Morphometric and Nanomechanical Features of Platelets from Women with Early Pregnancy Loss Provide New Evidence of the Impact of Inherited Thrombophilia
Abstract
1. Introduction
2. Results
2.1. Main Patient Characteristics and Blood Clinical Parameters Derived for the Participants under Study
2.2. Carriage of Thrombophilia Polymorphisms
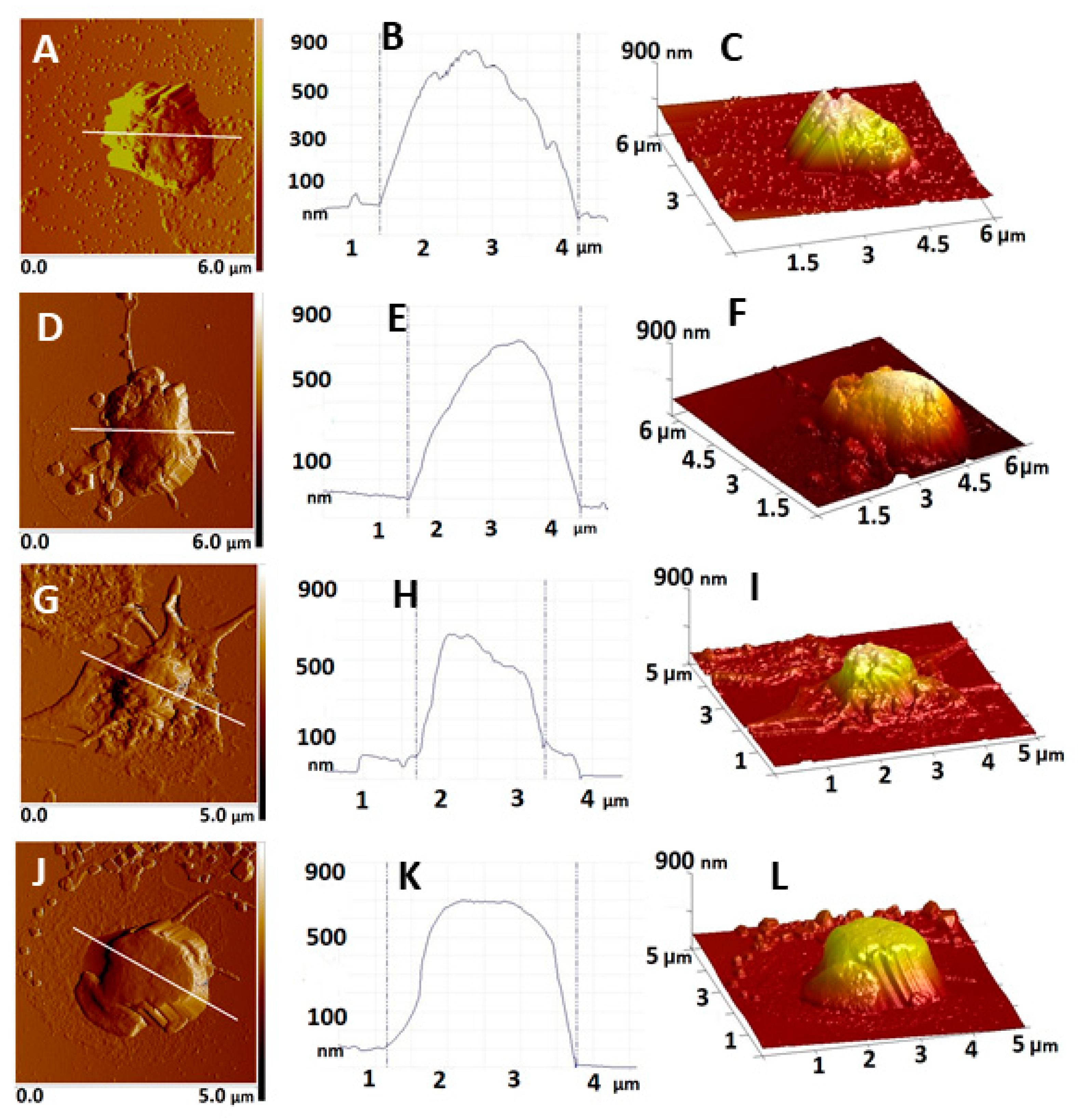
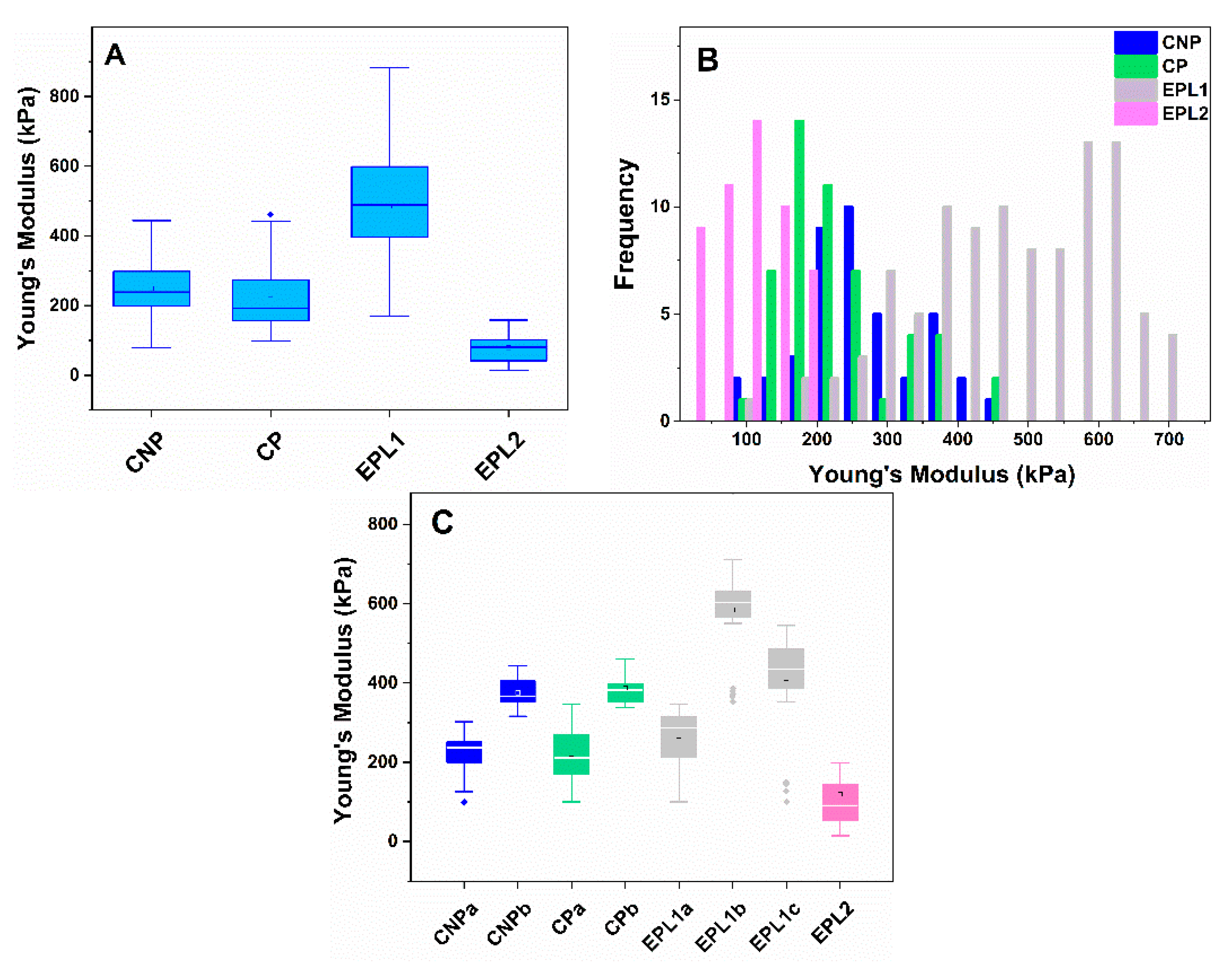
2.3. Atomic Force Microscopy on Platelets. Morphometric and Nanomechanical Characteristics of Platelets from Control and EPL Groups
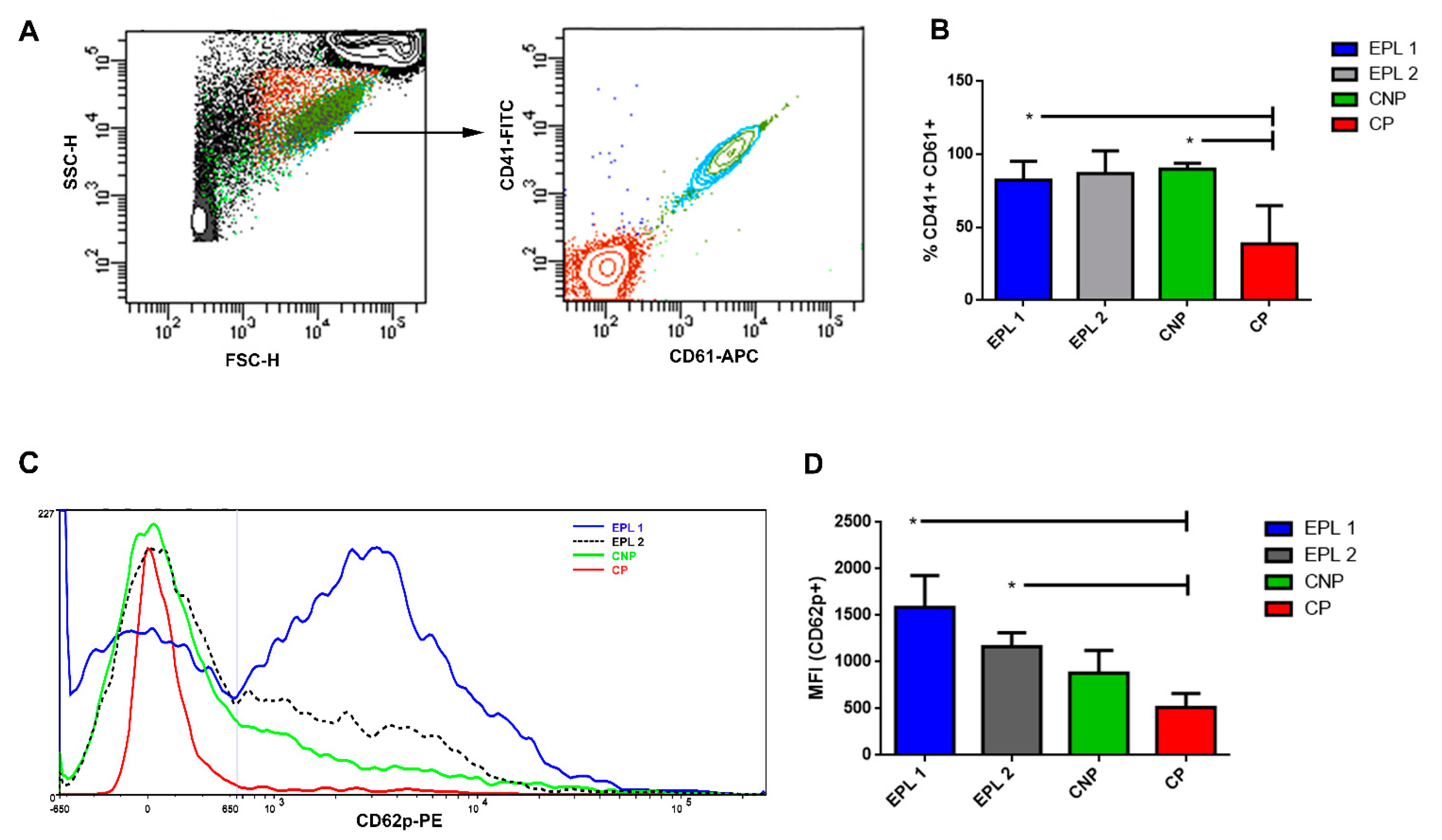
2.4. Flow Cytometry Analysis
3. Discussion
3.1. Increased EPL Platelet Activation Revealed by AFM
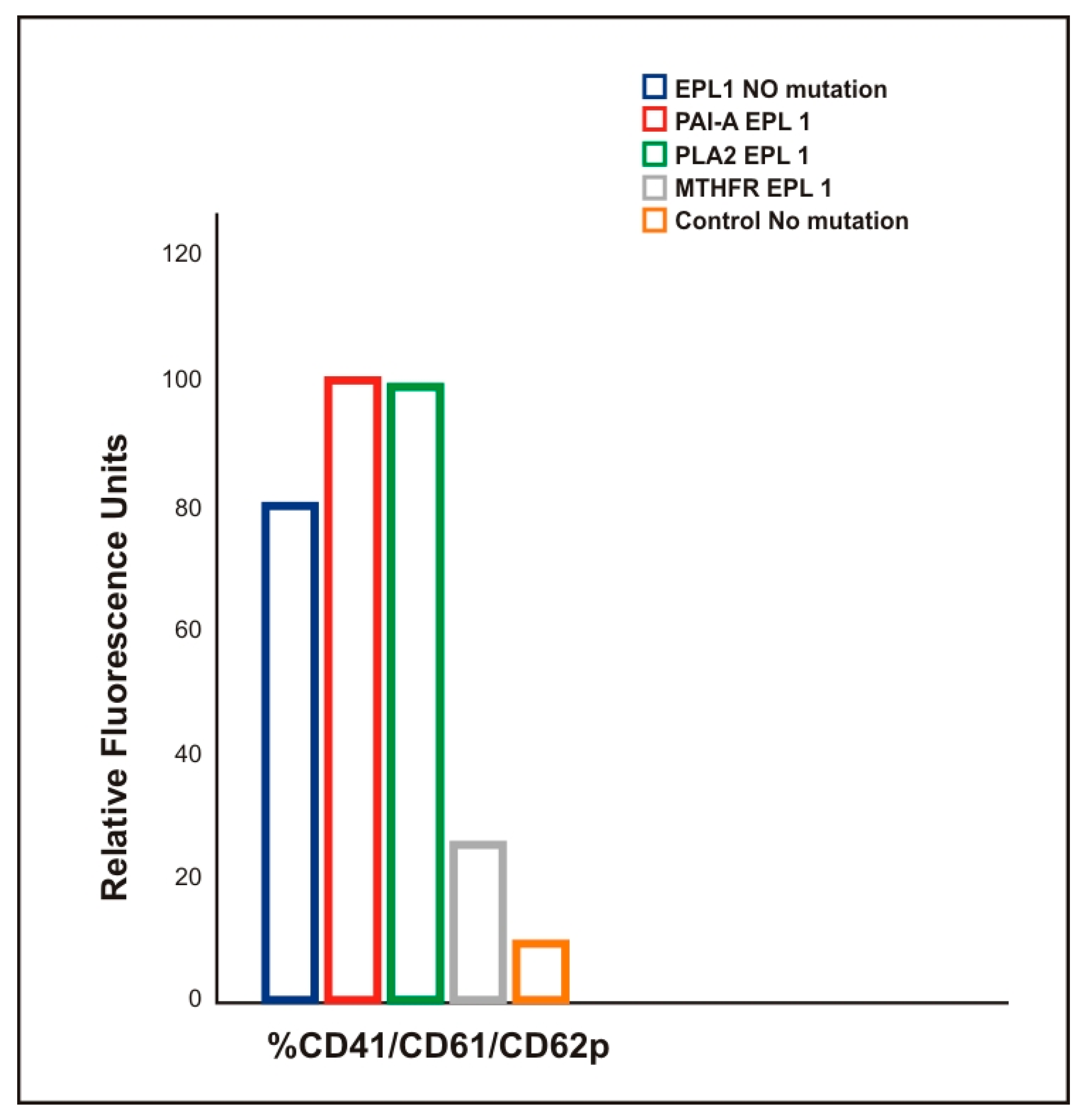
3.2. Correlation Between the Membrane Young’s Modulus and the Carriage of Thrombophilic Mutations in EPL Patients
3.3. Higher Production of PMPs in EPL Patients
4. Materials and Methods
4.1. Sample Selection
4.2. Blood Collection
4.3. DNA Analysis
4.4. Platelet Preparation and Immobilization for AFM Analysis
4.5. AFM Measurements
4.6. Blood Sample Preparation for Flow Cytometry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aslan, J.E. Platelet Shape Change. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N., Lopez, J., Page, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 321–336. [Google Scholar]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; LaValle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.L.; Lelah, M.D.; Lambrecht, L.K.; Cooper, S.L.; Albrecht, R.M. In vitro vs. ex vivo platelet deposition on polymer surfaces. Scanning Electron Microsc. 1984, 1, 279–290. [Google Scholar]
- Zarka, R.; Horev, M.B.; Volberg, T.; Neubauer, S.; Kessler, H.; Spatz, J.P.; Geiger, B. Differential modulation of platelet adhesion and spreading by adhesive ligand density. Nano Lett. 2019, 19, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Robier, C. Platelet morphology. J. Lab. Med. 2020, 44, 231–239. [Google Scholar] [CrossRef]
- D’Andrea, G.; Chetta, M.; Margaglione, M. Inherited platelet disorders: Thrombocytopenias and thrombocytopathies. High Speed Blood Transfus. Equip. 2009, 7, 278–292. [Google Scholar] [CrossRef]
- Stoiber, D.; Assinger, A. Platelet-leukocyte interplay in cancer development and progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Uchikova, E.H.; Ledjev, I.I. Changes in haemostasis during normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 119, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.-C.; Parant, O.; Vayssière, C.; Arnal, J.-F.; Payrastre, B. Physiologic and pathologic changes of platelets in pregnancy. Platelets 2010, 21, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Burke, N.; Flood, K.; Murray, A.; Cotter, B.; Dempsey, M.; Fay, L.; Dicker, P.; Geary, M.P.; Kenny, D.; Malone, F.D. Platelet reactivity changes significantly throughout all trimesters of pregnancy compared with the nonpregnant state: A prospective study. BJOG 2013, 120, 1599–1604. [Google Scholar] [CrossRef]
- Robb, A.O.; Din, J.N.; Mills, N.L.; Smith, I.B.J.; Blomberg, A.; Zikry, M.N.L.; Raftis, J.B.; Newby, D.E.; Denison, F.C. The influence of the menstrual cycle, normal pregnancyand pre-eclampsia on platelet activation. Thromb. Haemost. 2010, 103, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-R.; Hsiao, G.; Chen, T.-F.; Chien, Y.-Y.; Lin, C.-H.; Tzeng, C.-R.; Sheu, J.-R.; Lin, W.-Y. Mechanisms involved in agonist-induced hyperaggregability of platelets from normal pregnancy. J. Biomed. Sci. 2002, 9, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Brennand, J.; Conkie, J.A.; McCall, F.; Greer, I.A.; Walker, I.D.; Clark, P. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb. Haemost. 1998, 79, 1166–1170. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Bauer, K.A.; Kasthuri, R.; Cushman, M.; Streiff, M.; Lim, W.; Douketis, J.D. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J. Thromb. Thrombolysis 2016, 41, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Rull, K.; Nagirnaja, L.; Laan, M. Genetics of recurrent miscarriage: Challenges, current knowledge, future directions. Front. Genet. 2012, 3, 34. [Google Scholar] [CrossRef]
- Kupferminc, M.J. Thrombophilia and pregnancy. Reprod. Biol. Endocrinol. 2003, 1, 111. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.H.; Wu, X.-X.; Andree, H.A.; Lockwood, C.J.; Guller, S.; Scher, J.; Harpel, P.C. Pregnancy loss in the antiphospholipid-antibody syndrome—A possible thrombogenic mechanism. N. Engl. J. Med. 1997, 337, 154–160. [Google Scholar] [CrossRef]
- Carp, H.; Salomon, O.; Seidman, D.; Dardik, R.; Rosenberg, N.; Inbal, A. Prevalence of genetic markers for thrombophilia in recurrent pregnancy loss. Hum. Reprod. 2002, 17, 1633–1637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simcox, L.E.; Ormesher, L.; Tower, C.L.; Greer, I.A. Thrombophilia and pregnancy complications. Int. J. Mol. Sci. 2015, 16, 28418–28428. [Google Scholar] [CrossRef]
- Brenner, B.; Sarig, G.; Weiner, Z.; Younis, J.; Blumenfeld, Z.; Lanir, N. Thrombophilic polymorphisms are common in women with fetal loss without apparent cause. Thromb. Haemost. 1999, 82, 6–9. [Google Scholar]
- Yalcintepe, S.; Ozdemir, O.; Hacivelioglu, S.O.; Akurut, C.; Koc, E.; Uludag, A.; Cosar, E.; Silan, F. Multiple inherited thrombophilic gene polymorphisms in spontaneous abortions in Turkish population. Int. J. Mol. Cell. Med. 2015, 4, 120–127. [Google Scholar] [PubMed]
- Subrt, I.; Ulcova-Gallova, Z.; Cerna, M.; Hejnalova, M.; Slovanova, J.; Bibkova, K.; Micanova, Z. Recurrent pregnancy loss, plasminogen activator inhibitor-1 (-675) 4G/5G polymorphism and antiphospholipid antibodies in Czech women. Am. J. Reprod. Immunol. 2013, 70, 54–58. [Google Scholar] [CrossRef]
- Sadek, S.; El Kaffash, D.; El Mahdy, M.; Abd El-Latif, O. Platelet Glycoprotein iib/iiia (leu 33 pro) polymorphism and its role in recurrent early pregnancy loss. J. Clin. Cell Immunol. 2020, 11, 593. [Google Scholar]
- Faulk, W.P. Haemostasis and fibrinolysis in human normal placentae: Possible role in early pregnancy failure. In Early Pregnancy Loss; Sharp, F., Beard, R.W., Eds.; Springer: London, UK, 1988; pp. 193–198. [Google Scholar]
- Kupferminc, M.J.; Eldor, A.; Steinman, N.; Many, A.; Bar-Am, A.; Jaffa, A.; Fait, G.; Lessing, J.B. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N. Engl. J. Med. 1999, 340, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, J.M. Platelet adhesion signaling and the regulation of thrombus formation. J. Cell Sci. 2004, 117, 3415–3425. [Google Scholar] [CrossRef]
- Rivera, J.; Lozano, M.L.; Navarro-Nunez, L.; Vicente, V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef]
- Shet, A. Characterizing blood microparticles: Technical aspects and challenges. Vasc. Health Risk. Manag. 2008, 4, 769–774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toukh, M.; Black, A.; Siemens, R.D.; Othman, M. Evaluation of the hemostatic status in patients with prostate cancer using thromboelastography and tissue factor-microparticles. J. Canc. Res. 2012, 72 (Suppl. 8), 2690. [Google Scholar]
- Wang, Z.T.; Wang, Z.; Hu, Y.W. Possible roles of platelet-derived microparticles in atherosclerosis. Atherosclerosis 2016, 248, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, N.; Andrei, E.; Dragan, E.; Georgescu, A. Interaction of platelets with endothelial progenitor cells in the experimental atherosclerosis: Role of transplanted endothelial progenitor cells and platelet microparticles. Biol. Cell 2015, 107, 189–204. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Mairuhu, A.T.; Flaumenhaft, R. Clinical relevance of microparticles from platelets and megakaryocytes. J. Curr. Opin. Hematol. 2010, 17, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Herring, J.; McMichael, M.; Smith, S. Microparticles in health and disease. J. Veter. Intern. Med. 2013, 27, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Nikolaidou, B.; Gavriilaki, E.; Lazaridis, A.; Yiannaki, E.; Anyfanti, P.; Zografou, I.; Markala, D.; Douma, S. Increased erythrocyte- and platelet-derived macrovesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2019, 16, 458–465. [Google Scholar] [CrossRef]
- Lok, C.A.R.; Van der Post, A.M.J.; Sturk, A.; Sargent, I.L.; Nieuwland, R. The functions of microparticles in preeclampsia, Pregnancy Hypertension. Internat. J. Women’s Cardiovasc. Health. 2011, 1, 59–65. [Google Scholar]
- Linden, M.D.; Frelinger, I.A.L.; Barnard, M.R.; Przyklenk, K.; Furman, M.I.; Michelson, A.D. Application of flow cytometry to platelet disorders. Semin. Thromb. Hemost. 2004, 30, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, K.S.; Lee, E.S.; Lee, Y.J.; Park, Y.S.; Lee, K.R.; Lee, S.N. Optimized flow cytometric assay for the measurement of platelet microparticles in plasma: Pre-analytic and analytic considerations. Blood Coagul. Fibrinolysis 2002, 13, 393–397. [Google Scholar] [CrossRef]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of atomic force microscopy in cancer research. J. Nanobiotechnol. 2018, 16, 102. [Google Scholar] [CrossRef]
- Girasole, M.; Pompeo, G.; Cricenti, A.; Congiu-Castellano, A.; Andreola, F.; Serafino, A.; Frazer, B.; Boumis, G.; Amiconi, G. Roughness of the plasma membrane as an independent morphological parameter to study RBCs: A quantitative atomic force microscopy investigation. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R. Molecular basis of platelet granule secretion. Arter. Thromb. Vasc. Biol. 2003, 23, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, M.; Fritz, M.; Kacher, C.; Cleveland, J.; Hansma, P. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 1996, 70, 556–567. [Google Scholar] [CrossRef]
- Paul, B.Z.S.; Daniel, J.L.; Kunapuli, S.P. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. J. Biol. Chem. 1999, 274, 28293–28300. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Podor, T.J.; Manocha, B.; Lewis, J.D. Validation of flow cytometric detection of platelet microparticles and liposomes by atomic force microscopy. J. Thromb. Haemost. 2011, 9, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Obeid, S.; Ceroi, A.; Mourey, G.; Saas, P.; Elie-Caille, C.; Boireau, W. Development of a NanoBioAnalytical platform for “on-chip” qualification and quantification of platelet-derived microparticles. Biosens. Bioelectron. 2017, 93, 250–259. [Google Scholar] [CrossRef]
- Yuana, Y.; Oosterkamp, T.H.; Bahatyrova, S.; Ashcroft, B.; Garcia Rodriguez, P.; Bertina, R.M.; Osanto, S. Atomic force microscopy: A novel approach to the detection of nanosized blood microparticles. J. Thromb. Haemost. 2010, 8, 315–323. [Google Scholar] [CrossRef]
- Todinova, S.J.; Komsa-Penkova, R.; Krumova, S.B.; Taneva, S.G.; Golemanov, G.; Georgieva, G.; Tonchev, P.; Tsankov, B.; Beshev, L.; Balashev, K.; et al. PlA2 polymorphism in glycoprotein IIb/IIIa modulates the morphology and nanomechanics of platelets. J. Clin. Appl. Thromb. Hemost. 2017, 23, 951–960. [Google Scholar] [CrossRef]
- Van Rooy, M.-J.; Duim, W.; Ehlers, R.; Buys, A.V.; Pretorius, E. Platelet hyperactivity and fibrin clot structure in transient ischemic attack individuals in the presence of metabolic syndrome: A microscopy and thromboelastography study. Cardiovasc. Diabetol. 2015, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- García, D.L.; Rodríguez-Varela, M.; Martínez-Vieyra, I.; De La Mora, M.; Méndez-Méndez, J.V.; Durán-Álvarez, J.C.; Cerecedo, D. Alterations to the contents of plasma membrane structural lipids are associated with structural changes and compartmentalization in platelets in hypertension. Exp. Cell Res. 2019, 385, 111692. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, A.; Antonova, N.; Kyulavska, M.; Velcheva, I.; Ivanov, I.; Zvetkova, E. Emorheological and atomic force microscopy studies on the experimental clot formations in patients with type 2 diabetes mellitus. Ser. Biomech. 2018, 3, 63–73. [Google Scholar]
- Li, A.; Chen, J.; Liang, Z.-H.; Cai, J.; Cai, H.-H.; Chen, M. Comparison of ultrastructural and nanomechanical signature of platelets from acute myocardial infarction and platelet activation. Biochem. Biophys. Res. Commun. 2017, 486, 245–251. [Google Scholar] [CrossRef]
- Posch, S.; Neundlinger, I.; Leitner, M.; Siostrzonek, P.; Panzer, S.; Hinterdorfer, P.; Ebner, A. Activation induced morphological changes and integrin αIIbβ3 activity of living platelets. Methods 2013, 60, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Du Plooy, J.N.; Buys, A.; Duim, W.; Pretorius, E. Comparison of platelet ultrastructure and elastic properties in thrombo-embolic ischemic stroke and smoking using atomic force and scanning electron microscopy. PLoS ONE 2013, 8, e69774. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Wang, C.-C.; Lee, C.-H. Mechanoprofiling on membranes of living cells with atomic force microscopy and optical nano-profilometry. Adv. Phys. X 2017, 2, 608–621. [Google Scholar] [CrossRef]
- Cines, D.B.; Levine, L.D. Thrombocytopenia in pregnancy. Blood 2017, 130, 2271–2277. [Google Scholar] [CrossRef]
- Demchenko, A.P. Beyond annexin V: Fluorescence response of cellular membranes to apoptosis. Cytotechnology 2013, 65, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.G.; Grinstein, S. Sensing phosphatidylserine in cellular membranes. Sensors 2011, 11, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Research 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Guettler, J.; Forstner, D.; Gauster, M. Maternal platelets of the human placenta: Friend or Foe? Int. J. Mol. Sci. 2019, 20, 5639. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Zeng, B.-X.; Higuchi, T.; Yoshioka, S.; Fujii, S. Platelet-derived soluble factors induce human extravillous trophoblast migration and differentiation: Platelets are a possible regulator of trophoblast infiltration into maternal spiral arteries. Blood 2005, 106, 428–435. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sargent, I.L. Calculating microparticles in normal pregnancy and pre-eclampsia. Placenta 2008, 22, S73–S77. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.; Axelsson, O.; Wernroth, L.; Larsson, A.; Basu, S. Involvement of inflammation in normal pregnancy. Acta Obstet. Gynecol. Scand. 2013, 92, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Granot, I.; Gnainsky, Y.; Dekel, N.; Jiménez-Trejo, F.; Tapia-Rodríguez, M.; Cerbón, M.; Kuhn, D.M.; Manjarrez-Gutiérrez, G.; Mendoza-Rodríguez, C.A.; Picazo, O. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reprod 2012, 144, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, O.B.; Nielsen, H.S.; Kolte, A.M. Inflammation and miscarriage. Semin. Fetal Neonatal Med. 2006, 11, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, B.H.; Mokhtar, D.A.; Badr, A.M.; Gamal el Din, N.M. Plasminogen activator inhibitor-1 4G/5G gene polymorphism in hemodialysis patients with cardiovascular disease. Egypt. J. Haematol. 2013, 38, 29–35. [Google Scholar] [CrossRef]
- Galluzzi, M.; Tang, G.; Biswas, C.S.; Zhao, J.; Chen, S.; Stadler, F.J. Atomic force microscopy methodology and AFMech Suite software for nanomechanics on heterogeneous soft materials. Nat. Commun. 2018, 9, 3584. [Google Scholar] [CrossRef]

| Groups | Age | Gestational Weeks | Platelet Count × 109/L | CRP (mg/L) | Fibrinogen (g/L) | INR |
|---|---|---|---|---|---|---|
| CNP | 36 ± 6 | - | 318 ± 76 | 4.5 ± 1.1 | 3.2 ± 0.60 | 1.00 ± 0.04 |
| CP | 31 ± 4 | 7–12 | 296 ± 50 | 5.8 ± 0.9 | 3.5 ± 0.80 | 0.99 ± 0.05 |
| EPL1 | 34 ± 8 | 6–9 | 311 ± 39 | 3.3 ± 0.6 ** | 3.9 ± 0.86 | 0.95 ± 0.05 |
| EPL2 | 35 ± 4 | 10–12 | 323 ± 98 | 6.9 ± 1.3 | 3.0 ± 0.82 | 0.91 ± 0.04 |
| Genetic Factor | Carriage in CNP % | Carriage in EPL1 % | Pearson Chi-Squared | Fisher’s Exact Test | Mantel–Haenszel Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| FVL | 7.8 | 14.8 | 7.381 | 0.018 | 4.954 | 1.426–17.210 |
| FII20210A | 2.6 | 9.8 | 1.906 | 0.063 | 5.022 | 0.775–32.537 |
| 677 MTHFR(T) | 10.6 | 17.6 | 0.998 | 0.430 | 2.006 | 0.498–8.083 |
| PLA1/A2 | 21.2 | 31.7 | 0.898 | 0.404 | 1.857 | 0.428–8.055 |
| 4G/4G PAI-1 | 20.5 | 26.0 | 2.044 | 0.153 | 1.415 | 0.391–4.422 |
| Platelets | Height (nm) | Area (µm2) | Volume (µm3) | Rrms (nm) | Ea (kPa) |
|---|---|---|---|---|---|
| CNP | 1090 ± 131 | 4.25 ± 1.4 | 0.71 ± 0.12 | 39.1 ± 8 | 241 ± 103 |
| CP | 955 ± 88 | 4.85 ± 1.3 | 0.66 ± 0.10 | 28.9 ± 6 | 174 ± 77 |
| EPL1—embryonic stage | 692 ± 128 * | 3.89 ± 1.3 | 0.41 ± 0.08 * | 22.9 ± 6 * | 482 ± 131 * |
| EPL2—placentation stage | 873 ± 153 | 6.02 ± 2.2 | 0.78 ± 0.13 | 24.8 ± 8 * | 97 ± 48 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, T.; Komsa-Penkova, R.; Langari, A.; Krumova, S.; Golemanov, G.; Georgieva, G.B.; Taneva, S.G.; Giosheva, I.; Mihaylova, N.; Tchorbanov, A.; et al. Morphometric and Nanomechanical Features of Platelets from Women with Early Pregnancy Loss Provide New Evidence of the Impact of Inherited Thrombophilia. Int. J. Mol. Sci. 2021, 22, 7778. https://doi.org/10.3390/ijms22157778
Andreeva T, Komsa-Penkova R, Langari A, Krumova S, Golemanov G, Georgieva GB, Taneva SG, Giosheva I, Mihaylova N, Tchorbanov A, et al. Morphometric and Nanomechanical Features of Platelets from Women with Early Pregnancy Loss Provide New Evidence of the Impact of Inherited Thrombophilia. International Journal of Molecular Sciences. 2021; 22(15):7778. https://doi.org/10.3390/ijms22157778
Chicago/Turabian StyleAndreeva, Tonya, Regina Komsa-Penkova, Ariana Langari, Sashka Krumova, Georgi Golemanov, Galya B. Georgieva, Stefka G. Taneva, Ina Giosheva, Nikolina Mihaylova, Andrey Tchorbanov, and et al. 2021. "Morphometric and Nanomechanical Features of Platelets from Women with Early Pregnancy Loss Provide New Evidence of the Impact of Inherited Thrombophilia" International Journal of Molecular Sciences 22, no. 15: 7778. https://doi.org/10.3390/ijms22157778
APA StyleAndreeva, T., Komsa-Penkova, R., Langari, A., Krumova, S., Golemanov, G., Georgieva, G. B., Taneva, S. G., Giosheva, I., Mihaylova, N., Tchorbanov, A., & Todinova, S. (2021). Morphometric and Nanomechanical Features of Platelets from Women with Early Pregnancy Loss Provide New Evidence of the Impact of Inherited Thrombophilia. International Journal of Molecular Sciences, 22(15), 7778. https://doi.org/10.3390/ijms22157778







