Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells
Abstract
1. Introduction
2. Results
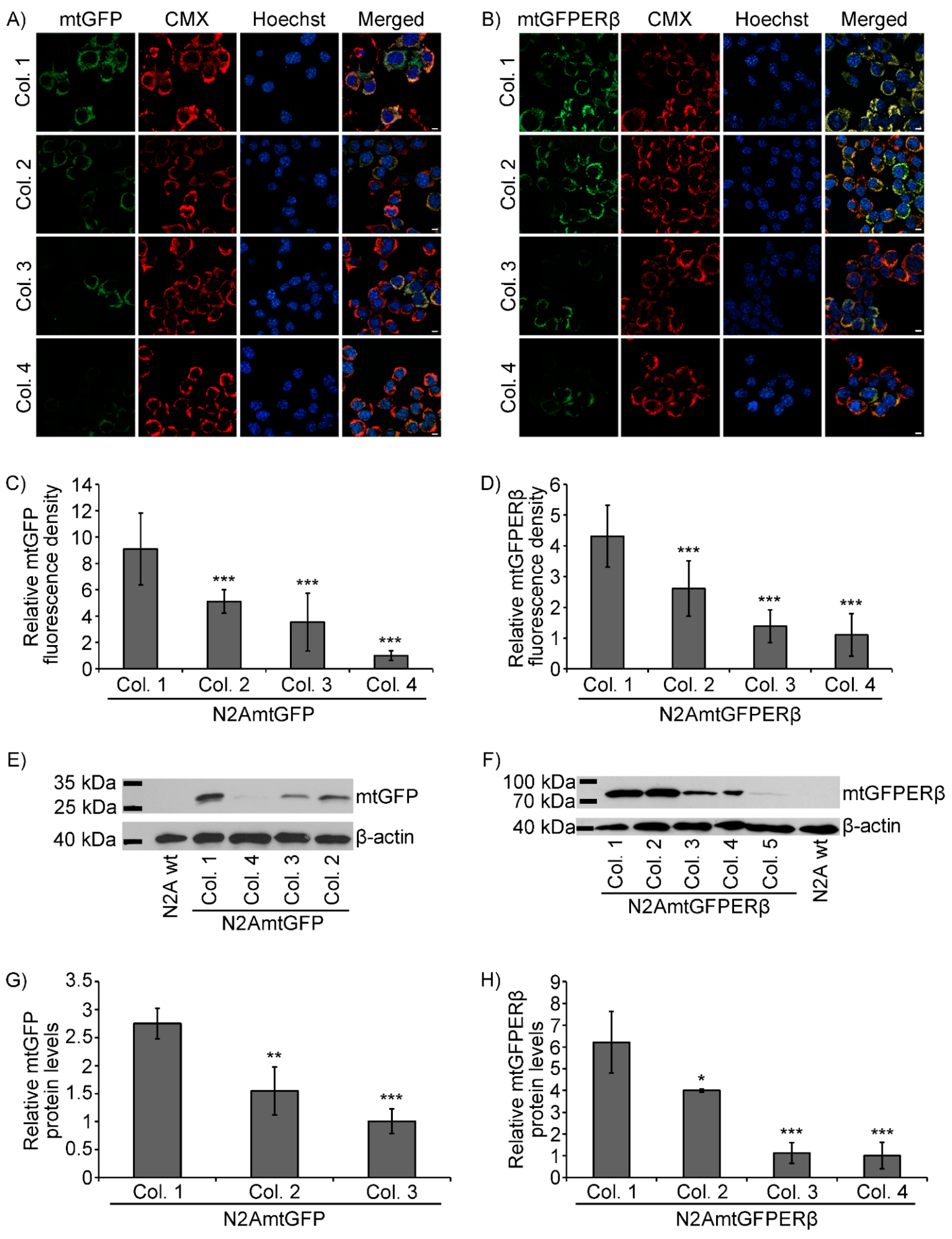
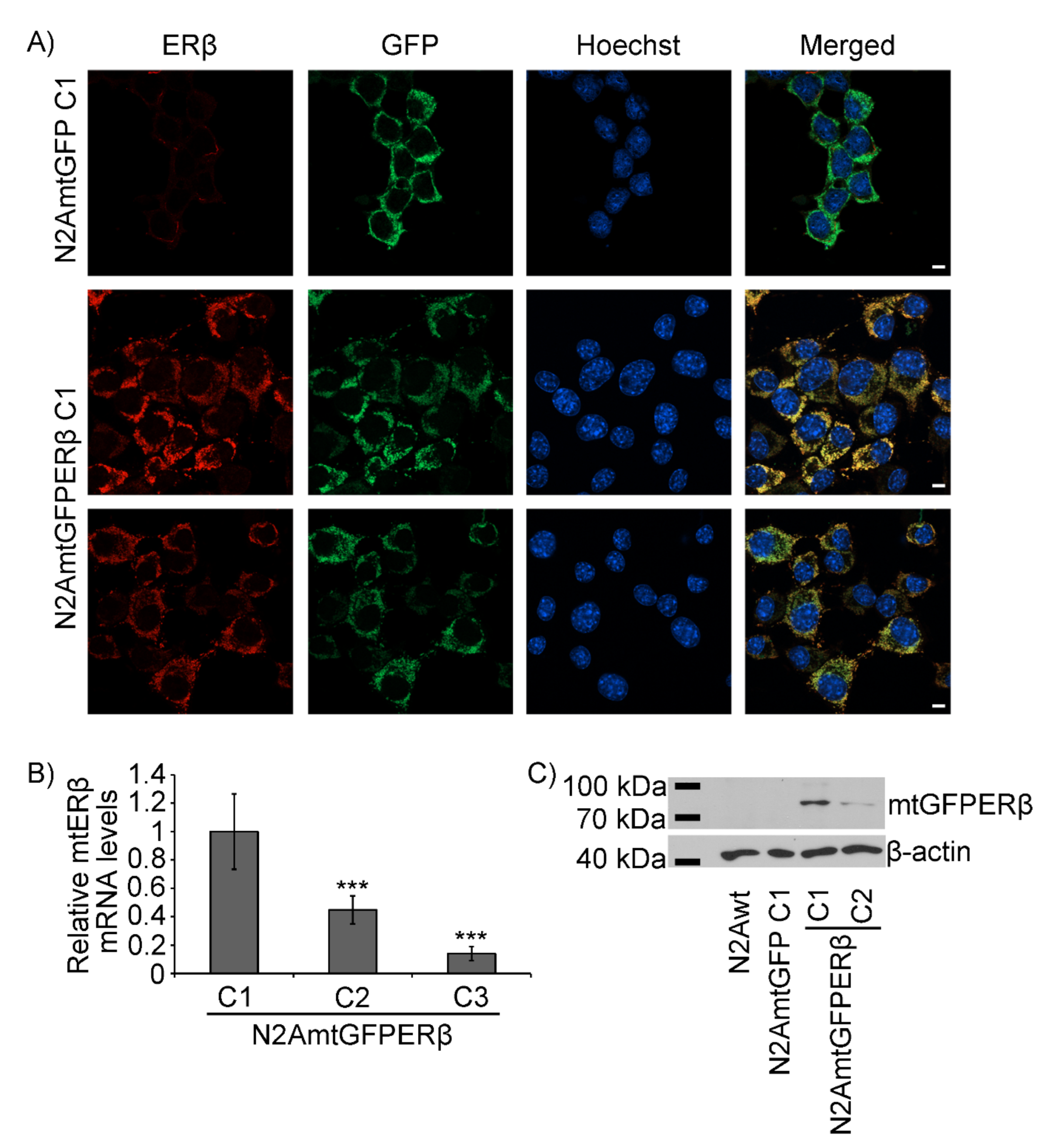
2.1. Generation of N2A Cells Stably Overexpressing a Mitochondrial-Targeted GFPERβ Protein
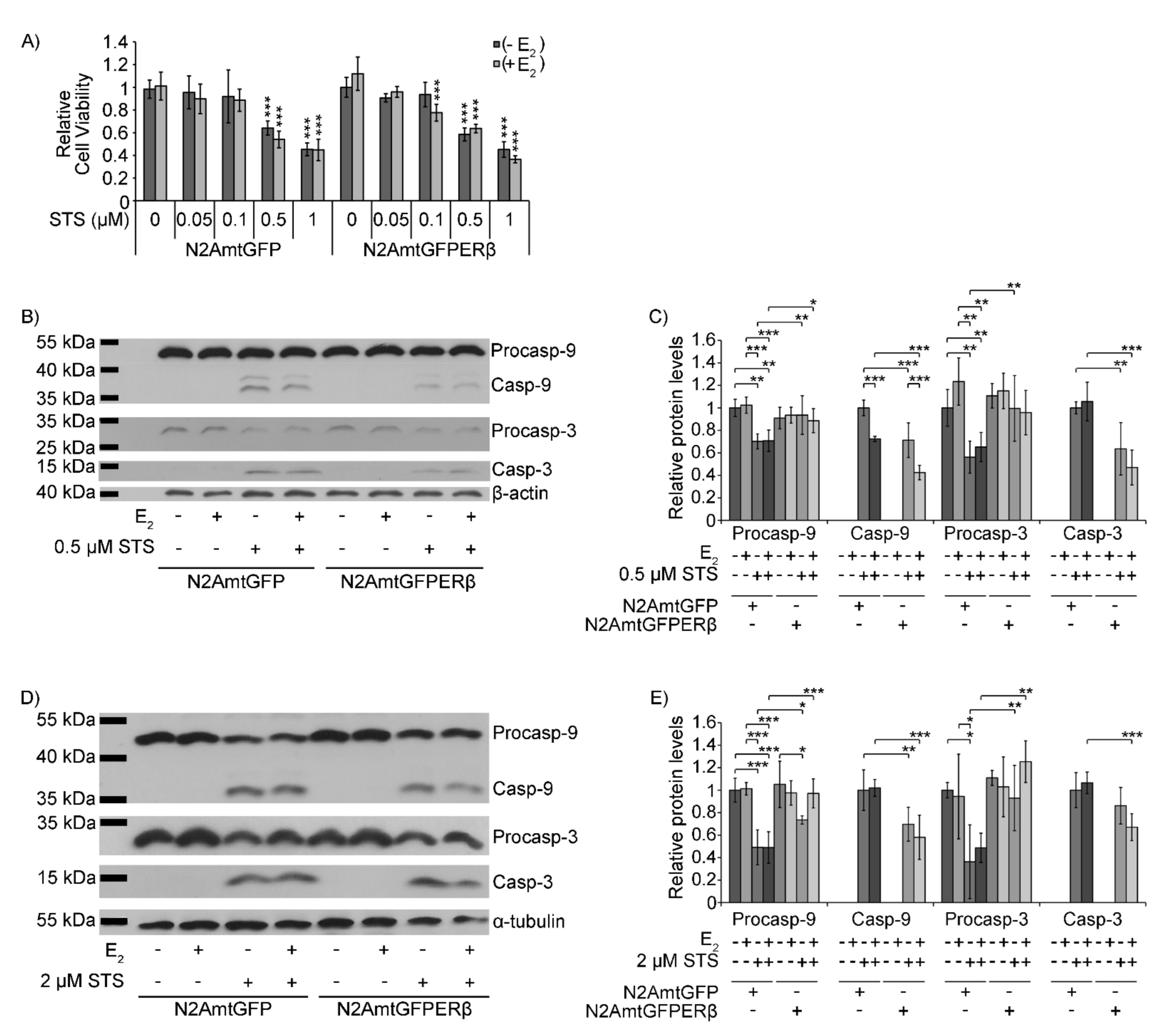
2.2. Effect of mtERβ on Staurosporine-Induced Apoptosis
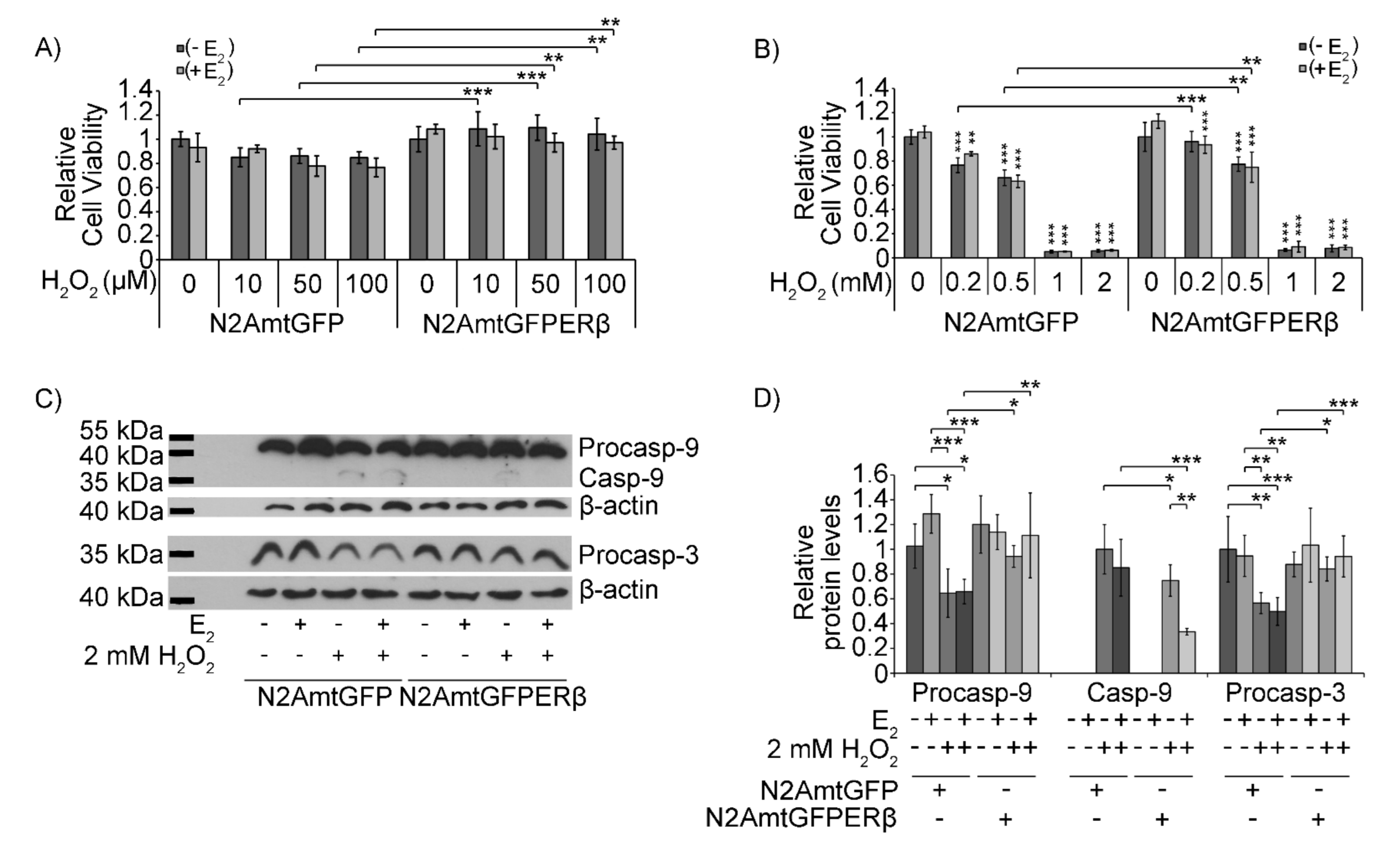
2.3. Effect of mtERβ on H2O2-Induced Apoptosis
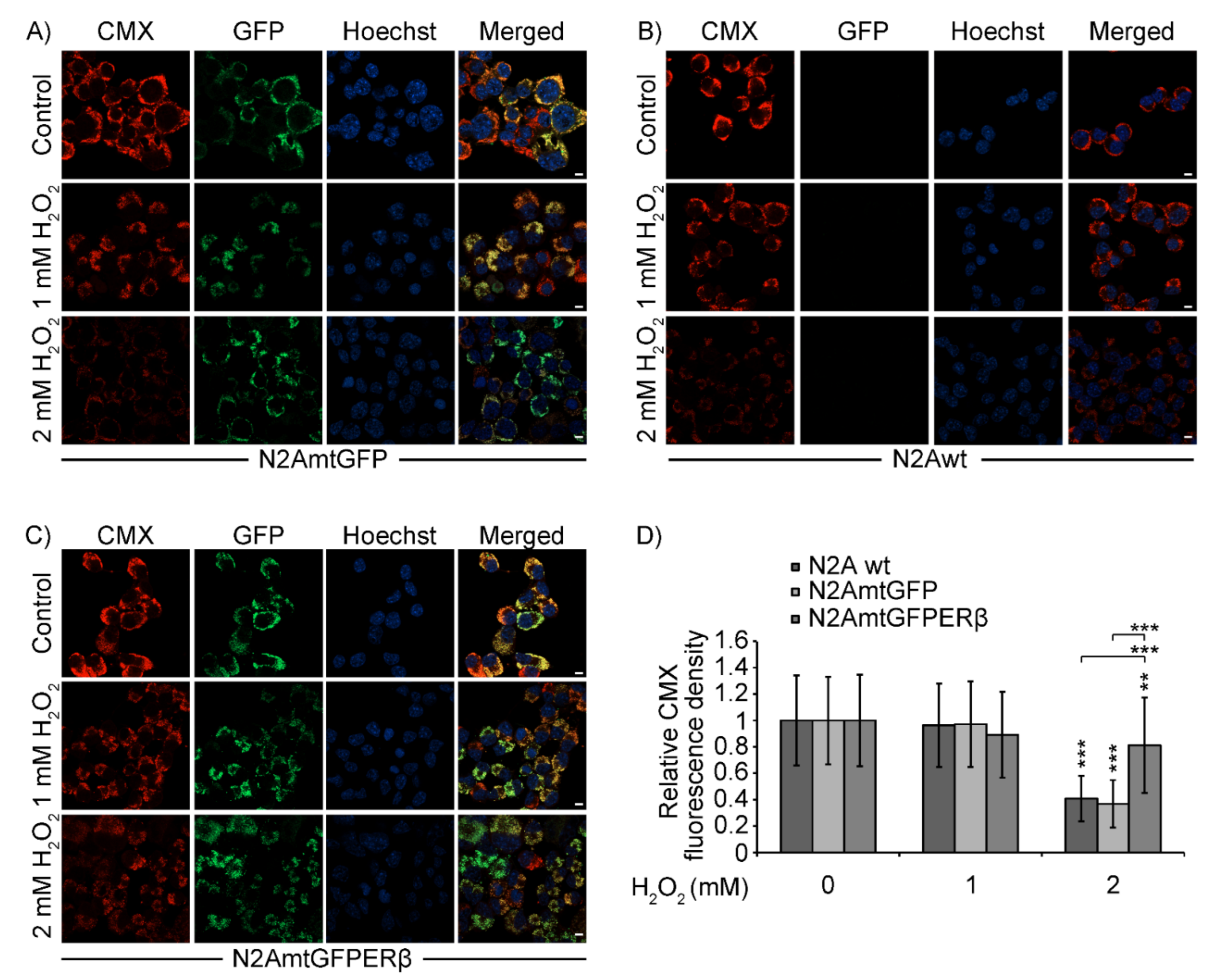
2.4. Effect of mtERβ on H2O2-Induced Mitochondrial Impairment in N2A Cells
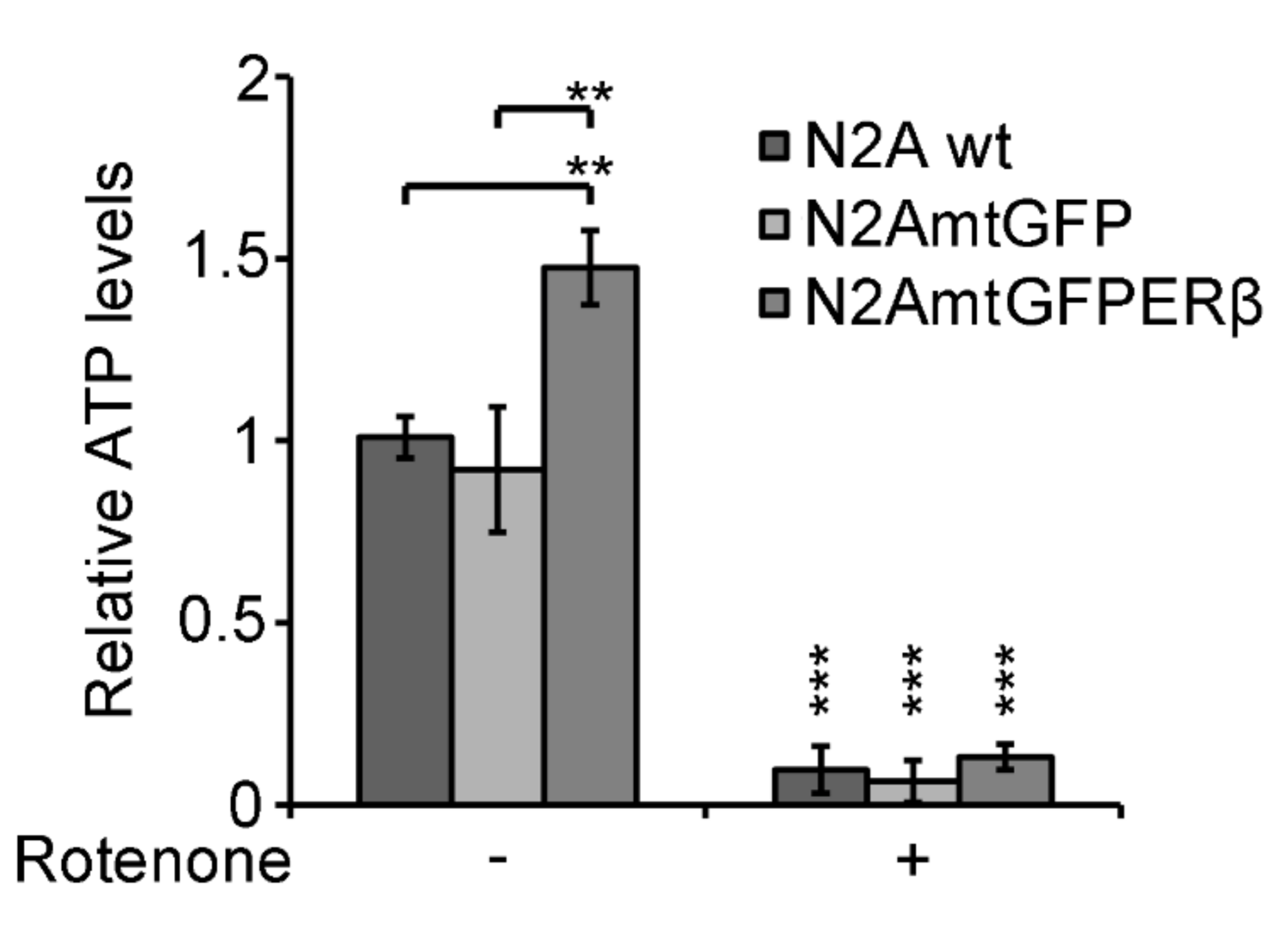
2.5. Effect of Mitochondrial ERβ on ATP Content
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Antibodies
4.3. Cell Culture—Generation of N2A Cells Stably Expressing a Mitochondrial Targeted GFPERβ Fusion Protein
4.4. Western Blot Analysis
4.5. Immunofluorescence—Confocal Microscopy
4.6. Cell Viability Assay
4.7. ATP Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hess, R.A.; Bunick, D.; Lee, K.-H.; Bahr, J.; Taylor, J.A.; Korach, K.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nat. Cell Biol. 1997, 390, 509–512. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.; Lacey, D.L.; Spelsberg, T.C.; Riggs, B.L. Estrogen Stimulates Gene Expression and Protein Production of Osteoprotegerin in Human Osteoblastic Cells. Endocrinology 1999, 140, 4367–4370. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Ganjam, V.K.; Taylor, J.A.; Yuan, X.; Stiehr, J.R.; Hardy, M.P.; Lubahn, D.B. Transcription and Translation of Estrogen Receptor-? in the Male Reproductive Tract of Estrogen Receptor-? Knock-Out and Wild-Type Mice. Endocrinology 1998, 139, 2982–2987. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogens regulate life and death in mitochondria. J. Bioenerg. Biomembr. 2017, 49, 307–324. [Google Scholar] [CrossRef]
- Kocanova, S.; Mazaheri, M.; Caze-Subra, S.; Bystricky, K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010, 11, 98. [Google Scholar] [CrossRef]
- Psarra, A.-M.G.; Sekeris, C.E. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life 2008, 60, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Levin, E.R. Extranuclear Steroid Receptors Are Essential for Steroid Hormone Actions. Annu. Rev. Med. 2015, 66, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Psarra, A.-M.; Solakidi, S.; Sekeris, C. The mitochondrion as a primary site of action of steroid and thyroid hormones: Presence and action of steroid and thyroid hormone receptors in mitochondria of animal cells. Mol. Cell. Endocrinol. 2006, 246, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, J.W.; Yang, S.-H.; Sarkar, S.N.; Pearce, V. Estrogen actions on mitochondria—Physiological and pathological implications. Mol. Cell. Endocrinol. 2008, 290, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Cammarata, P.R.; Baines, C.; Yager, J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009, 1793, 1540–1570. [Google Scholar] [CrossRef]
- Mattingly, K.A.; Ivanova, M.M.; Riggs, K.A.; Wickramasinghe, N.S.; Barch, M.J.; Klinge, C.M. Estradiol Stimulates Transcription of Nuclear Respiratory Factor-1 and Increases Mitochondrial Biogenesis. Mol. Endocrinol. 2008, 22, 609–622. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Emmanuelle, N.-E.; Marie-Cécile, V.; Florence, T.; Jean-François, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical Role of Estrogens on Bone Homeostasis in Both Male and Female: From Physiology to Medical Implications. Int. J. Mol. Sci. 2021, 22, 1568. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Adachi, Y.; Liu, P.; Fukuma, N.; Takimoto, E. Regulatory Actions of Estrogen Receptor Signaling in the Cardiovascular System. Front. Endocrinol. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocr. 2019, 55, 100787. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015, 16, 17–29. [Google Scholar] [CrossRef]
- Baez-Jurado, E.; Rincón-Benavides, M.; Lanussa, O.A.H.; Guio-Vega, G.; Ashraf, G.M.; Sahebkar, A.; Echeverria, V.; Garcia-Segura, L.; Barreto, G. Molecular mechanisms involved in the protective actions of Selective Estrogen Receptor Modulators in brain cells. Front. Neuroendocr. 2019, 52, 44–64. [Google Scholar] [CrossRef]
- Saldanha, C.J. Estrogen as a Neuroprotectant in Both Sexes: Stories from the Bird Brain. Front. Neurol. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Rahman, M.M.; Jakaria, M.; Rahman, M.S.; Hossain, M.S.; Islam, A.; Ahmed, M.; Mathew, B.; Omar, U.M.; Barreto, G.E.; et al. Estrogen Signaling in Alzheimer’s Disease: Molecular Insights and Therapeutic Targets for Alzheimer’s Dementia. Mol. Neurobiol. 2020, 57, 2654–2670. [Google Scholar] [CrossRef]
- Kann, O.; Kovács, R. Mitochondria and neuronal activity. Am. J. Physiol. Physiol. 2007, 292, C641–C657. [Google Scholar] [CrossRef]
- Johnson, J.; Mercado-Ayón, E.; Mercado-Ayón, Y.; Na Dong, Y.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 108698, 108698. [Google Scholar] [CrossRef]
- Tsialtas, I.; Gorgogietas, V.A.; Michalopoulou, M.; Komninou, A.; Liakou, E.; Georgantopoulos, A.; Kalousi, F.D.; Karra, A.G.; Protopapa, E.; Psarra, A.-M.G. Neurotoxic effects of aluminum are associated with its interference with estrogen receptors signaling. NeuroToxicology 2020, 77, 114–126. [Google Scholar] [CrossRef]
- Méndez, P.; Garcia-Segura, L.M. Phosphatidylinositol 3-Kinase and Glycogen Synthase Kinase 3 Regulate Estrogen Receptor-Mediated Transcription in Neuronal Cells. Endocrinology 2006, 147, 3027–3039. [Google Scholar] [CrossRef][Green Version]
- Su, C.; Rybalchenko, N.; Schreihofer, D.A.; Singh, M.; Abbassi, B.; Cunningham, R.L. Cell Models for the Study of Sex Steroid Hormone Neurobiology. J. Steroids Horm. Sci. 2012; 3, (Suppl. 2). [Google Scholar] [CrossRef]
- Li, H.-L.; Wang, H.-H.; Liu, S.-J.; Deng, Y.-Q.; Zhang, Y.-J.; Tian, Q.; Wang, X.-C.; Chen, X.-Q.; Yang, Y.; Zhang, J.-Y.; et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Li, C.-C.; Cai, J.-X. Aniracetam attenuates H2O2-induced deficiency of neuron viability, mitochondria potential and hippocampal long-term potentiation of mice in vitro. Neurosci. Bull. 2006, 22, 274–280. [Google Scholar] [PubMed]
- Maharjan, S.; Oku, M.; Tsuda, M.; Hoseki, J.; Sakai, Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 2014, 4, 05896. [Google Scholar] [CrossRef] [PubMed]
- Chiaradonna, F.; Gaglio, D.; Vanoni, M.; Alberghina, L. Expression of transforming K-Ras oncogene affects mitochondrial function and morphology in mouse fibroblasts. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 1338–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen Attenuates Traumatic Brain Injury by Inhibiting the Activation of Microglia and Astrocyte-Mediated Neuroinflammatory Responses. Mol. Neurobiol. 2021, 58, 1052–1061. [Google Scholar] [CrossRef]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Zhang, Q.; Tang, F.-L.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Neuron-Derived Estrogen Is Critical for Astrocyte Activation and Neuroprotection of the Ischemic Brain. J. Neurosci. 2020, 40, 7355–7374. [Google Scholar] [CrossRef]
- Guo, H.; Liu, M.; Zhang, L.; Wang, L.; Hou, W.; Ma, Y.; Ma, Y. The Critical Period for Neuroprotection by Estrogen Replacement Therapy and the Potential Underlying Mechanisms. Curr. Neuropharmacol. 2020, 18, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Moos, W.H.; Howell, N. Development of 17?-Estradiol as a Neuroprotective Therapeutic Agent: Rationale and Results from a Phase I Clinical Study. Ann. N. Y. Acad. Sci. 2005, 1052, 116–135. [Google Scholar] [CrossRef]
- Stanojlovic, M.; Guševac, I.; Grković, I.; Mitrovic, N.; Zlatković, J.; Horvat, A.; Drakulić, D.; Martinovic, J. Repeated Estradiol Treatment Attenuates Chronic Cerebral Hypoperfusion-Induced Neurodegeneration in Rat Hippocampus. Cell. Mol. Neurobiol. 2015, 36, 989–999. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Yi, K.D.; Yang, S.-H.; Dykens, J.A. Mitochondrial mechanisms of estrogen neuroprotection. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 1113–1120. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Yager, J.D.; Russo, J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim. Biophys. Acta (BBA)-Bioenerg. 2005, 1746, 1–17. [Google Scholar] [CrossRef]
- Chen, J.Q.; Eshete, M.; Alworth, W.L.; Yager, J.D. Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors and to human mitochondrial dna estrogen response elements. J. Cell. Biochem. 2004, 93, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Liu, R.; Perez, E.J.; Wen, Y.; Stevens, S.M.; Valencia, T.; Brun-Zinkernagel, A.-M.; Prokai, L.; Will, Y.; Dykens, J.; et al. Mitochondrial localization of estrogen receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4130–4135. [Google Scholar] [CrossRef]
- Psarra, A.-M.G.; Sekeris, C.E. Nuclear receptors and other nuclear transcription factors in mitochondria: Regulatory molecules in a new environment. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1783, 1–11. [Google Scholar] [CrossRef]
- Borrás, C.; Gambini, J.; Lopez-Grueso, R.; Pallardó, F.V.; Viña, J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Membrane estrogen receptors signal to determine transcription factor function. Steroids 2018, 132, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Delannoy, M.; Cooke, C.; Yager, J.D. Mitochondrial localization of ERα and ERβ in human MCF7 cells. Am. J. Physiol. Metab. 2004, 286, E1011–E1022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, J.; Du, Y. Estrogen receptor beta interacts and colocalizes with HADHB in mitochondria. Biochem. Biophys. Res. Commun. 2012, 427, 305–308. [Google Scholar] [CrossRef]
- Burstein, S.R.; Kim, H.J.; Fels, J.A.; Qian, L.; Zhang, S.; Zhou, P.; Starkov, A.A.; Iadecola, C.; Manfredi, G. Estrogen receptor beta modulates permeability transition in brain mitochondria. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 423–433. [Google Scholar] [CrossRef]
- Álvarez-Delgado, C.; Mendoza-Rodriguez, C.A.; Picazo, O.; Cerbón, M. Different expression of α and β mitochondrial estrogen receptors in the aging rat brain: Interaction with respiratory complex V. Exp. Gerontol. 2010, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yanamala, N.; Lathrop, K.L.; Zhang, L.; Klein-Seetharaman, J.; Srinivas, H. Ligand-Independent Antiapoptotic Function of Estrogen Receptor-β in Lung Cancer Cells. Mol. Endocrinol. 2010, 24, 1737–1747. [Google Scholar] [CrossRef]
- Liang, J.; Xie, Q.; Li, P.; Zhong, X.; Chen, Y. Mitochondrial estrogen receptor β inhibits cell apoptosis via interaction with Bad in a ligand-independent manner. Mol. Cell. Biochem. 2014, 401, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.; Veličković, N.; Stanojević, I.; Milosevic, M.; Drakulic, D.; Stanojlovic, M.; Horvat, A. Inhibition of mitochondrial Na+-dependent Ca2+ efflux by 17β-estradiol in the rat hippocampus. Neuroscience 2011, 192, 195–204. [Google Scholar] [CrossRef]
- Petrović, S.; Milosevic, M.; Drakulic, D.; Grkovic, I.; Stanojlovic, M.; Mitrovic, N.; Horvat, A. 17β-estradiol modulates mitochondrial Ca2+ flux in rat caudate nucleus and brain stem. Neuroscience 2012, 220, 32–40. [Google Scholar] [CrossRef]
- Crespo-Castrillo, A.; Arevalo, M.-A. Microglial and Astrocytic Function in Physiological and Pathological Conditions: Estrogenic Modulation. Int. J. Mol. Sci. 2020, 21, 3219. [Google Scholar] [CrossRef]
- Nelson, A.W.; Groen, A.J.; Miller, J.L.; Warren, A.Y.; Holmes, K.A.; Tarulli, G.A.; Tilley, W.; Katzenellenbogen, B.S.; Hawse, J.R.; Gnanapragasam, V.J.; et al. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol. Cell. Endocrinol. 2017, 440, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, M.; Shang, D.; Yan, X.; Zhao, B.; Zhang, X. Mitochondrial Behavior in Axon Degeneration and Regeneration. Front. Aging Neurosci. 2021, 13, 650038. [Google Scholar] [CrossRef]
- Kent, A.C.; El Baradie, K.B.Y.; Hamrick, M.W. Targeting the Mitochondrial Permeability Transition Pore to Prevent Age-Associated Cell Damage and Neurodegeneration. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; He, W.; Liou, Y.-C. The redox language in neurodegenerative diseases: Oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2019, 54, 1–26. [Google Scholar] [CrossRef]
- Green, P.S.; Simpkins, J.W. Neuroprotective effects of estrogens: Potential mechanisms of action. Int. J. Dev. Neurosci. 2000, 18, 347–358. [Google Scholar] [CrossRef]
- Green, P.S.; Simpkins, J.W. Estrogens and estrogen-like non-feminizing compounds. Their role in the prevention and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2006, 924, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Dubal, D.B.; E Wilson, M.; Rau, S.W.; Böttner, M.; Rosewell, K.L. Estradiol is a protective factor in the adult and aging brain: Understanding of mechanisms derived from in vivo and in vitro studies. Brain Res. Rev. 2001, 37, 313–319. [Google Scholar] [CrossRef]
- McEwen, B.S. Invited review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. J. Appl. Physiol. 2001, 91, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.-U.-D.; Baghel, K.S.; Kanchan, R.K.; Shrivastava, R.; A Malik, S.; Tewari, B.N.; Tripathi, C.; Negi, M.P.S.; Garg, V.K.; Sharma, M.; et al. Physical interaction of estrogen receptor with MnSOD: Implication in mitochondrial O2. − upregulation and mTORC2 potentiation in estrogen-responsive breast cancer cells. Oncogene 2016, 36, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Razandi, M.; Pedram, A.; Jordan, V.C.; Fuqua, S.; Levin, E.R. Tamoxifen regulates cell fate through mitochondrial estrogen receptor beta in breast cancer. Oncogene 2013, 32, 3274–3285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gou, Y.; Zhang, H.; Zuo, H.; Zhang, H.; Liu, Z.; Yao, D. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biol. 2014, 3, 88–99. [Google Scholar] [CrossRef]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Bohm, M.; Nickenig, G. Modulation of Antioxidant Enzyme Expression and Function by Estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef]
- Chiueh, C.C.; Lee, S.Y.; Andoh, T.; Murphy, D.L. Induction of Antioxidative and Antiapoptotic Thioredoxin Supports Neuroprotective Hypothesis of Estrogen. Endocrine 2003, 21, 27–32. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem. 2008, 105, 1342–1351. [Google Scholar] [CrossRef]
- Ogawaab, S.; Inoueab, S.; Watanabea, T.; Hiroia, H.; Orimoa, A.; Hosoib, T.; Ouchib, Y.; Muramatsu, M. The Complete Primary Structure of Human Estrogen Receptor β (hERβ) and Its Heterodimerization with ER αin Vivoandin Vitro. Biochem. Biophys. Res. Commun. 1998, 243, 122–126. [Google Scholar] [CrossRef]
- Psarra, A.-M.G.; Sekeris, C.E. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: Role of the mitochondrial glucocorticoid receptor. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1813, 1814–1821. [Google Scholar] [CrossRef]
- Psarra, A.-M.; Solakidi, S.; Trougakos, I.P.; Margaritis, L.H.; Spyrou, G.; Sekeris, C.E. Glucocorticoid receptor isoforms in human hepatocarcinoma HepG2 and SaOS-2 osteosarcoma cells: Presence of glucocorticoid receptor alpha in mitochondria and of glucocorticoid receptor beta in nucleoli. Int. J. Biochem. Cell Biol. 2005, 37, 2544–2558. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsialtas, I.; Georgantopoulos, A.; Karipidou, M.E.; Kalousi, F.D.; Karra, A.G.; Leonidas, D.D.; Psarra, A.-M.G. Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells. Int. J. Mol. Sci. 2021, 22, 7620. https://doi.org/10.3390/ijms22147620
Tsialtas I, Georgantopoulos A, Karipidou ME, Kalousi FD, Karra AG, Leonidas DD, Psarra A-MG. Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells. International Journal of Molecular Sciences. 2021; 22(14):7620. https://doi.org/10.3390/ijms22147620
Chicago/Turabian StyleTsialtas, Ioannis, Achilleas Georgantopoulos, Maria E. Karipidou, Foteini D. Kalousi, Aikaterini G. Karra, Demetrios D. Leonidas, and Anna-Maria G. Psarra. 2021. "Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells" International Journal of Molecular Sciences 22, no. 14: 7620. https://doi.org/10.3390/ijms22147620
APA StyleTsialtas, I., Georgantopoulos, A., Karipidou, M. E., Kalousi, F. D., Karra, A. G., Leonidas, D. D., & Psarra, A.-M. G. (2021). Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells. International Journal of Molecular Sciences, 22(14), 7620. https://doi.org/10.3390/ijms22147620








