LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds
2.1.1. Ellagitannins
2.1.2. Gallic Acid and Galloyl Derivatives
2.1.3. Other Polyphenols
2.2. Antioxidant Effects
2.3. Enzyme Inhibitory Activities
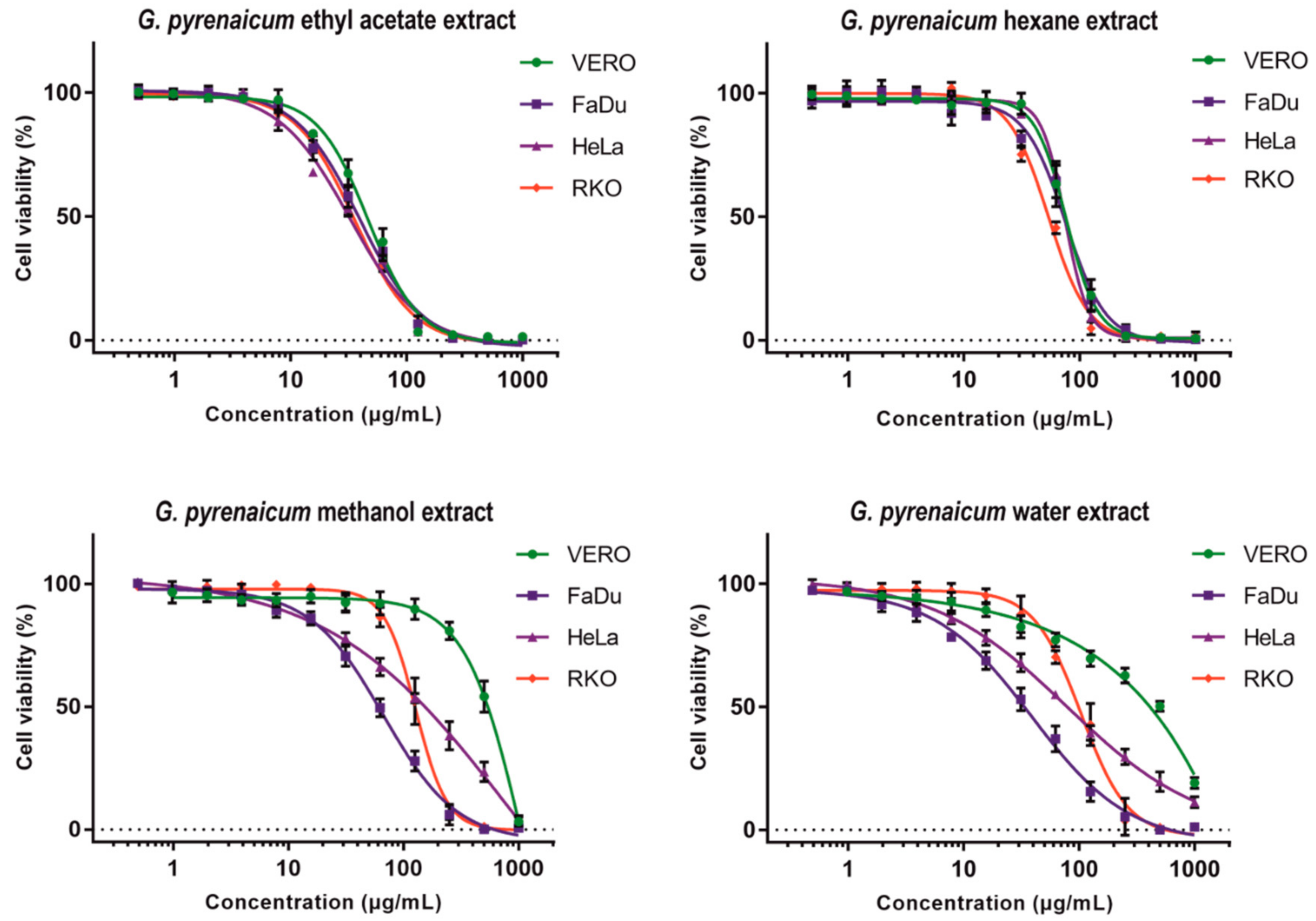
2.4. Cytotoxic Evaluation
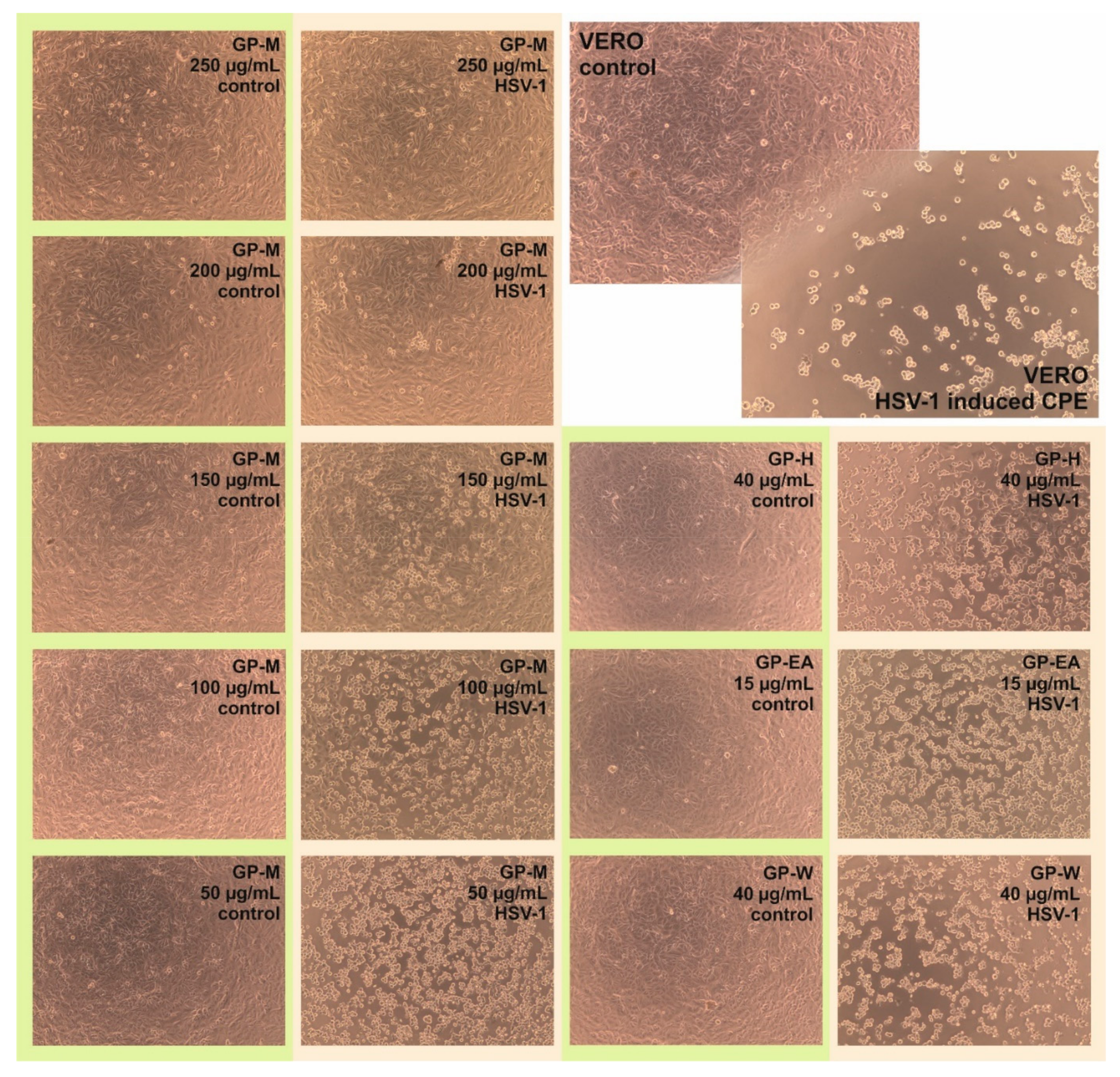
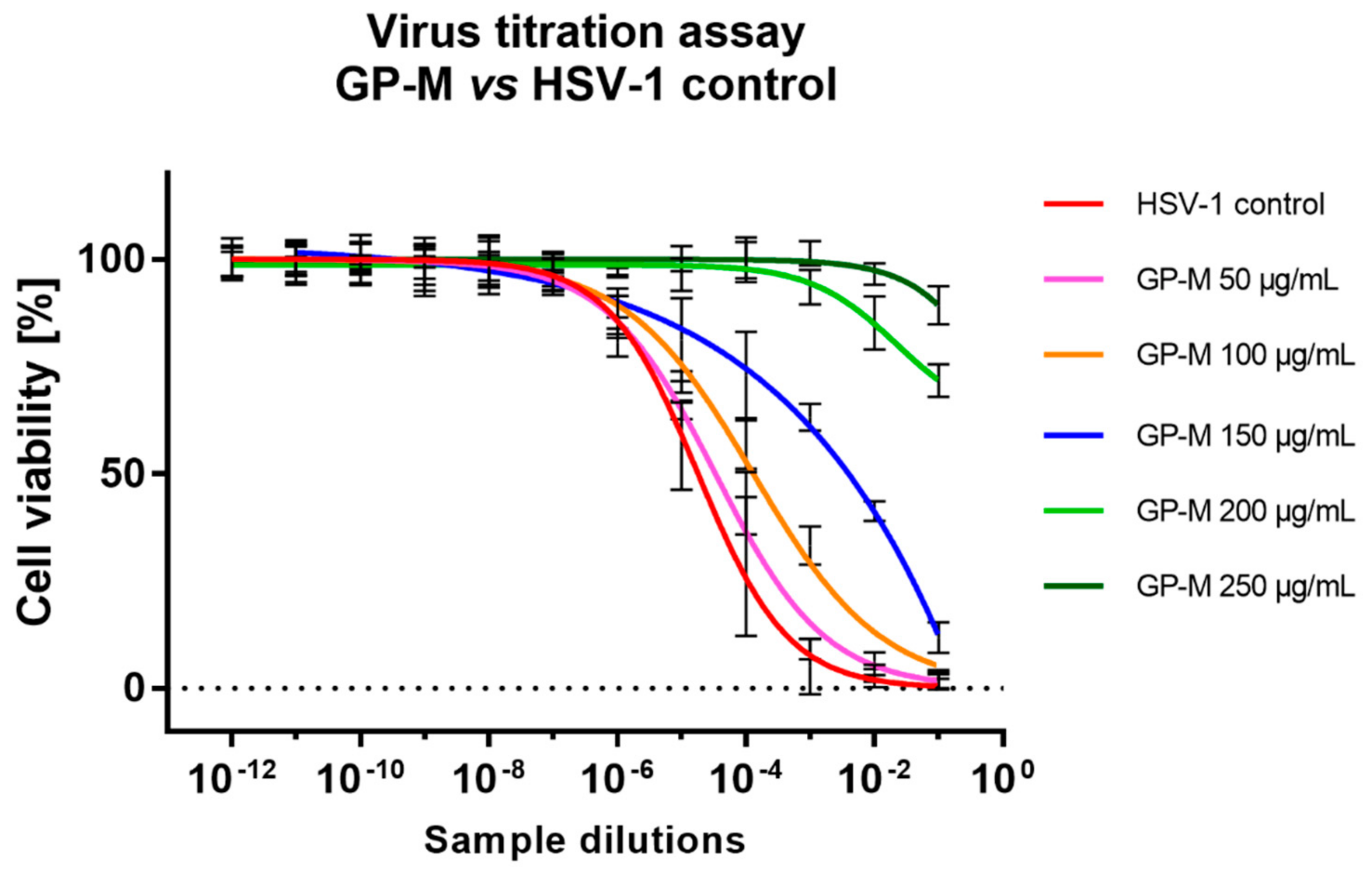
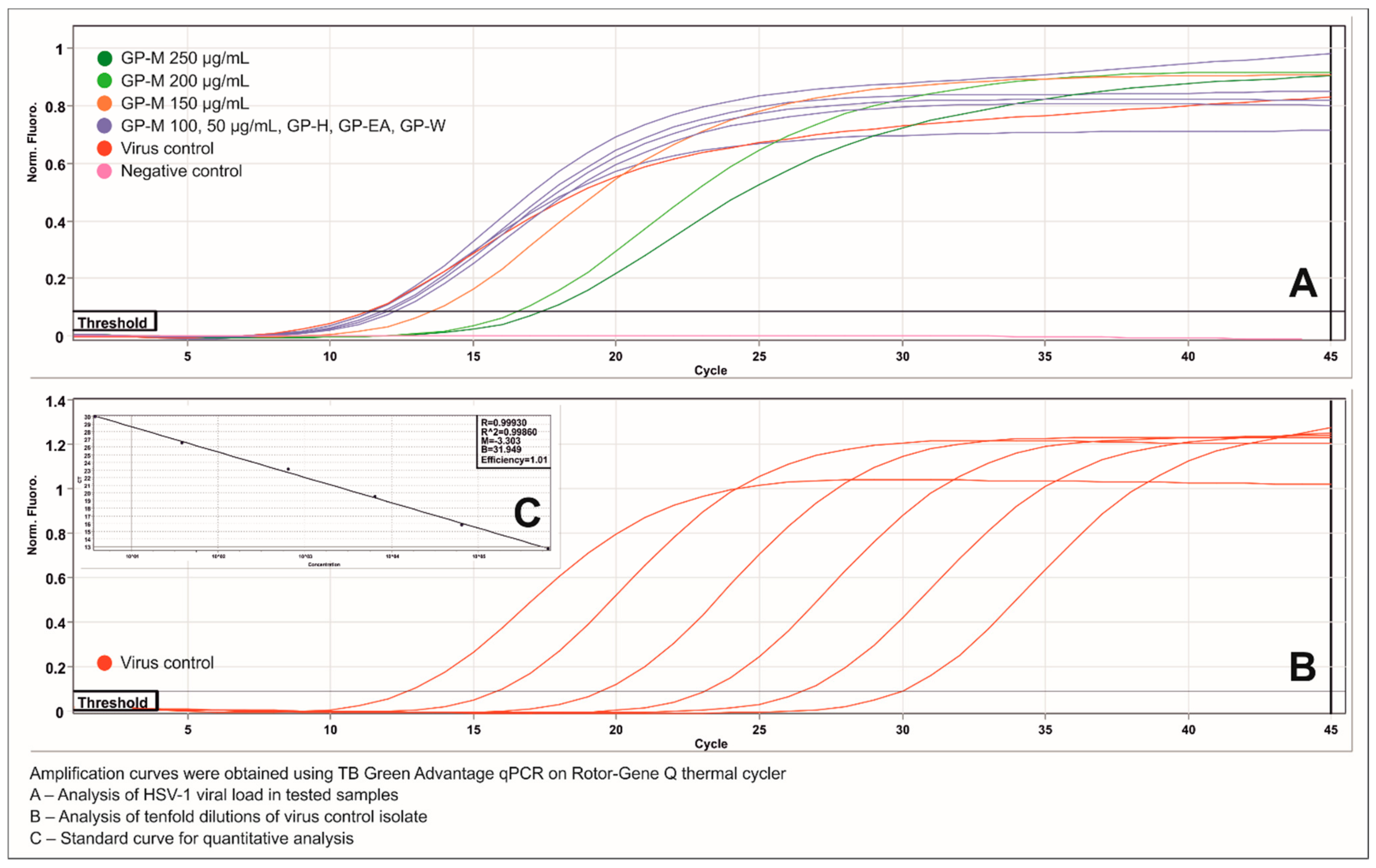
2.5. Antiviral Activity
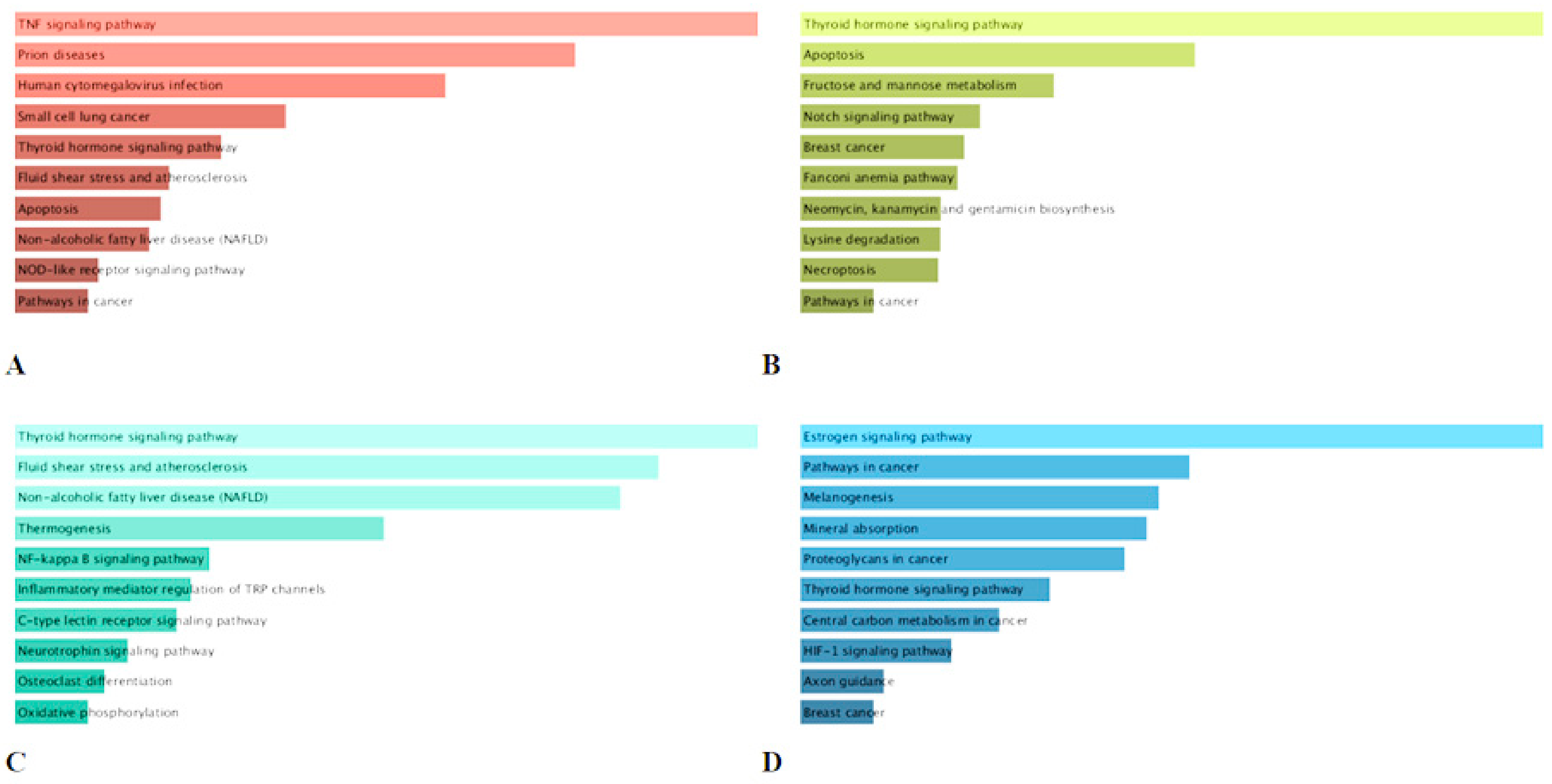
2.6. KEGG Pathway Enrichment Analysis of the Major Compounds
3. Materials and Methods
3.1. Plant Material and Preparation of Extracts
3.2. Profile of Bioactive Compounds
3.3. LC-ESI-QTOF-MS/MS Analysis
3.4. Determination of Antioxidant and Enzyme Inhibitory Effects
3.5. Cell Line Maintenance and Sample Preparation for In Vitro Assays
3.6. Cytotoxicity Assessment
3.7. Evaluation of Antiviral Activity—Influence on CPE Formation
3.8. End-Point Dilution Assay for HSV-1 Titration
3.9. Viral DNA Isolation and Real-Time PCR Analysis
3.10. Statistical Analysis
3.11. Bioinformatics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ilić, M.D.; Marčetić, M.D.; Zlatković, B.K.; Lakušić, B.S.; Kovačević, N.N.; Drobac, M.M. Chemical Composition of Volatiles of Eight Geranium L. Species from Vlasina Plateau (South Eastern Serbia). Chem. Biodivers. 2020, 17, e1900544. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.B.; de Lúcio, J.A.G.; Mendoza, N.V.; González, C.V.; De la O Arciniega, M.; Vargas, G.A. Geranium species as antioxidants. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; IntechOpen Limited: London, UK, 2013; p. 113. [Google Scholar]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef]
- Sharopov, F.; Ahmed, M.; Satyal, P.; Setzer, W.N.; Wink, M. Antioxidant activity and cytotoxicity of methanol extracts of Geranium macrorrhizum and chemical composition of its essential oil. J. Med. Act. Plants 2017, 5, 53–58. [Google Scholar]
- Herrera-Calderon, O.; Alvarado-Puray, C.; Arroyo-Acevedo, J.L.; Rojas-Armas, J.P.; Chumpitaz-Cerrate, V.; Hañari-Quispe, R.; Valenzuela-Herrera, R. Phytochemical screening, total phenolic content, antioxidant, and cytotoxic activity of five peruvian plants on human tumor cell lines. Pharmacogn. Res. 2018, 10, 161. [Google Scholar] [CrossRef]
- Kim, H.-S. The anti-melanogenic effect of Geranium krameri extract. Korean J. Food Sci. Technol. 2016, 48, 72–76. [Google Scholar] [CrossRef][Green Version]
- Nam, H.H.; Choo, B.K. Geranium koreanum, a medicinal plant Geranii Herba, ameliorate the gastric mucosal injury in gastritis-induced mice. J. Ethnopharmacol. 2021, 265, 113041. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Genç, Y.; Harput, Ş. Comparative evaluation of phenolic profile, antioxidative and cytotoxic activities of different Geranium species. Iran. J. Pharm. Res. IJPR 2017, 16, 178. [Google Scholar]
- Boisvert, W.A.; Yu, M.; Choi, Y.; Jeong, G.H.; Zhang, Y.-L.; Cho, S.; Choi, C.; Lee, S.; Lee, B.-H. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complementary Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- De Mello, C.P.; Bloom, D.C.; Paixão, I.C. Herpes simplex virus type-1: Replication, latency, reactivation and its antiviral targets. Antivir. Ther. 2016, 21, 277–286. [Google Scholar] [CrossRef]
- Yildirim, A.; Duran, G.G.; Duran, N.; Jenedi, K.; Bolgul, B.S.; Miraloglu, M.; Muz, M. Antiviral activity of hatay propolis against replication of herpes simplex virus type 1 and type 2. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 422. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-G.; Kim, Y.S.; Kim, J.H.; Chung, H.-S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Serkedjieva, J.; Ivancheva, S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J. Ethnopharmacol. 1998, 64, 59–68. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Hay, A.J. In vitro anti-influenza virus activity of a plant preparation from Geranium sanguineum L. Antivir. Res. 1998, 37, 121–130. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Zhou, W.; Feng, M.; Zhou, P. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol. Pharm. Bull. 2008, 31, 743–747. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Rocchetti, G.; Lucini, L.; Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M.; Senkardes, I.; Aktümsek, A. Chemical Characterization and Bioactive Properties of Different Extracts from Fibigia clypeata, an Unexplored Plant Food. Foods 2020, 9, 705. [Google Scholar] [CrossRef]
- Human Metabolome Database (HMDB). Available online: https://hmdb.ca/ (accessed on 15 April 2021).
- Metlin Database. Available online: https://metlin.scripps.edu (accessed on 15 April 2021).
- Masike, K.; Mhlongo, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- PubChem—An Open Chemistry Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 15 April 2021).
- Clifford, M.N.; Stoupi, S.; Kuhnert, N. Profiling and characterization by LC-MS n of the galloylquinic acids of green tea, tara tannin, and tannic acid. J. Agric. Food Chem. 2007, 55, 2797–2807. [Google Scholar] [CrossRef]
- MassBank of North America (MoNA) Database. Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 15 April 2021).
- Graça, V.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Santos, P.; Ferreira, I.C. Fractionation of the more active extracts of Geranium molle L.: A relationship between their phenolic profile and biological activity. Food Funct. 2018, 9, 2032–2042. [Google Scholar] [CrossRef]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Santos, P.F.; Ferreira, I.C. Chemical characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016, 7, 3807–3814. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Sinan, K.I.; Ak, G.; Etienne, O.K.; Sieniawska, E.; Maciejewska-Turska, M.; Świątek, Ł.; Rajtar, B. Chemical composition, biological properties and bioinformatics analysis of two Caesalpina species: A new light in the road from nature to pharmacy shelf. J. Pharm. Biomed. Anal. 2021, 198, 114018. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 1934578X1601100227. [Google Scholar] [CrossRef]
- D’Urso, G.; Maldini, M.; Pintore, G.; d’Aquino, L.; Montoro, P.; Pizza, C. Characterisation of Fragaria vesca fruit from Italy following a metabolomics approach through integrated mass spectrometry techniques. LWT 2016, 74, 387–395. [Google Scholar] [CrossRef]
- Montoro, P.; Serreli, G.; Gil, K.A.; D’Urso, G.; Kowalczyk, A.; Tuberoso, C.I.G. Evaluation of bioactive compounds and antioxidant capacity of edible feijoa (Acca sellowiana (O. Berg) Burret) flower extracts. J. Food Sci. Technol. 2020, 57, 2051–2060. [Google Scholar] [CrossRef]
- Grace, M.H.; Warlick, C.W.; Neff, S.A.; Lila, M.A. Efficient preparative isolation and identification of walnut bioactive components using high-speed counter-current chromatography and LC-ESI-IT-TOF-MS. Food Chem. 2014, 158, 229–238. [Google Scholar] [PubMed]
- Yang, B.; Kortesniemi, M.; Liu, P.; Karonen, M.; Salminen, J.-P. Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid chromatography–electrospray ionization mass spectrometry. J. Agric. Food Chem. 2012, 60, 8672–8683. [Google Scholar] [CrossRef]
- Tuominen, A.; Salminen, J.-P. Hydrolyzable tannins, flavonol glycosides, and phenolic acids show seasonal and ontogenic variation in Geranium sylvaticum. J. Agric. Food Chem. 2017, 65, 6387–6403. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar]
- Pérez-Ramírez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Comprehensive Characterization of Extractable and Nonextractable Phenolic Compounds by High-Performance Liquid Chromatography-Electrospray Ionization-Quadrupole Time-of-Flight of a Grape/Pomegranate Pomace Dietary Supplement. J. Agric. Food Chem. 2018, 66, 661–673. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Flavonoids and ellagitannins characterization, antioxidant and cytotoxic activities of Phyllanthus acuminatus Vahl. Plants 2017, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Ding, S.-T. A Review of the Phytochemistry and Pharmacology of Phyllanthus urinaria L. Front. Pharmacol. 2018, 9, 1109. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Yokota, T.; Lean, M.E.; Crozier, A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC–MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- De Andrade Neves, N.; Stringheta, P.C.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Flavonols and ellagic acid derivatives in peels of different species of jabuticaba (Plinia spp.) identified by HPLC-DAD-ESI/MSn. Food Chem. 2018, 252, 61–71. [Google Scholar] [CrossRef]
- Li, C.; Seeram, N.P. Ultra-fast liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry for the rapid phenolic profiling of red maple (Acer rubrum) leaves. J. Sep. Sci. 2018, 41, 2331–2346. [Google Scholar] [CrossRef]
- Ercil, D.; Kaloga, M.; Radtke, O.A.; Sakar, M.K.; Kiderlen, A.F.; Kolodziej, H. O-galloyl flavonoids from Geranium pyrenaicum and their in vitro antileishmanial activity. Turk. J. Chem. 2005, 29, 437–443. [Google Scholar]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and content of phenolic compounds in leaves, flowers, roots, and stalks of Sanguisorba officinalis L. determined with the LC-DAD-ESI-QTOF-MS/MS analysis and their in vitro antioxidant, antidiabetic, antiproliferative potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- Li, Z.-H.; Guo, H.; Xu, W.-B.; Ge, J.; Li, X.; Alimu, M.; He, D.-J. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Fan, X.; Qin, C.; Liu, J. Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorganic Med. Chem. Lett. 2012, 22, 2209–2211. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Aguilar, C.N.; Flores-Gallegos, A.C.; Sepúlveda, L.; Rodríguez-Herrera, R.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J. Preliminary Testing of Ultrasound/Microwave-Assisted Extraction (U/M-AE) for the Isolation of Geraniin from Nephelium lappaceum L. (Mexican Variety) Peel. Processes 2020, 8, 572. [Google Scholar] [CrossRef]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Xin, X.; Aisa, H.A. A LC/QTOF–MS/MS application to investigate chemical compositions in a fraction with protein tyrosine phosphatase 1B inhibitory activity from Rosa rugosa flowers. Phytochem. Anal. 2013, 24, 661–670. [Google Scholar] [CrossRef]
- Chao, C.-H.; Lin, Y.-J.; Cheng, J.-C.; Huang, H.-C.; Yeh, Y.-J.; Wu, T.-S.; Hwang, S.-Y.; Wu, Y.-C. Chemical constituents from Flueggea virosa and the structural revision of dehydrochebulic acid trimethyl ester. Molecules 2016, 21, 1239. [Google Scholar] [CrossRef]
- Sobeh, M.; Rezq, S.; Sabry, O.M.; Abdelfattah, M.A.; El Raey, M.A.; El-Kashak, W.A.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. Albizia anthelmintica: HPLC-MS/MS profiling and in vivo anti-inflammatory, pain killing and antipyretic activities of its leaf extract. Biomed. Pharmacother. 2019, 115, 108882. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Sinan, K.I.; Ferrarese, I.; Sut, S.; Bene, K.; Mahomoodally, M.F.; Bibi Sadeer, N.; Ak, G.; Zengin, G. Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts. Molecules 2020, 25, 5537. [Google Scholar] [CrossRef]
- Sinan, K.I.; Mahomoodally, M.F.; Eyupoglu, O.E.; Etienne, O.K.; Sadeer, N.B.; Ak, G.; Behl, T.; Zengin, G. HPLC-FRAP methodology and biological activities of different stem bark extracts of Cajanus cajan (L.) Millsp. J. Pharm. Biomed. Anal. 2021, 192, 113678. [Google Scholar] [CrossRef] [PubMed]
- Segura Campos, M.R.; Ruiz Ruiz, J.; Chel-Guerrero, L.; Betancur Ancona, D. Coccoloba uvifera (L.) (Polygonaceae) Fruit: Phytochemical Screening and Potential Antioxidant Activity. J. Chem. 2015, 2015, 534954. [Google Scholar] [CrossRef]
- Nandini, H.; Naik, P.R. Action of corilagin on hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 299, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.-P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef]
- Zeb, A. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Mol. Cell. Biochem. 2018, 448, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Ou, Y.; Chen, H.; Xiao, S.; Liu, G.; Cao, Y.; Huang, Q. Cellular antioxidant activities of polyphenols isolated from Eucalyptus leaves (Eucalyptus grandis × Eucalyptus urophylla GL9). J. Funct. Foods 2014, 7, 737–745. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Nat. C J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activities of selected polyphenols—A short report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chen, J. Screening and identification of acetylcholinesterase inhibitors from Terminalia chebula fruits based on ultrafiltration and ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Microchem. J. 2021, 168, 106438. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Awad, O.M.E.; Shehata, M.G.; El-Sohaimy, S.A. Antioxidant and anti-acetylcholinesterase potential of artichoke phenolic compounds. Food Biosci. 2021, 41, 101006. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini. Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Yang, Y.; Lian, G.; Yu, B. Naturally occurring polyphenolic glucosidase inhibitors. Isr. J. Chem. 2015, 55, 268–284. [Google Scholar] [CrossRef]
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 34993. [Google Scholar] [CrossRef]
- Bulusu, K.C.; Guha, R.; Mason, D.J.; Lewis, R.P.I.; Muratov, E.; Kalantar Motamedi, Y.; Cokol, M.; Bender, A. Modelling of compound combination effects and applications to efficacy and toxicity: State-of-the-art, challenges and perspectives. Drug Discov. Today 2016, 21, 225–238. [Google Scholar] [CrossRef]
- Cho, S.-M.; Kim, J.-H.; Lee, M.-W. Inhibitory effects of tannins on tyrosinase activity. Korean J. Pharmacogn. 2001, 32, 68–71. [Google Scholar]
- Ozer, O.C.; Orhan, I.E.; Çalışkan, B.; Deniz, F.S.S.; Gokbulut, A.; Maz, T.G.; Aysal, A.; Emerce, E.; Shekfeh, S.; Kahraman, A. Exploration of anti-tyrosinase effect of Geranium glaberrimum Boiss. & Heldr. with in silico approach and survey of 21 Geranium species. J. Herb. Med. 2021, 27, 100431. [Google Scholar]
- Mazzio, E.; Badisa, R.; Mack, N.; Deiab, S.; Soliman, K. High throughput screening of natural products for anti-mitotic effects in MDA-MB-231 human breast carcinoma cells. Phytother. Res. 2014, 28, 856–867. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A. Natural products as antiviral agents. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 24, pp. 473–572. [Google Scholar]
- Nonaka, G.-I.; Nishioka, I.; Nishizawa, M.; Yamagishi, T.; Kashiwada, Y.; Dutschman, G.E.; Bodner, A.J.; Kilkuskie, R.E.; Cheng, Y.-C.; Lee, K.-H. Anti-AIDS agents, 2: Inhibitory effect of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells. J. Nat. Prod. 1990, 53, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Stefaniu, A.; Pirvu, L.; Albu, B.; Pintilie, L. Molecular Docking Study on Several Benzoic Acid Derivatives against SARS-CoV-2. Molecules 2020, 25, 5828. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Mohammed, H.H.H.; Dutta, K.; Li, C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CLpro: An in silico approach with 19 structural different hydrolysable tannins. J. Food Biochem. 2020, 44, e13432. [Google Scholar] [CrossRef]
- Yeo, S.-G.; Song, J.H.; Hong, E.-H.; Lee, B.-R.; Kwon, Y.S.; Chang, S.-Y.; Kim, S.H.; won Lee, S.; Park, J.-H.; Ko, H.-J. Antiviral effects of Phyllanthus urinaria containing corilagin against human enterovirus 71 and Coxsackievirus A16 in vitro. Arch. Pharmacal Res. 2015, 38, 193–202. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sharma, G.; Bag, P.; Roy, C.L.; Sudha, G.; Tandon, H.; Dave, P.; Shukla, A. A natural small molecule inhibitor corilagin blocks HCV replication and modulates oxidative stress to reduce liver damage. Antivir. Res. 2018, 150, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Zhang, S.-J.; Liu, P.; Wang, Y.-Q.; Chen, Z.-L.; Wang, Y.-J.; Zhou, J.-B.; Guo, Y.-J.; Zhao, L. Corilagin interferes with Toll-like receptor 3-mediated immune response in herpes simplex encephalitis. Front. Mol. Neurosci. 2019, 12, 83. [Google Scholar] [CrossRef]
- Chen, F.; Yang, L.; Zhai, L.; Huang, Y.; Chen, F.; Duan, W.; Yang, J. Methyl brevifolincarboxylate, a novel influenza virus PB2 inhibitor from Canarium Album (Lour.) Raeusch. Chem. Biol. Drug Des. 2020, 96, 1280–1291. [Google Scholar] [CrossRef]
- Yang, C.-M.; Cheng, H.-Y.; Lin, T.-C.; Chiang, L.-C.; Lin, C.-C. The in vitro activity of geraniin and 1, 3, 4, 6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007, 110, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Grauzdytė, D.; Koishi, A.C.; Viranaicken, W.; Venskutonis, P.R.; Nunes Duarte dos Santos, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. The geraniin-rich extract from Reunion Island endemic medicinal plant Phyllanthus phillyreifolius inhibits Zika and dengue virus infection at non-toxic effect doses in zebrafish. Molecules 2020, 25, 2316. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.-W.; Kan, C.-W.; Hau, D.K.-P. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.T.; Gohil, V.M.; Bhutani, K.K. Modulating TNF-α signaling with natural products. Drug Discov. Today 2006, 11, 725–732. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.-I.; Yamaguchi, Y. Estrogen signaling pathway and hormonal therapy. Breast Cancer 2008, 15, 256–261. [Google Scholar] [CrossRef]
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030. [Google Scholar] [CrossRef]
- Chi, H.-C.; Tsai, C.-Y.; Tsai, M.-M.; Yeh, C.-T.; Lin, K.-H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Cipolletti, M.; Solar Fernandez, V.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the antioxidant activity of dietary polyphenols in cancer: The modulation of estrogen receptors (ers) signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef]
- Da-Silva, W.S.; Harney, J.W.; Kim, B.W.; Li, J.; Bianco, S.D.; Crescenzi, A.; Christoffolete, M.A.; Huang, S.A.; Bianco, A.C. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes 2007, 56, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Zengin, G.; Sieniawska, E.; Senkardes, I.; Picot-Allain, M.C.N.; Sinan, K.I.; Mahomoodally, M.F. Antioxidant abilities, key enzyme inhibitory potential and phytochemical profile of Tanacetum poteriifolium Grierson. Ind. Crop. Prod. 2019, 140, 111629. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Lagunin, A.; Ivanov, S.; Rudik, A.; Filimonov, D.; Poroikov, V. DIGEP-Pred: Web service for in silico prediction of drug-induced gene expression profiles based on structural formula. Bioinformatics 2013, 29, 2062–2063. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
| Solvents | TPC (mg GAE/g) | TFC (mg RE/g) | PBD (mmol TE/g) |
|---|---|---|---|
| Hexane | 25.03 ± 0.27 d | 28.26 ± 1.61 c | 1.59 ± 0.07 c |
| EA | 31.25 ± 3.15 c | 43.95 ± 0.50 a | 2.13 ± 0.11 b |
| MeOH | 133.22 ± 0.34 b | 38.66 ± 0.73 b | 3.08 ± 0.15 a |
| Water | 170.50 ± 0.46 a | 25.11 ± 0.31 d | 3.15 ± 0.09 a |
| Comp. No | Tentative Identification | Retention Time | Molecular Formula | Molecular Weight | [M-H]− | Fragments (m/z) | Extracts | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid glucoside | 1.520 | C12H22O11 | 342.1109 | 341.1109 | 179.0572; 161.0482; 135.0455 | 1, 2, 3, 4 | [16] |
| 2 | Quinic acid derivative | 1.654 | - | 534.1755 | 533.1755 | 191.0583; 173.0415; 111.0477 | 3, 4 | [16] |
| 3 | Quinic acid | 1.771 | C7H12O6 | 192.0584 | 191.0584 | 173.0184; 111.0078; 93.0373; 85.0305 | 1, 2, 3, 4 | [17] |
| 4 | Malic acid | 1.917 | C4H6O5 | 134.0154 | 133.0154 | 115.0046; 89.268; 71.0147 | 1, 2, 3, 4 | [18] |
| 5 | Citric acid | 2.246 | C6H8O7 | 192.0209 | 191.0209 | 111.0131; 87.0128; 57.0408 | 1, 3, 4 | [19,20] |
| 6 | 3-O-Galloylquinic acid | 2.658 | C14H16O10 | 344.0695 | 343.0695 | 191.0516; 169.0091; 125.0179; 111.0505 107.0141 | 3 | [21] |
| 7 | Glucogallic acid/Glucosyl gallate | 2.900 | C13H16O10 | 332.0694 | 331.0694 | 271.0432; 211.0585; 169.0143; 151.0054; 125.0211; | 1,2,3 | [22] |
| 8 | Gallic acid | 4.049 | C7H6O5 | 170.0158 | 169.0158 | 125.0247; 106.9624; 83.0489; 79.0247; 51.0242 | 2, 3, 4 | [17] |
| 9 | 4-O-Galloylquinic acid | 5.143 | C14H16O10 | 344.0695 | 343.0695 | 191.0550; 173.0496; 169.0131; 125.0298; 85.0290 | 1, 2, 3, 4 | [21] |
| 10 | Dihydroxybenzoic acid glucoside | 6.337 | C13H16O9 | 316.0748 | 315.0748 | 153.0217; 108.0278 | 3 | [18,20,22] |
| 11 | Glycerol gallate | 6.454 | C10H12O7 | 244.0508 | 243.0508 | 169.0111; 125.0275; 124.0175 | 3 | [17] |
| 12 | Dihydroxybenzoic acid | 7.476 | C7H6O4 | 154.0208 | 153.0208 | 109.0302; 108.0203; 91.0152; 53.0379 | 2, 3, 4 | [18] |
| 13 | Gallic acid O-(6-galloylglucoside)/Di-galloyl-hexoside | 8.103 | C20H20O14 | 484.0812 | 483.0812 | 331.0590; 313.0453; 271.0677; 169.0110; 150.9913; 125.0221 | 3, 4 | [17,23,24] |
| 14 | 2-Isopropylmalic acid | 8.243 | C7H12O5 | 176.0614 | 175.0614 | 157.0537; 131.0713; 115.0409; 113.0609; 85.0661 | 3, 4 | [18,25] |
| 15 | Galloylshikimic acid | 9.056 | C14H14O9 | 326.0585 | 325.0585 | 173.0437; 169.0163; 137.0592; 125.0260 | 3 | [25,26,27] |
| 16 | di-HHDP-glucose isomer 1 (Pedunculagin I structure) | 9.121 | C34H24O22 | 784.0720 | 783.0720 | 481.0677; 301.0031; 275.0225; 249.0320; 169.0301 | 3 | [28,29] |
| 17 | Dihydrocaffeic acid | 10.037 | C9H10O4 | 182.0157 | 181.0157 | 137.0283; 109.0356; | 3, 4 | [18] |
| 18 | Unknown | 10.592 | - | 442.1640 | 441.1640 | 377. 1378; 317.1239; 275.1158; 233. 1032; 173.0836; 119.0361 | 1,2,3 | |
| 19 | Unknown | 10.636 | - | 452.2188 | 451.2188 | 405.1966 | 1, 2, 3, 4 | |
| 20 | Methyl gallate | 10.832 | C8H8O5 | 184.0315 | 183.0315 | 168.0088; 124.0184; 78.0135 | 1, 2, 3, 4 | [22] |
| 21 | di-HHDP-glucose isomer 2 (Pedunculagin I structure) | 12.438 | C34H24O22 | 784.0692 | 783.0692 | 481.0698; 301.0021; 275.0229; 249.0429; 169.0448 | 3 | [27,28,29] |
| 22 | Caffeoylmalic Acid | 13.375 | C13H12O8 | 296.0480 | 295.0480 | 251.0566; 219.0290 | 3, 4 | [20] |
| 23 | Benzyl alcohol D-xylopyranosyl D-glucopyranoside | 14.119 | C18H26O10 | 402.1448 | 447.1522 [M+HCOOH]− | 401.1448; 269.1076; 161.0426; 149.0446 | 2, 3, 4 | [20] |
| 24 | HDDP-galloyl-glucose isomer 1- (Corilagin structure) | 14.299 | C27H22O18 | 634.0689 | 633.0689 | 463.0484; 300.9942; 275.0090; 169.0082 | 3 | [27,30,31] |
| 25 | Di-galloylo-quinic acid | 15.179 | C21H20O14 | 496.0805 | 495.0805 | 343.0615; 191.0564; 169.0102 | 3, 4 | [32] |
| 26 | Methyl brevifolincarboxylate derivative | 15.897 | - | 382.0480 | 381.0480 | 337.0593; 305.0301; 273.0018; 261.0420 | 3 | [20] |
| 27 | Brevifolin | 17.761 | C10H12O4 | 248.0268 | 247.0268 | 219.0324; 191.0495; 173.0356; 145.0434; 117.0400 | 3, 4 | [20,33,34] |
| 28 | Brevifolincarboxylic acid | 18.026 | C13H8O8 | 292.0169 | 291.0169 | 247.0269; 191.0358; 173.0274; 163.0419; 145.0339 | 3, 4 | [20,32,35] |
| 29 | HDDP-galloyl-glucose isomer 2 (Corilagin structure) | 19.130 | C27H22O18 | 634.0768 | 633.0768 | 463.0507; 300.9996; 275.0242; 245.0085; 169.0145; 125.0232 | 3, 4 | [27,30,31] |
| 30 | di-galloyl-HHDP-glucoside isomer 1 (Tellimagrandin I structure) | 19.268 | C34H26O22 | 786.0725 | 785.0725 | 633.0786; 615.0878; 483.0649; 300.9964; 275.0119; 249.0340; 169.0128I | 3, 4 | [30] |
| 31 | Geraniin | 20.417 | C41H27O27 | 952.0548 | 951.0548 | 933.0557; 915.0390; 802.8552; 463.0388; 300.9928; 169.0060 | 3 | [31,36] |
| 32 | Methyl brevifolincarboxylate isomer 1 | 20.886 | C14H10O8 | 306.0325 | 305.0325 | 273.0017; 245.0049; 217.0084; 173.0185; 161.0211; 145.0259; 133.0261; 117.0297; 105.0318 | 3, 4 | [33,35] |
| 33 | Dehydrochebulic acid trimethyl ester | 22.134 | C17H16O11 | 396.0647 | 395.0647 | 363.0370; 351.0818; 319.0430; 287.0224 | 3 | [20,37] |
| 34 | Shikimic acid derivative | 22.992 | - | 296.0462 | 295.0462 | 173.0056; 154.9935; 129.0224; 111.0080 | 3, 4 | [20] |
| 35 | Methyl brevifolincarboxylate isomer 2 | 23.398 | C14H10O8 | 306.0312 | 305.0312 | 273.0026; 245.0060; 217.0143; 201.0204; 189.0203; 173.0235; 161.0230; 145.0310; 133.0298; | 3 | [33,35] |
| 36 | Ferulic acid | 23.563 | C10H10O4 | 194.0509 | 193.0509 | 178.0311; 149.0628; 135.0424; 134.0364; 106.0464 | 3, 4 | [18] |
| 37 | Quercetin-O-hexoside derivative | 23.773 | - | 774.1598 | 773.1598 | 463.0868; 301.0189; 300.0253; 193.0506 | 3, 4 | [18] |
| 38 | Quercetin-O-hexoside | 23.900 | C21H20O12 | 464.0909 | 463.0909 | 301.0241; 300.0241; 271.0248; 255.0258 178.9943; 151.0021 | 1, 2, 3, 4 | [18] |
| 39 | Ellagic acid | 24.240 | C14H6O8 | 302.0022 | 301.0022 | 300.9990; 283.9952; 245.0101; 229.0198; 200.0098; 173.0274; 157.2608; 161.0196; 145.0282 | 3, 4 | [18,38] |
| 40 | Nonanedioic acid | 24.305 | C9H16O4 | 188.0981 | 187.0981 | 169.0836; 143.0994; 125.0960; 97.0642 | 2 | [18] |
| 41 | Quercetin-O-glucuronide | 24.569 | C21H18O13 | 478.0684 | 477.0684 | 301.0353; 178.9961; 151.0043 | 3, 4 | [38,39] |
| 42 | Quercetin-O-(galloyl)-glucoside | 24.856 | C28H24O16 | 616.1008 | 615.1008 | 301.0359; 179.0011; 151.0061 | 2, 3, 4 | [40] |
| 43 | Kaempferol-O-galloylglucoside | 25.318 | C28H24O15 | 600.1022 | 599.1022 | 447.0780; 313.0565; 285.0410; 284.0369; 255.0288; 169.0141; 151.0032; 125.0251 | 3 | [18,41] |
| 44 | Kaempferol-O-glucoside | 25.640 | C21H20O11 | 448.0974 | 447.0974 | 285.0379; 284.0314; 255.0266; 151.0010 | 3, 4 | [18,41] |
| 45 | HHDP-galloyl glucovanillin | 26.653 | C35H28O20 | 768.1081 | 767.1081 | 615.1026; 465.0779; 313.0672; 169.0220; 125.0308 | 3, 4 | [20,27] |
| 46 | Ellagic acid-galloyl-glucoside derivative | 28.253 | - | 998.1205 | 997.1205 | 827.0874; 615.0581; 463.0464; 300.9983; 169.0161 | 3 | [27] |
| 47 | HHDP-galloyl derivative | 28.467 | - | 752.1191 | 751.1191 | 599.1070; 465.0743; 313.0625; 169.0130; 125.0217 | 3 | [27] |
| 48 | Quercetin | 30.914 | C15H10O7 | 302.0022 | 301.0022 | 257.0624; 178.9998; 151.0052 | 1, 2, 3, 4 | [18] |
| 49 | Kaempferol-3-O-rutinoside | 30.969 | C27H30O15 | 594.1334 | 593.1334 | 285.0414; 284.0318; 255.0296; 151.0048 | 3 | [33] |
| 50 | Luteolin | 31.018 | C15H10O6 | 286.0428 | 285.0428 | 199.0523; 175.0417; 149.0235; 133.0306 | 1, 2, 3, 4 | [18] |
| 51 | Fatty acid | 31.965 | C18H32O5 | 328.2197 | 327.2197 | - | 1, 2, 3, 4 | |
| 52 | Fatty acid | 33.680 | C18H34O5 | 330.2349 | 329.2349 | - | 1, 2, 3, 4 | |
| 53 | Fatty acid derivative | 37.072 | - | 536.2399 | 581.2461 [M+HCOOH]− | - | 1, 2, 3, 4 | |
| 54 | Unknown | 37.698 | - | 308.1939 | 307.1939 | 289.1870; 235.1350; 185.1188; 121.0060 | 1, 2, 3, 4 | |
| 55 | Fatty acid derivative | 38.986 | - | 550.2535 | 595.2635 [M+HCOOH]− | - | 1, 2, 3, 4 | |
| 56 | Fatty acid derivative | 41.062 | - | 564.2696 | 609.2767 [M+HCOOH]− | - | 1, 2, 3, 4 |
| Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|
| Hexane | na | 6.02 ± 0.67 d | 64.18 ± 2.37 d | 30.68 ± 0.18 d | 12.13 ± 0.25 d |
| EA | 6.23 ± 0.53 c | 22.43 ± 1.74 c | 89.59 ± 1.07 c | 40.43 ± 0.29 c | 25.84 ± 2.26 c |
| MeOH | 199.26 ± 0.13 a | 448.84 ± 3.67 b | 514.79 ± 15.17 b | 294.54 ± 4.00 b | 36.53 ± 1.10 b |
| Water | 191.20 ± 0.18 b | 469.82 ± 0.34 a | 613.27 ± 4.64 a | 364.10 ± 1.71 a | 52.39 ± 0.15 a |
| Solvents | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|
| Hexane | 3.41 ± 0.09 b | 10.05 ± 0.13 ab | 113.33 ± 0.37 b | 0.92 ± 0.01 b | 2.23 ± 0.04 b |
| EA | 4.49 ± 0.09 a | 12.26 ± 0.98 a | 109.11 ± 0.65 c | 1.04 ± 0.03 a | 2.18 ± 0.01 b |
| MeOH | 4.39 ± 0.30 a | 8.49 ± 1.60 b | 121.42 ± 0.33 a | 0.87 ± 0.03 b | 2.39 ± 0.03 a |
| Water | 0.65 ± 0.09 c | na | 28.53 ± 1.11 d | 0.21 ± 0.01 c | 2.04 ± 0.03 c |
| Geranium pyrenaicum | VERO | FaDu | HeLa | RKO | |||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | Sample Code | CC50 | CC10 | CC50 | SI | CC50 | SI | CC50 | SI |
| Hexane | GP-H | 76.07 ± 3.63 | 40.1 ± 9.06 | 75.46 ± 6.40 | 1.0 | 73.28 ± 3.37 | 1.0 | 53.72 ± 2.07 | 1.4 |
| Ethyl acetate | GP-EA | 46.53 ± 1.61 | 15.75 ± 3.20 | 39.49 ± 5.18 | 1.2 | 32.34 ± 1.27 | 1.4 | 35.27 ± 1.14 | 1.3 |
| Methanol | GP-M | 481.50 ± 47.09 | 222.61 ± 35.1 | 66.92 ± 8.0 * | 7.2 | 132.44 ± 11.22 * | 3.6 | 124.77 ± 14.79 * | 3.9 |
| Water | GP-W | 435.93 ± 32.31 | 36.23 ± 5.90 | 40.22 ± 2.89 * | 10.8 | 63.21 ± 8.67 * | 6.9 | 96.27 ± 13.72 * | 4.5 |
| Geranium pyrenaicum Extracts | ||||
|---|---|---|---|---|
| Solvent | Sample Code | Concentration (µg/mL) | Reduction of HSV-1 Infectious Titer (Δlog) * | Reduction of HSV-1 Viral Load (Δlog’) ** |
| Hexane | GP-H | 40 | 0.09 ± 0.28 | 0.17 ± 0.19 |
| Ethyl acetate | GP-EA | 15 | −0.24 ± 0.39 | 0.17 ± 0.18 |
| Methanol | GP-M | 250 | >4 | 1.72 ± 0.19 |
| 200 | >4 | 1.56 ± 0.04 | ||
| 150 | 2.1 ± 0.25 | 0.64 ± 0.06 | ||
| 100 | 1.29 ± 0.17 | 0.23 ± 0.04 | ||
| 50 | 0.06 ± 0.27 | 0.32 ± 0.03 | ||
| Water | GP-W | 40 | 0.16 ± 0.18 | 0.24 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F.; et al. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. https://doi.org/10.3390/ijms22147621
Świątek Ł, Sieniawska E, Sinan KI, Maciejewska-Turska M, Boguszewska A, Polz-Dacewicz M, Senkardes I, Guler GO, Bibi Sadeer N, Mahomoodally MF, et al. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. International Journal of Molecular Sciences. 2021; 22(14):7621. https://doi.org/10.3390/ijms22147621
Chicago/Turabian StyleŚwiątek, Łukasz, Elwira Sieniawska, Kouadio Ibrahime Sinan, Magdalena Maciejewska-Turska, Anastazja Boguszewska, Małgorzata Polz-Dacewicz, Ismail Senkardes, Gokalp Ozmen Guler, Nabeelah Bibi Sadeer, Mohamad Fawzi Mahomoodally, and et al. 2021. "LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure" International Journal of Molecular Sciences 22, no. 14: 7621. https://doi.org/10.3390/ijms22147621
APA StyleŚwiątek, Ł., Sieniawska, E., Sinan, K. I., Maciejewska-Turska, M., Boguszewska, A., Polz-Dacewicz, M., Senkardes, I., Guler, G. O., Bibi Sadeer, N., Mahomoodally, M. F., & Zengin, G. (2021). LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. International Journal of Molecular Sciences, 22(14), 7621. https://doi.org/10.3390/ijms22147621










