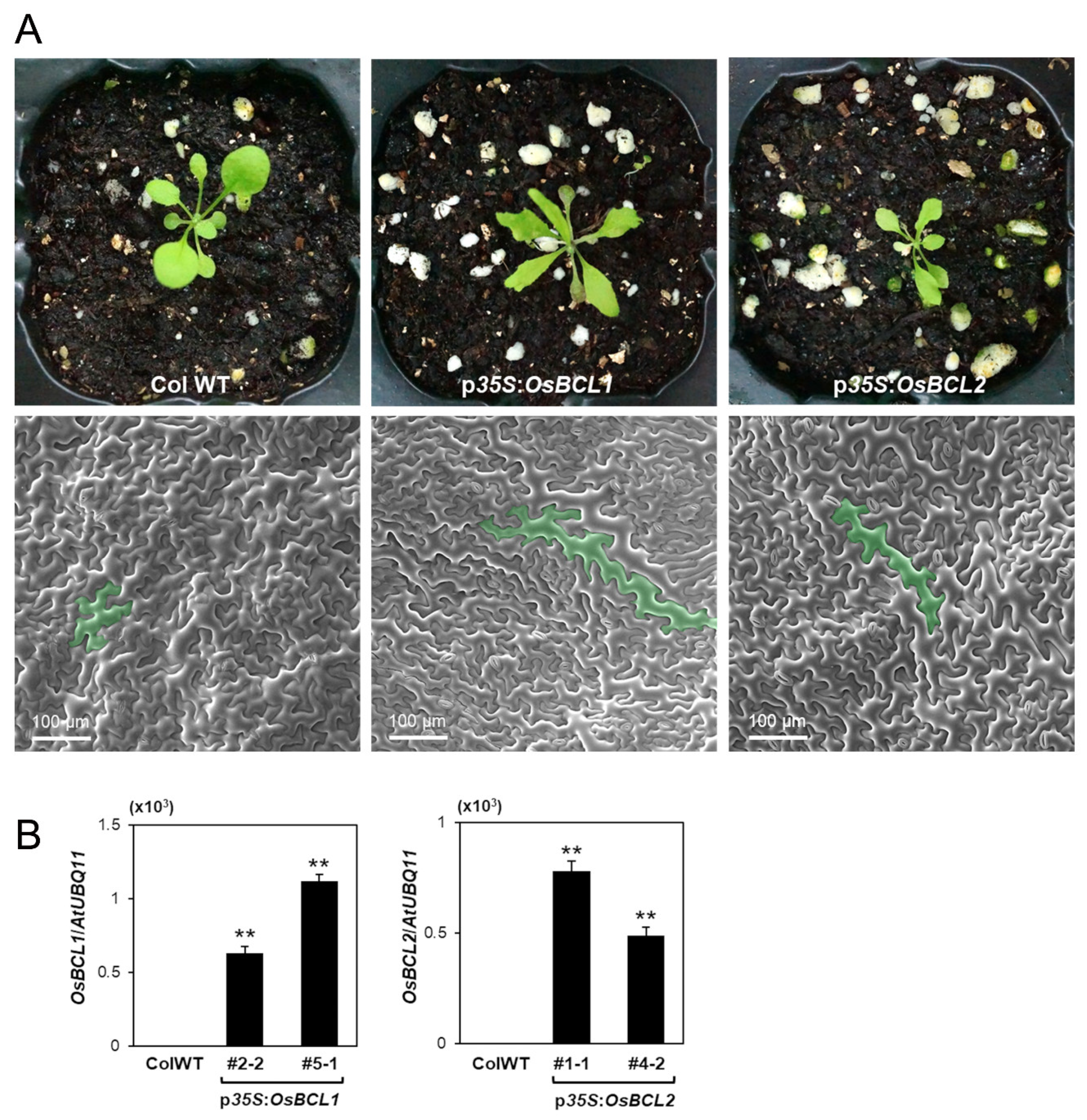
2.7. Overexpression of OsBCL1 and OsBCL2 in Arabidopsis Causes Cell Elongation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. GA3 Treatment
4.3. Vector Construction and Plant Transformation
4.4. RNA Extraction and Expression Analyses
4.5. GUS Staining
4.6. Phytohormone Sample Preparation
4.7. UPLC-MS Analysis
4.8. Histological Analyses and Microscopy
4.9. Subcellular Localization of Proteins
4.10. Yeast Two-Hybrid Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.-Q.; Hu, J.; Guo, L.-B.; Qian, Q.; Xue, H.-W. Rice leaf inclination2, a VIN3-like protein, regulates leaf angle through modulating cell division of the collar. Cell Res. 2010, 20, 935–947. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; An, G.; Li, H.-Y. Rice Leaf Angle and Grain Size Are Affected by the OsBUL1 Transcriptional Activator Complex. Plant Physiol. 2017, 173, 688–702. [Google Scholar] [CrossRef]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 2000, 12, 1591–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.Y.; Bai, M.Y.; Wu, J.; Zhu, J.Y.; Wang, H.; Zhang, Z.; Wang, W.; Sun, Y.; Zhao, J.; Sun, X.; et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; Zhang, B.; Wang, N.; Zhou, Y.; Xiong, L. Increased Leaf Angle1, a Raf-Like MAPKKK That Interacts with a Nuclear Protein Family, Regulates Mechanical Tissue Formation in the Lamina Joint of Rice. Plant Cell 2011, 23, 4334–4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Sun, J.; Cao, X.; Song, X. Epigenetic Mutation of RAV6 Affects Leaf Angle and Seed Size in Rice. Plant Physiol. 2015, 169, 2118–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wang, S.; Xu, Y.; Yu, C.; Shen, C.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y. The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Wu, C.; Wang, C.; Roh, J.; Zhang, L.; Chen, J.; Zhang, S.; Zhang, H.; Yang, C.; Hu, J.; et al. SLG controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice. J. Exp. Bot. 2016, 67, 4241–4253. [Google Scholar] [CrossRef] [Green Version]
- Mantilla-Perez, M.B.; Salas Fernandez, M.G. Differential manipulation of leaf angle throughout the canopy: Current status and prospects. J. Exp. Bot. 2017, 68, 5699–5717. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Kim, S.H.; Lee, B.D.; Lim, J.H.; Lee, S.J.; An, G.; Paek, N.C. The Rice Basic Helix-Loop-Helix 79 (OsbHLH079) Determines Leaf Angle and Grain Shape. Int. J. Mol. Sci. 2020, 21, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.-J.; Xiao, L.-T.; Xue, H.-W. Dynamic Cytology and Transcriptional Regulation of Rice Lamina Joint Development. Plant Physiol. 2017, 174, 1728–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, A.; Ueguchi-Tanaka, M.; Sakamoto, T.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Sazuka, T.; Ashikari, M.; Matsuoka, M. The rice SPINDLY gene functions as a negative regulator of gibberellin signaling by controlling the suppressive function of the DELLA protein, SLR1, and modulating brassinosteroid synthesis. Plant J. 2006, 48, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, L.; Jin, Y.; Du, L.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING Acts as a Direct Downstream Target of a GSK3/SHAGGY-Like Kinase to Mediate Brassinosteroid Responses in Rice. Plant Cell 2012, 24, 2562–2577. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, D.; Li, X.; Qiao, S.; Shi, C.; Li, C.; Shen, H.; Wang, X. Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Dev. Cell 2015, 34, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-y.; Trieu, A.; Radhakrishnan, P.; Kwok, S.F.; Harris, S.; Zhang, K.; Wang, J.; Wan, J.; Zhai, H.; Takatsuto, S.; et al. Brassinosteroids regulate grain filling in rice. Plant Cell 2008, 20, 2130–2145. [Google Scholar] [CrossRef] [Green Version]
- Hong, Z.; Ueguchi-Tanaka, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 2005, 17, 2243–2254. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, L.; Wang, M.; Xu, Y.Y.; Luo, W.; Liu, Y.J.; Xu, Z.H.; Li, J.; Chong, K. Engineering OsBAK1 gene as a molecular tool to improve rice architecture for high yield. Plant Biotechnol. J. 2009, 7, 791–806. [Google Scholar] [CrossRef]
- Potter, C.J.; Xu, T. Mechanisms of size control. Curr. Opin. Genet. Dev. 2001, 11, 279–286. [Google Scholar] [CrossRef]
- Sugimoto-Shirasu, K.; Roberts, K. “Big it up”: Endoreduplication and cell-size control in plants. Curr. Opin. Plant Biol. 2003, 6, 544–553. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Bai, M.-y.; Chong, K. Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep. 2014, 33, 683–696. [Google Scholar] [CrossRef]
- Tong, H.; Chu, C. Reply: Brassinosteroid Regulates Gibberellin Synthesis to Promote Cell Elongation in Rice: Critical Comments on Ross and Quittenden’s Letter. Plant Cell 2016, 28, 833–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.J.; Quittenden, L.J. Interactions between Brassinosteroids and Gibberellins: Synthesis or Signaling? Plant Cell 2016, 28, 829–832. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Xu, Y.; Joo, S.H.; Kim, S.K.; Xue, Z.; Xu, Z.; Wang, Z.; Chong, K. OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J. 2009, 57, 498–510. [Google Scholar] [CrossRef]
- Wei, L.; Gu, L.; Song, X.; Cui, X.; Lu, Z.; Zhou, M.; Wang, L.; Hu, F.; Zhai, J.; Meyers, B.C.; et al. Dicer-like 3 produces transposable element-associated 24-nt siRNAs that control agricultural traits in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 3877–3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Liu, H.; Guo, S.; Wang, B.; Li, Z.; Chong, K.; Xu, Y. OsmiR396d Affects Gibberellin and Brassinosteroid Signaling to Regulate Plant Architecture in Rice. Plant Physiol. 2018, 176, 946–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, N.; Dolan, L. Early evolution of bHLH proteins in plants. Plant Signal. Behav. 2010, 5, 911–912. [Google Scholar] [CrossRef]
- Li, X.; Duan, X.; Jiang, H.; Sun, Y.; Tang, Y.; Yuan, Z.; Guo, J.; Liang, W.; Chen, L.; Yin, J.; et al. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006, 141, 1167–1184. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.-H.; Han, M.-J.; Lee, Y.-S.; Kim, Y.-W.; Hwang, I.; Kim, M.-J.; Kim, Y.-K.; Nahm, B.H.; An, G. Rice Undeveloped Tapetum1 is a major regulator of early tapetum development. Plant Cell 2005, 17, 2705–2722. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, T.; Kyozuka, J. Two-Step Regulation of LAX PANICLE1 Protein Accumulation in Axillary Meristem Formation in Rice. Plant Cell 2009, 21, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Ogo, Y.; Itai, R.N.; Nakanishi, H.; Kobayashi, T.; Takahashi, M.; Mori, S.; Nishizawa, N.K. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 2007, 51, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, Y.; Cai, Y.; Niu, M.; Feng, Z.; Jing, R.; Mou, C.; Liu, X.; Xiao, L.; Zhang, X.; et al. Overexpression of OsbHLH107, a member of the basic helix-loop-helix transcription factor family, enhances grain size in rice (Oryza sativa L.). Rice 2018, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Moon, S.; Jang, S.; Lee, S.; An, G.; Jung, K.-H.; Park, S.K. OsbHLH073 Negatively Regulates Internode Elongation and Plant Height by Modulating GA Homeostasis in Rice. Plants 2020, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Friedrichsen, D.M.; Nemhauser, J.; Muramitsu, T.; Maloof, J.N.; Alonso, J.; Ecker, J.R.; Furuya, M.; Chory, J. Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 2002, 162, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Yang, K.-Y.; Kim, Y.-M.; Park, S.-Y.; Kim, S.Y.; Soh, M.-S. Overexpression of PRE1 and its Homologous Genes Activates Gibberellin-dependent Responses in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Kathare, P.K.; Kim, J.I.; Huq, E. Expanding Roles of PIFs in Signal Integration from Multiple Processes. Mol. Plant 2017, 10, 1035–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Song, J.H.; Park, S.U.; Jeong, Y.S.; Kim, S.H. Brassinosteroid-Induced Transcriptional Repression and Dephosphorylation-Dependent Protein Degradation Negatively Regulate BIN2-Interacting AIF2 (a BR Signaling-Negative Regulator) bHLH Transcription Factor. Plant Cell Physiol. 2017, 58, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, M.-q.; Chang, Y.-p.; Zhang, B.; Zhao, Q.-z.; Zhao, W.-l. The basic helix-loop-helix transcription factor OsBLR1 regulates leaf angle in rice via brassinosteroid signalling. Plant Mol. Biol. 2020, 102, 589–602. [Google Scholar] [CrossRef]
- Wei, K.; Chen, H. Comparative functional genomics analysis of bHLH gene family in rice, maize and wheat. BMC Plant Biol. 2018, 18, 309. [Google Scholar] [CrossRef]
- Guo, J.; Li, W.; Shang, L.; Wang, Y.; Yan, P.; Bai, Y.; Da, X.; Wang, K.; Guo, Q.; Jiang, R.; et al. OsbHLH98 regulates leaf angle in rice through transcriptional repression of OsBUL1. New Phytol. 2021, 230, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Guo, T.; Li, X.-M.; Zhang, Y.-M.; Yang, Y.-B.; Ye, W.-W.; Dong, N.-Q.; Shi, C.-L.; Kan, Y.; Xiang, Y.-H.; et al. Translational Regulation of Plant Response to High Temperature by a Dual-Function tRNAHis Guanylyltransferase in Rice. Mol. Plant 2019, 12, 1123–1142. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Guo, M.; Xu, L.; Wang, X.; Zhao, H.; Wang, J.; Yi, K. An SPX-RLI1 Module Regulates Leaf Inclination in Response to Phosphate Availability in Rice. Plant Cell 2018, 30, 853–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-Y.; Wang, H.-M.; Jang, S. Rice lamina joint inclination assay. Bio-Protocol 2017, 7, e2409. [Google Scholar] [CrossRef]
- Kim, S.-R.; Lee, D.-Y.; Yang, J.-I.; Moon, S.; An, G. Cloning Vectors for Rice. J. Plant Biol. 2009, 52, 73. [Google Scholar] [CrossRef]
- Jeon, J.S.; Lee, S.; Jung, K.H.; Jun, S.H.; Jeong, D.H.; Lee, J.; Kim, C.; Jang, S.; Yang, K.; Nam, J.; et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. 2000, 22, 561–570. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Caldana, C.; Scheible, W.-R.; Mueller-Roeber, B.; Ruzicic, S. A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods 2007, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wang, M.; Zuo, J.; Feng, X.; Liang, X.; Wu, Z.; Ye, H. Cytosolic BolA Plays a Repressive Role in the Tolerance against Excess Iron and MV-Induced Oxidative Stress in Plants. PLoS ONE 2015, 10, e0124887. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, R.A. The GUS reporter gene system. Nature 1989, 342, 837–838. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Clément, C.; Burrus, M.; Audran, J.-C. Floral organ growth and carbohydrate content during pollen development in Lilium. Am. J. Bot. 1996, 83, 459–469. [Google Scholar] [CrossRef]
- Jang, S.; Li, H.Y. Oryza sativa BRASSINOSTEROID UPREGULATED1 LIKE1 Induces the Expression of a Gene Encoding a Small Leucine-Rich-Repeat Protein to Positively Regulate Lamina Inclination and Grain Size in Rice. Front. Plant Sci. 2017, 8, 1253. [Google Scholar] [CrossRef] [PubMed]
- Jang, S. A novel trimeric complex in plant cells that contributes to the lamina inclination of rice. Plant Signal. Behav. 2017, 12, e1274482. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Cho, J.-Y.; Do, G.-R.; Kang, Y.; Li, H.-Y.; Song, J.; Kim, H.-Y.; Kim, B.-G.; Hsing, Y.-I. Modulation of Rice Leaf Angle and Grain Size by Expressing OsBCL1 and OsBCL2 under the Control of OsBUL1 Promoter. Int. J. Mol. Sci. 2021, 22, 7792. https://doi.org/10.3390/ijms22157792
Jang S, Cho J-Y, Do G-R, Kang Y, Li H-Y, Song J, Kim H-Y, Kim B-G, Hsing Y-I. Modulation of Rice Leaf Angle and Grain Size by Expressing OsBCL1 and OsBCL2 under the Control of OsBUL1 Promoter. International Journal of Molecular Sciences. 2021; 22(15):7792. https://doi.org/10.3390/ijms22157792
Chicago/Turabian StyleJang, Seonghoe, Jwa-Yeong Cho, Gyung-Ran Do, Yeeun Kang, Hsing-Yi Li, Jaeeun Song, Ho-Youn Kim, Beom-Gi Kim, and Yue-Ie Hsing. 2021. "Modulation of Rice Leaf Angle and Grain Size by Expressing OsBCL1 and OsBCL2 under the Control of OsBUL1 Promoter" International Journal of Molecular Sciences 22, no. 15: 7792. https://doi.org/10.3390/ijms22157792
APA StyleJang, S., Cho, J.-Y., Do, G.-R., Kang, Y., Li, H.-Y., Song, J., Kim, H.-Y., Kim, B.-G., & Hsing, Y.-I. (2021). Modulation of Rice Leaf Angle and Grain Size by Expressing OsBCL1 and OsBCL2 under the Control of OsBUL1 Promoter. International Journal of Molecular Sciences, 22(15), 7792. https://doi.org/10.3390/ijms22157792








