Anandamide Concentration-Dependently Modulates Toll-Like Receptor 3 Agonism or UVB-Induced Inflammatory Response of Human Corneal Epithelial Cells
Abstract
1. Introduction
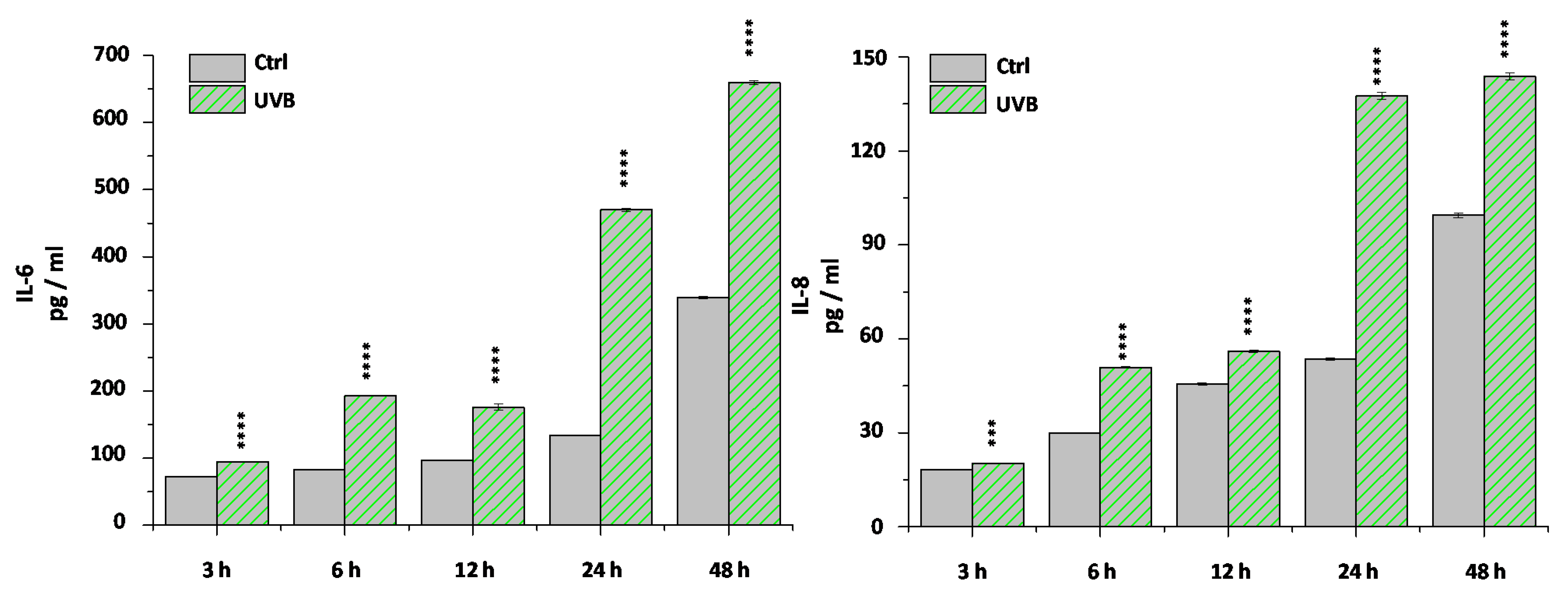
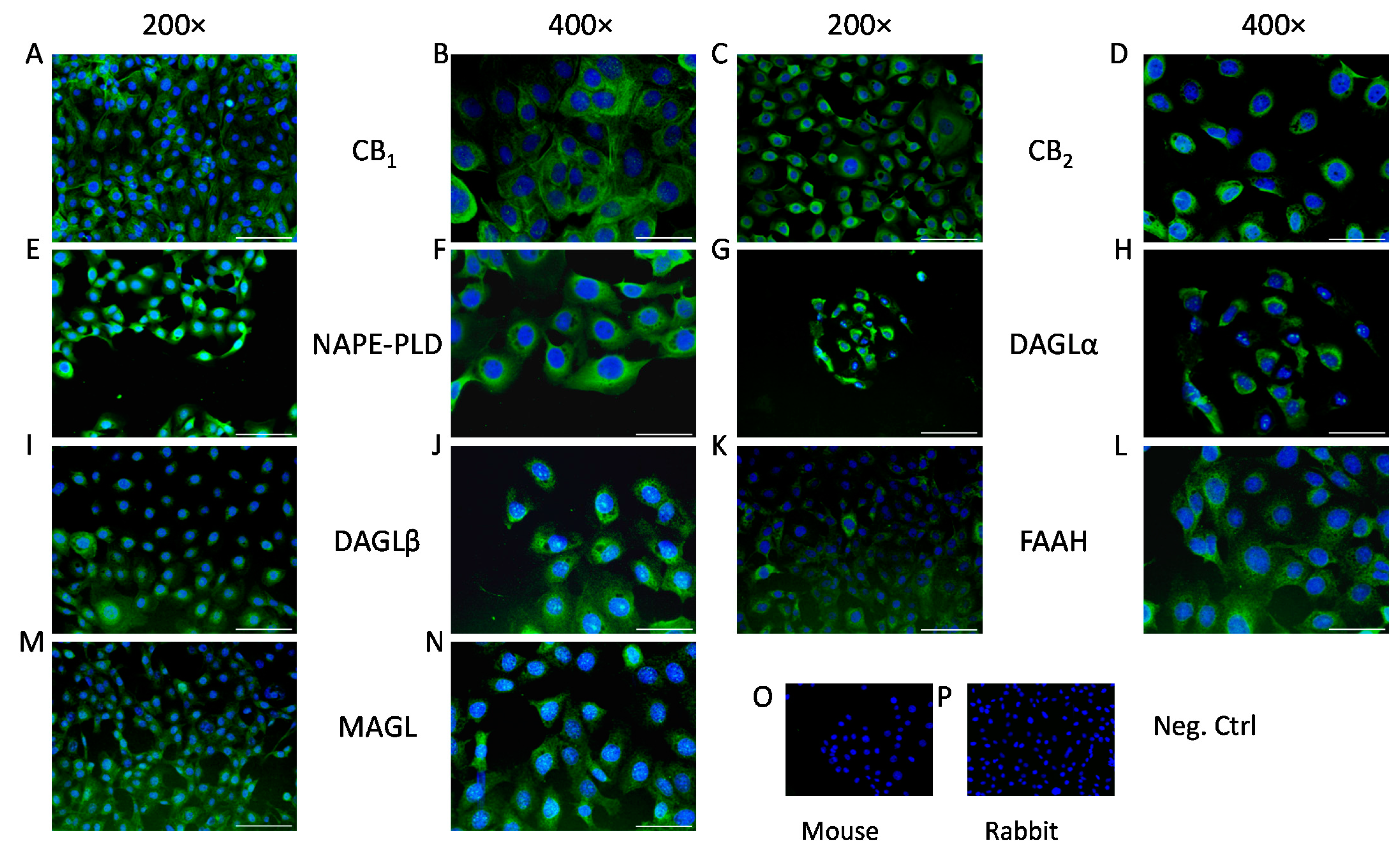
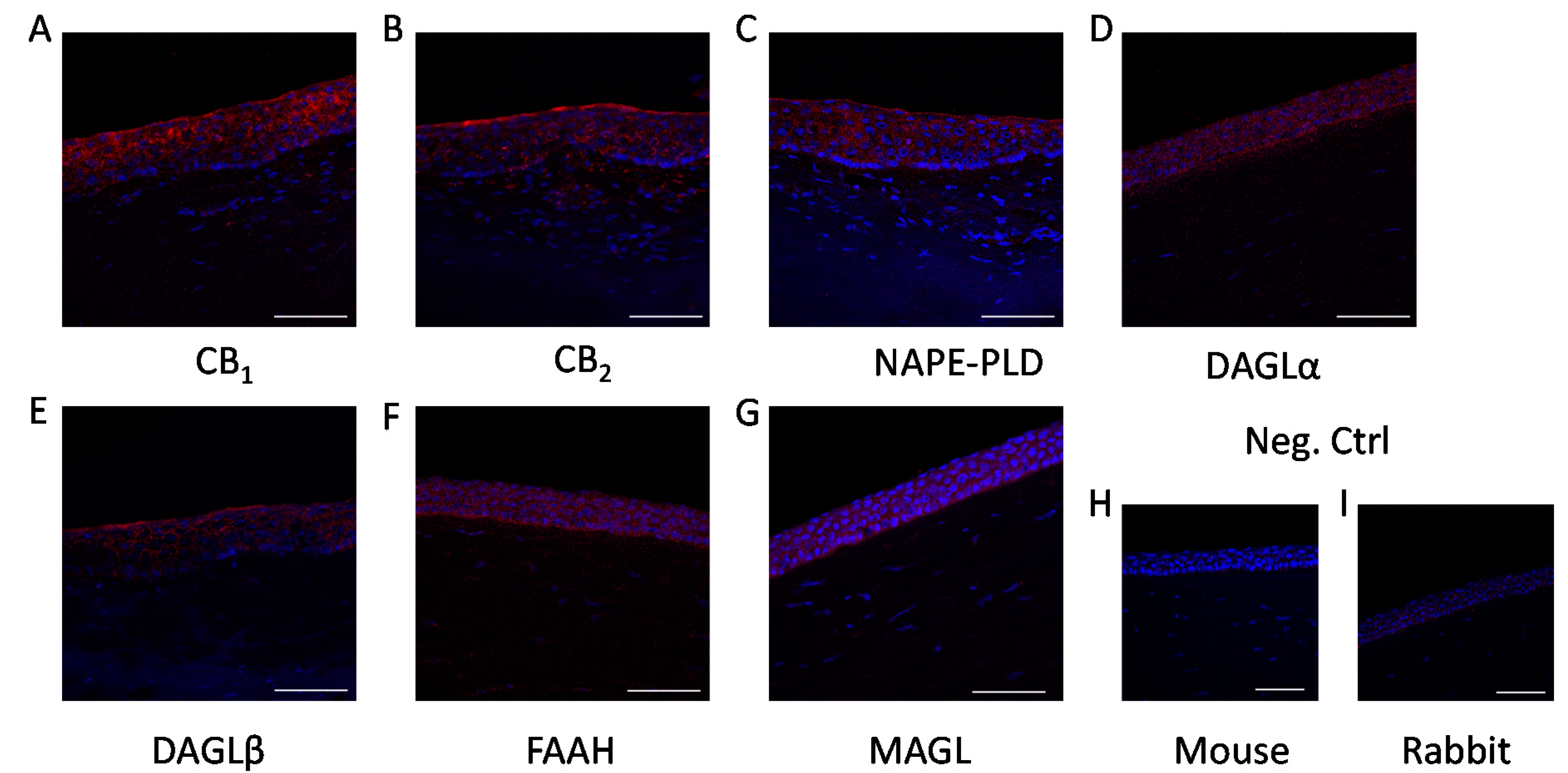
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culturing
4.3. UVB Irradiation
4.4. Immunohistofluorescence
4.5. Immunocytofluorescence
4.6. RNA Isolation, Reverse Transcription, and Quantitative “Real-Time” PCR (Q-PCR)
4.7. Determination of Cytokine Release (ELISA)
4.8. MTT Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Bashir, H.; Seykora, J.T.; Lee, V. Invisible Shield: Review of the Corneal Epithelium as a Barrier to UV Radiation, Pathogens, and Other Environmental Stimuli. J. Ophthalmic Vis. Res. 2017, 12, 305–311. [Google Scholar] [CrossRef]
- Meduri, A.; Grenga, P.L.; Scorolli, L.; Ceruti, P.; Ferreri, G. Role of Cysteine in Corneal Wound Healing after Photorefractive Keratectomy. Ophthalmic Res. 2009, 41, 76–82. [Google Scholar] [CrossRef]
- Scalinci, S.Z.; Scorolli, L.; Meduri, A.; Grenga, P.L.; Corradetti, G.; Metrangolo, C. Effect of Basic Fibroblast Growth Factor and Cytochrome c Peroxidase Combination in Transgenic Mice Corneal + Epithelial Healing Process after Excimer Laser Photoablation. Clin. Ophthalmol. 2011, 5, 215–221. [Google Scholar] [CrossRef]
- Meduri, A.; Scorolli, L.; Scalinci, S.Z.; Grenga, P.L.; Lupo, S.; Rechichi, M.; Meduri, E. Effect of the Combination of Basic Fibroblast Growth Factor and Cysteine on Corneal Epithelial Healing after Photorefractive Keratectomy in Patients Affected by Myopia. Indian J. Ophthalmol. 2014, 62, 424–428. [Google Scholar] [CrossRef]
- Murata, Y.; Masuko, S. Peripheral and Central Distribution of TRPV1, Substance P and CGRP of Rat Corneal Neurons. Brain Res. 2006, 1085, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, H.; Wang, Z.; Mergler, S.; Liu, H.; Kawakita, T.; Tachado, S.D.; Pan, Z.; Capó-Aponte, J.E.; Pleyer, U.; et al. Transient Receptor Potential Vanilloid 1 Activation Induces Inflammatory Cytokine Release in Corneal Epithelium through MAPK Signaling. J. Cell. Physiol. 2007, 213, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Mori, Y. Transient Receptor Potential (TRP) Channels: Biosensors for Redox Environmental Stimuli and Cellular Status. Free Radic. Biol. Med. 2020, 146, 36–44. [Google Scholar] [CrossRef]
- Okada, Y.; Reinach, P.S.; Shirai, K.; Kitano, A.; Kao, W.W.-Y.; Flanders, K.C.; Miyajima, M.; Liu, H.; Zhang, J.; Saika, S. TRPV1 Involvement in Inflammatory Tissue Fibrosis in Mice. Am. J. Pathol. 2011, 178, 2654–2664. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M.; Annicelli, L.L.; Krause, J.E.; Cortright, D.N. The Cloned Rat Vanilloid Receptor VR1 Mediates Both R-Type Binding and C-Type Calcium Response in Dorsal Root Ganglion Neurons. Mol. Pharmacol. 1999, 56, 581–587. [Google Scholar] [CrossRef]
- Bates, B.; Mitchell, K.; Keller, J.M.; Chan, C.-C.; Swaim, W.D.; Yaskovich, R.; Mannes, A.J.; Iadarola, M.J. Prolonged Analgesic Response of Cornea to Topical Resiniferatoxin, a Potent TRPV1 Agonist. Pain 2010, 149, 522–528. [Google Scholar] [CrossRef]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. In Recent Advances in Cannabinoid Physiology and Pathology; Bukiya, A.N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 151–165. ISBN 978-3-030-21737-2. [Google Scholar]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(Ut)Annabinoid” System. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Gayer, H. Pharmakologische Wertbestimmung von orientalischem Haschisch und Herba cannabis indicae. Naunyn Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie 1928, 129, 312–318. [Google Scholar] [CrossRef]
- Carlini, E.A.; Santos, M.; Claussen, U.; Bieniek, D.; Korte, F. Structure Activity Relationship of Four Tetrahydrocannabinols and the Pharmacological Activity of Five Semi-Purified Extracts of Cannabis Sativa. Psychopharmacologia 1970, 18, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A.; Karniol, I.G.; Renault, P.F.; Schuster, C.R. Effects of Marihuana in Laboratory Animals and in Man. Br. J. Pharmacol. 1974, 50, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bereiter, D.A.; Bereiter, D.F.; Hirata, H. Topical Cannabinoid Agonist, WIN55,212-2, Reduces Cornea-Evoked Trigeminal Brainstem Activity in the Rat. Pain 2002, 99, 547–556. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Capó-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal Growth Factor Receptor Transactivation by the Cannabinoid Receptor (CB1) and Transient Receptor Potential Vanilloid 1 (TRPV1) Induces Differential Responses in Corneal Epithelial Cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef]
- Tóth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Juhász, I.; Sugawara, K.; Paus, R.; Bíró, T. Endocannabinoids Modulate Human Epidermal Keratinocyte Proliferation and Survival via the Sequential Engagement of Cannabinoid Receptor-1 and Transient Receptor Potential Vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104. [Google Scholar] [CrossRef]
- Chen, J.; Matias, I.; Dinh, T.; Lu, T.; Venezia, S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Finding of Endocannabinoids in Human Eye Tissues: Implications for Glaucoma. Biochem. Biophys. Res. Commun. 2005, 330, 1062–1067. [Google Scholar] [CrossRef]
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. Biosynthesis and Degradation of Anandamide and 2-Arachidonoylglycerol and Their Possible Physiological Significance. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 173–192. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, J.; Yu, F.-S.X. Toll-like Receptor 3 Agonist Poly(I:C)-Induced Antiviral Response in Human Corneal Epithelial Cells. Immunology 2006, 117, 11–21. [Google Scholar] [CrossRef]
- Kennedy, M.; Kim, K.H.; Harten, B.; Brown, J.; Planck, S.; Meshul, C.; Edelhauser, H.; Rosenbaum, J.T.; Armstrong, C.A.; Ansel, J.C. Ultraviolet Irradiation Induces the Production of Multiple Cytokines by Human Corneal Cells. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2483–2491. [Google Scholar]
- Delic, N.C.; Lyons, J.G.; Girolamo, N.D.; Halliday, G.M. Damaging Effects of Ultraviolet Radiation on the Cornea. Photochem. Photobiol. 2017, 93, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Murataeva, N.; Miller, S.; Dhopeshwarkar, A.; Leishman, E.; Daily, L.; Taylor, X.; Morton, B.; Lashmet, M.; Bradshaw, H.; Hillard, C.J.; et al. Cannabinoid CB2R Receptors Are Upregulated with Corneal Injury and Regulate the Course of Corneal Wound Healing. Exp. Eye Res. 2019, 182, 74–84. [Google Scholar] [CrossRef]
- Qazi, Y.; Wong, G.; Monson, B.; Stringham, J.; Ambati, B.K. Corneal Transparency: Genesis, Maintenance and Dysfunction. Brain Res. Bull. 2010, 81, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, E.; Sun, Y.; Roy, S.; Karmakar, M.; Hise, A.G.; Szczotka-Flynn, L.; Ghannoum, M.; Chinnery, H.R.; McMenamin, P.G.; Rietsch, A. Host Defense at the Ocular Surface. Int. Rev. Immunol. 2013, 32, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.J.; Elliott, M.H.; Le, Y.Z.; Carr, D.J.J. Corneal Epithelial Cells Exhibit Myeloid Characteristics and Present Antigen via MHC Class II. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, H.S.; Joo, C.-K. TRPV1 Antagonist Suppresses Allergic Conjunctivitis in a Murine Model. Ocul. Immunol. Inflamm. 2018, 26, 440–448. [Google Scholar] [CrossRef]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 Antagonists That Cause Hypothermia, Instead of Hyperthermia, in Rodents: Compounds’ Pharmacological Profiles, in Vivo Targets, Thermoeffectors Recruited and Implications for Drug Development. Acta Physiol. 2018, 223. [Google Scholar] [CrossRef]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef]
- Matias, I.; Wang, J.W.; Moriello, A.S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Changes in Endocannabinoid and Palmitoylethanolamide Levels in Eye Tissues of Patients with Diabetic Retinopathy and Age-Related Macular Degeneration. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 413–418. [Google Scholar] [CrossRef]
- Petrocellis, L.D.; Cascio, M.G.; Marzo, V.D. The Endocannabinoid System: A General View and Latest Additions. Br. J. Pharmacol. 2004, 141, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.M.; Ku, E.S.; Dwarakanathan, S. Herpes Simplex Keratitis. Dis. Mon. 2014, 60, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Willmann, G. Ultraviolet Keratitis: From the Pathophysiological Basis to Prevention and Clinical Management. High. Alt. Med. Biol. 2015, 16, 277–282. [Google Scholar] [CrossRef]
- Németh, J.; Helyes, Z.; Thán, M.; Jakab, B.; Pintér, E.; Szolcsányi, J. Concentration-Dependent Dual Effect of Anandamide on Sensory Neuropeptide Release from Isolated Rat Tracheae. Neurosci. Lett. 2003, 336, 89–92. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ(9) -Tetrahydrocannabinol and N-Arachidonyl Glycine Are Full Agonists at GPR18 Receptors and Induce Migration in Human Endometrial HEC-1B Cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Chemin, J.; Monteil, A.; Perez-Reyes, E.; Nargeot, J.; Lory, P. Direct Inhibition of T-Type Calcium Channels by the Endogenous Cannabinoid Anandamide. EMBO J. 2001, 20, 7033–7040. [Google Scholar] [CrossRef]
- Maingret, F.; Patel, A.J.; Lazdunski, M.; Honoré, E. The Endocannabinoid Anandamide Is a Direct and Selective Blocker of the Background K(+) Channel TASK-1. EMBO J. 2001, 20, 47–54. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Starowicz, K.; Moriello, A.S.; Vivese, M.; Orlando, P.; Di Marzo, V. Regulation of Transient Receptor Potential Channels of Melastatin Type 8 (TRPM8): Effect of CAMP, Cannabinoid CB(1) Receptors and Endovanilloids. Exp. Cell Res. 2007, 313, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Poling, J.S.; Rogawski, M.A.; Salem, N.; Vicini, S. Anandamide, an Endogenous Cannabinoid, Inhibits Shaker-Related Voltage-Gated K+ Channels. Neuropharmacology 1996, 35, 983–991. [Google Scholar] [CrossRef]
- Caldwell, M.D.; Hu, S.S.-J.; Viswanathan, S.; Bradshaw, H.; Kelly, M.E.M.; Straiker, A. A GPR18-Based Signalling System Regulates IOP in Murine Eye. Br. J. Pharmacol. 2013, 169, 834–843. [Google Scholar] [CrossRef]
- Murataeva, N.; Daily, L.; Taylor, X.; Dhopeshwarkar, A.; Hu, S.S.-J.; Miller, S.; McHugh, D.; Oehler, O.; Li, S.; Bonanno, J.A.; et al. Evidence for a GPR18 Role in Chemotaxis, Proliferation, and the Course of Wound Closure in the Cornea. Cornea 2019, 38, 905–913. [Google Scholar] [CrossRef]
- Lucius, A.; Khajavi, N.; Reinach, P.S.; Köhrle, J.; Dhandapani, P.; Huimann, P.; Ljubojevic, N.; Grötzinger, C.; Mergler, S. 3-Iodothyronamine Increases Transient Receptor Potential Melastatin Channel 8 (TRPM8) Activity in Immortalized Human Corneal Epithelial Cells. Cell. Signal. 2016, 28, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mihály, J.; Angyal, Á.; Szilágyi, S.B.; Tubak, V.; Soeberdt, M.; Abels, C.; Oláh, A.; Bíró, T. 303 Establishment and Optimization of Pro-Inflammatory Model Systems in Human Keratinocytes. J. Investig. Dermatol. 2016, 136, S212. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angyal, Á.; Pénzes, Z.; Alimohammadi, S.; Horváth, D.; Takács, L.; Vereb, G.; Zsebik, B.; Bíró, T.; Tóth, K.F.; Lisztes, E.; et al. Anandamide Concentration-Dependently Modulates Toll-Like Receptor 3 Agonism or UVB-Induced Inflammatory Response of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 7776. https://doi.org/10.3390/ijms22157776
Angyal Á, Pénzes Z, Alimohammadi S, Horváth D, Takács L, Vereb G, Zsebik B, Bíró T, Tóth KF, Lisztes E, et al. Anandamide Concentration-Dependently Modulates Toll-Like Receptor 3 Agonism or UVB-Induced Inflammatory Response of Human Corneal Epithelial Cells. International Journal of Molecular Sciences. 2021; 22(15):7776. https://doi.org/10.3390/ijms22157776
Chicago/Turabian StyleAngyal, Ágnes, Zsófia Pénzes, Shahrzad Alimohammadi, Dorottya Horváth, Lili Takács, György Vereb, Barbara Zsebik, Tamás Bíró, Kinga Fanni Tóth, Erika Lisztes, and et al. 2021. "Anandamide Concentration-Dependently Modulates Toll-Like Receptor 3 Agonism or UVB-Induced Inflammatory Response of Human Corneal Epithelial Cells" International Journal of Molecular Sciences 22, no. 15: 7776. https://doi.org/10.3390/ijms22157776
APA StyleAngyal, Á., Pénzes, Z., Alimohammadi, S., Horváth, D., Takács, L., Vereb, G., Zsebik, B., Bíró, T., Tóth, K. F., Lisztes, E., Tóth, B. I., Oláh, A., & Szöllősi, A. G. (2021). Anandamide Concentration-Dependently Modulates Toll-Like Receptor 3 Agonism or UVB-Induced Inflammatory Response of Human Corneal Epithelial Cells. International Journal of Molecular Sciences, 22(15), 7776. https://doi.org/10.3390/ijms22157776









