A Novel Allele Encoding 7-Hydroxymethyl Chlorophyll a Reductase Confers Bacterial Blight Resistance in Rice
Abstract
:1. Introduction
2. Results
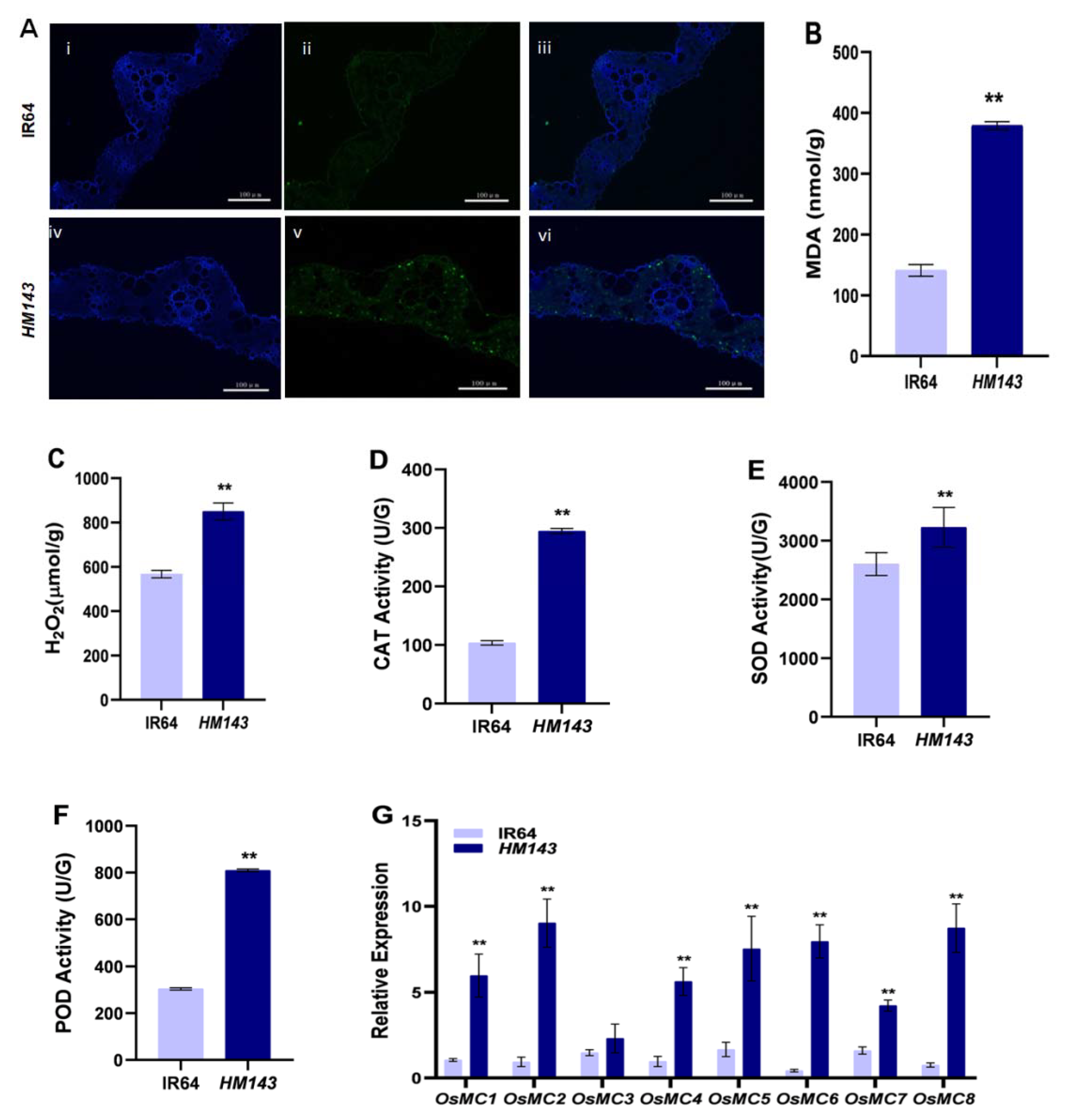
2.1. PCD Is Activated in HM143
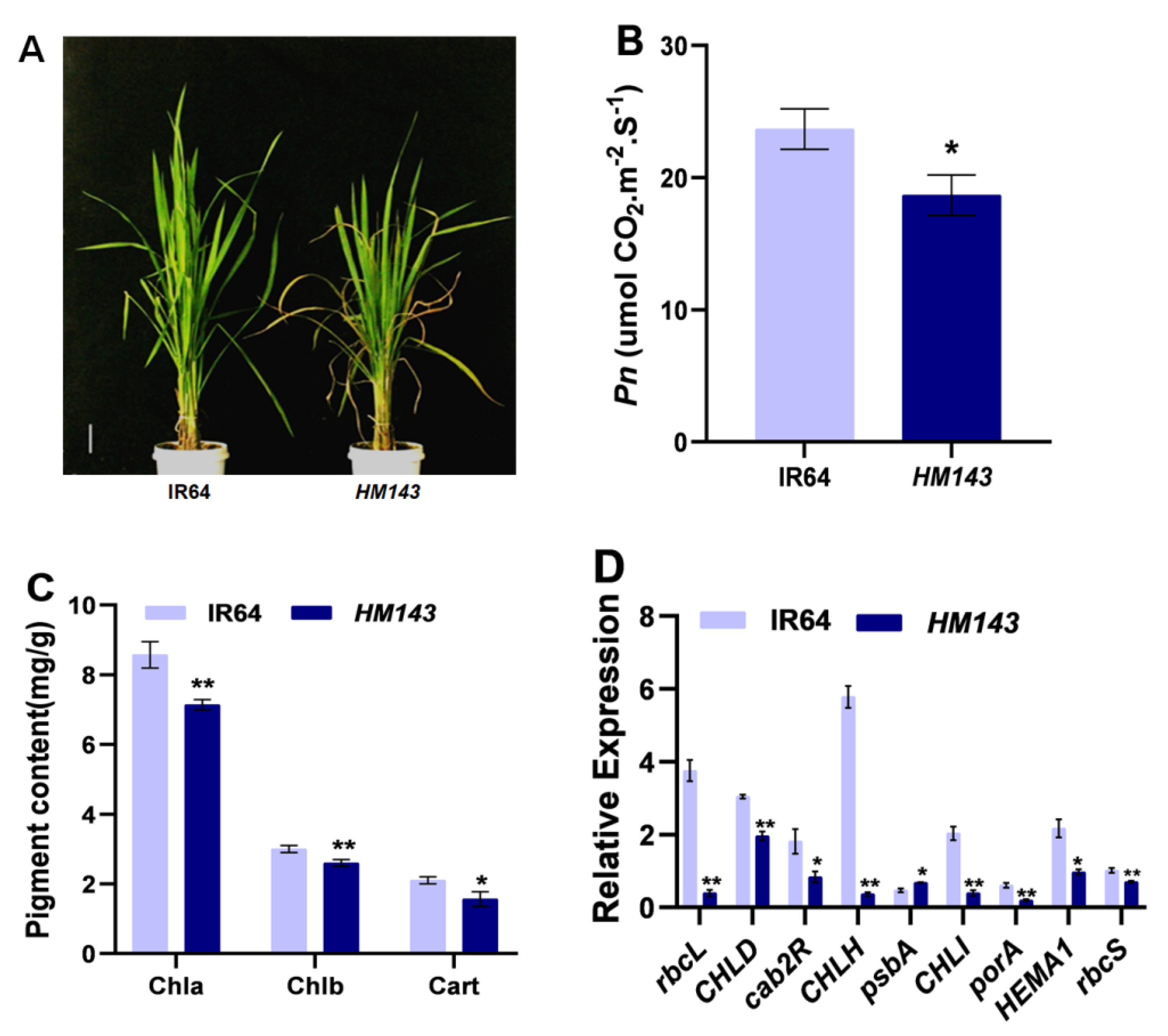
2.2. HM143 Is Deficient in Chlorophyll Metabolism and Photosynthesis
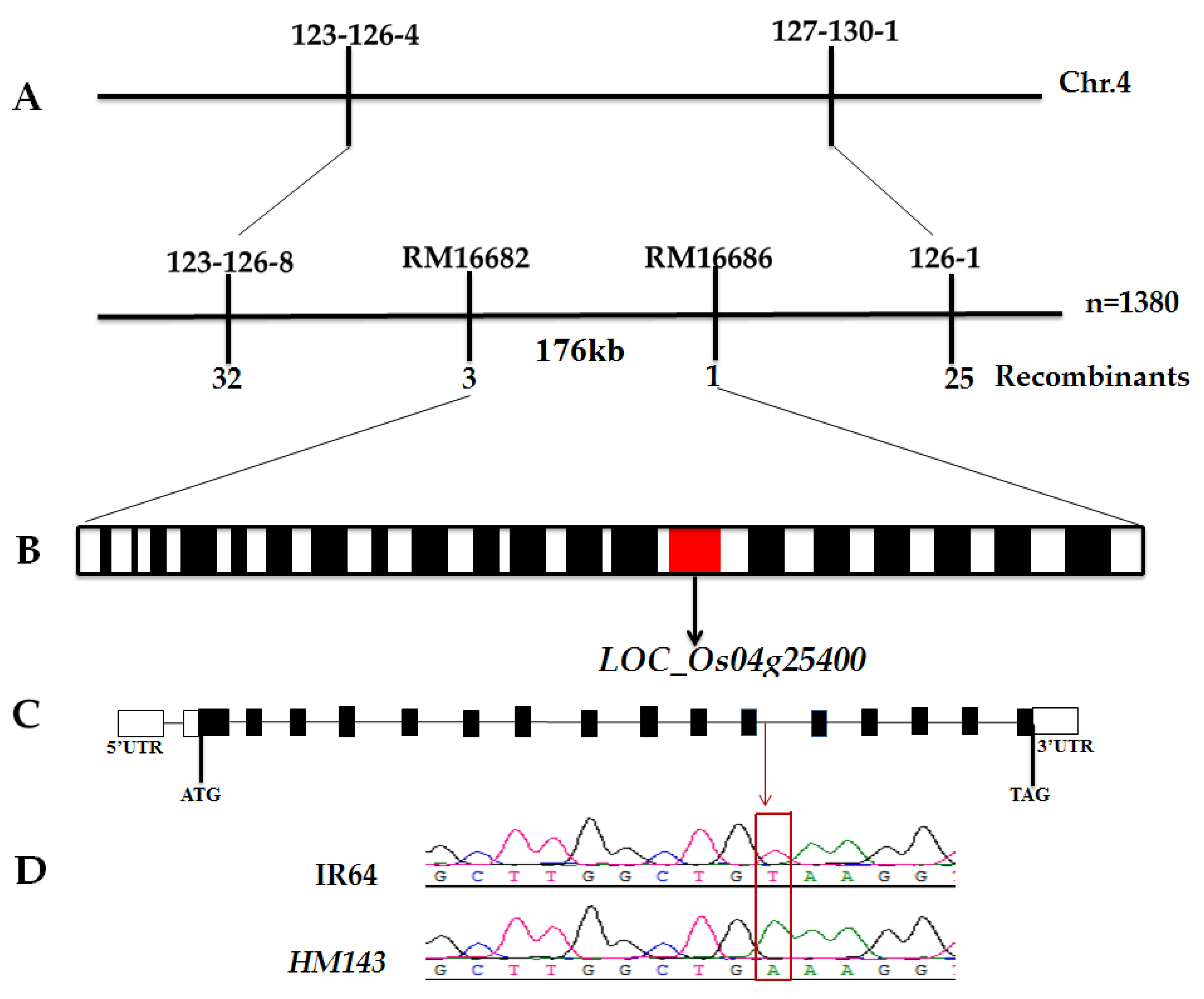
2.3. splHM143 Encodes 7-Hydroxymethyl Chlorophyll a Reductase (OsHCAR)
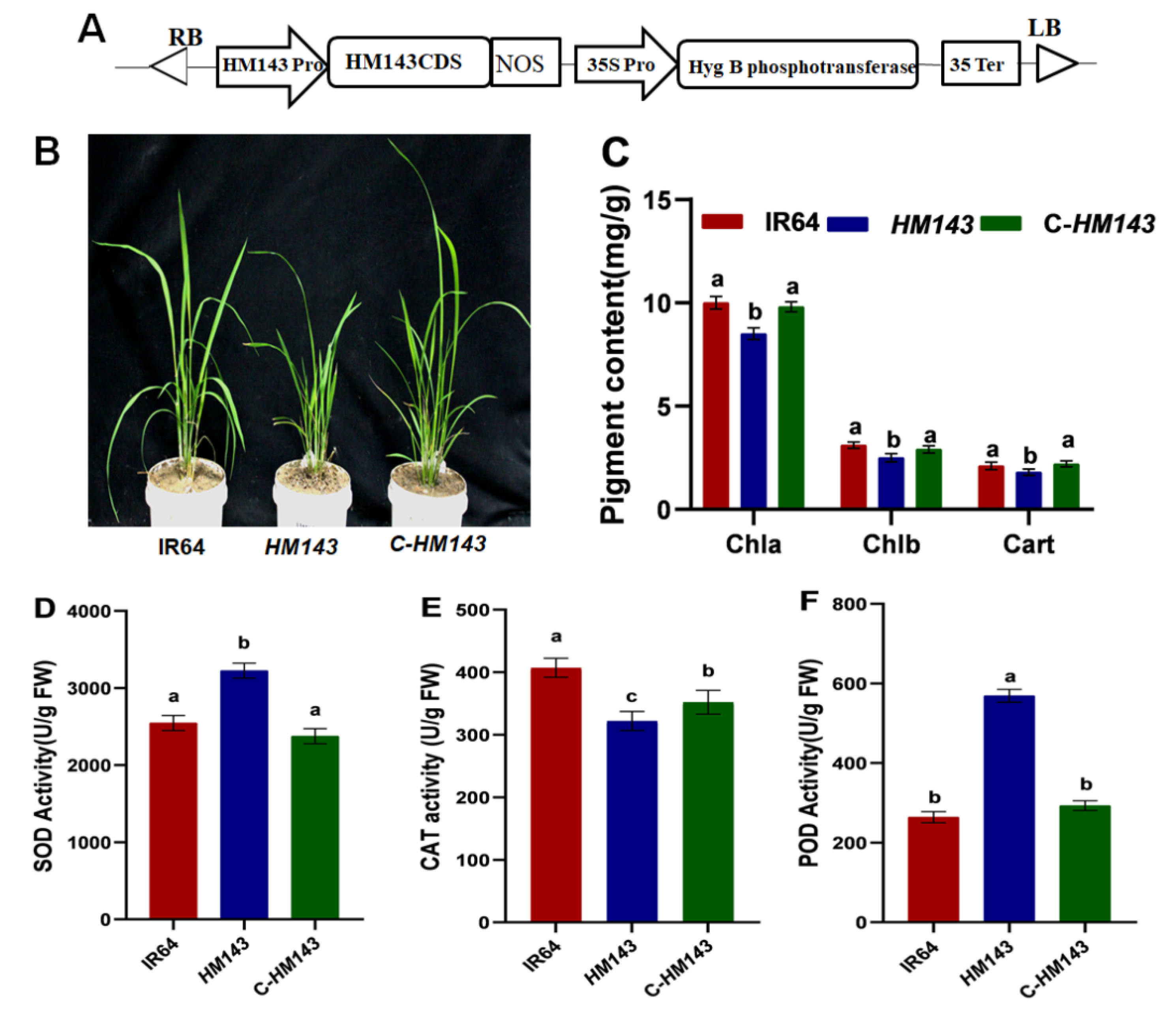
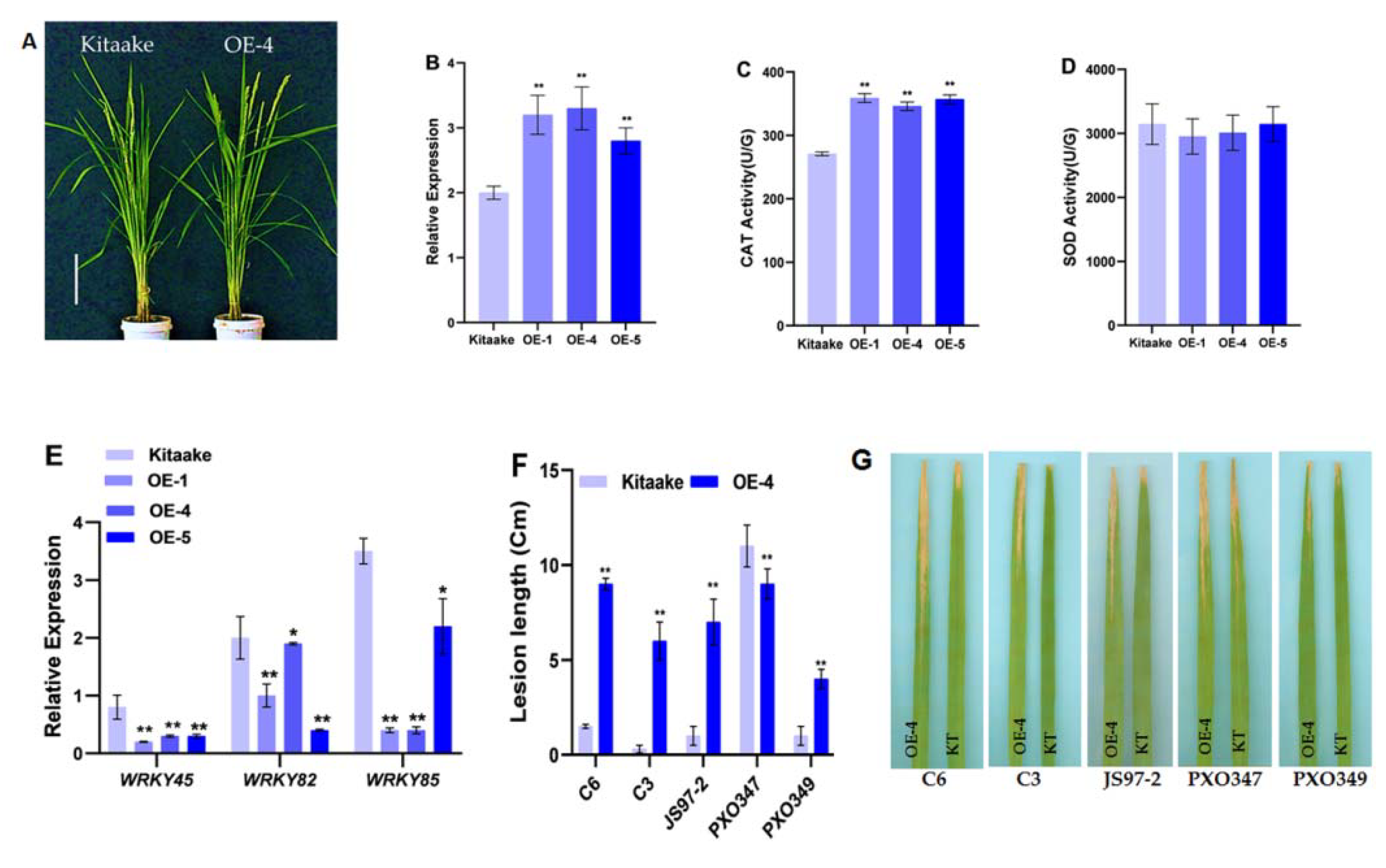
2.4. Overexpression of OsHCAR Enhances Susceptibility to Xoo
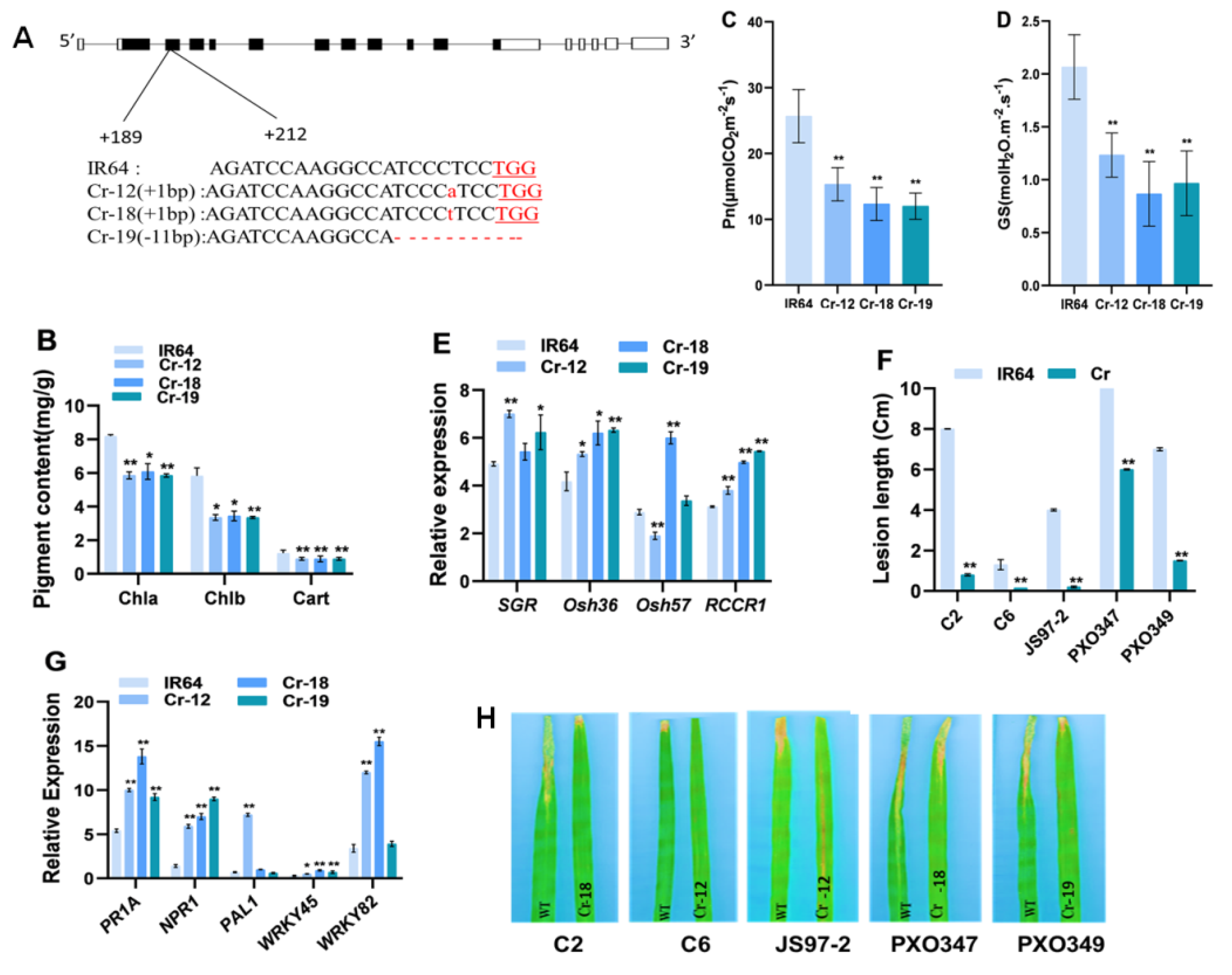
2.5. Knockout of OsHCAR Promotes Resistance to Xoo
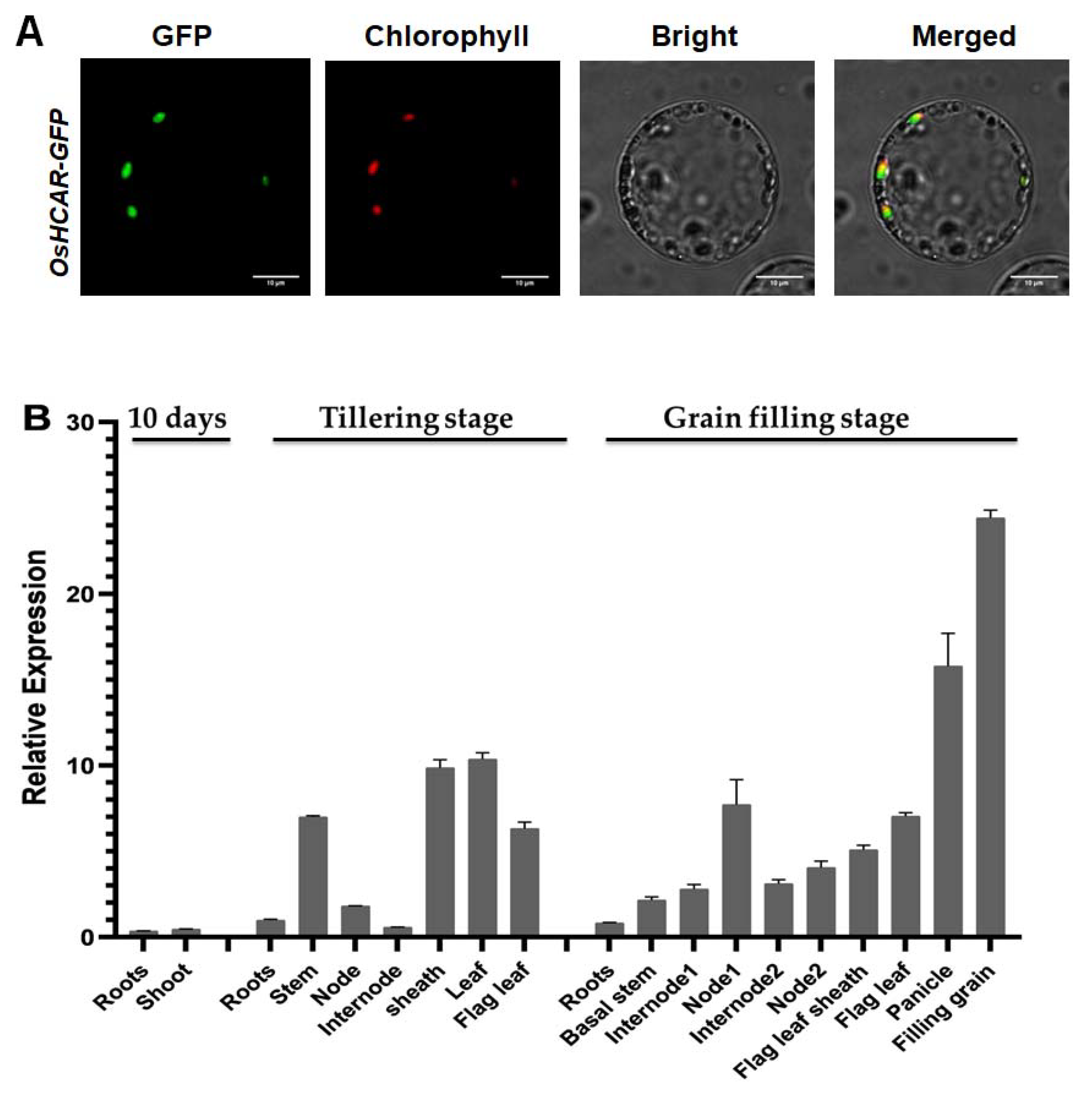
2.6. Constitutively Expressed OsHCAR Is Localized to Chloroplasts
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Physico-Biochemical Parameters Measurement
4.3. TUNEL Assay
4.4. Vector Construction
4.5. Quantitative RT-PCR
4.6. Disease Evaluation
4.7. Subcellular Localization
4.8. Map-Based Cloning
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Jain, P.; Venkadesan, S.; Karkute, S.G.; Bhati, J.; Abdin, M.Z.; Sevanthi, A.M.; Mishra, D.C.; Chaturvedi, K.K.; Rai, A.; et al. Understanding Rice-Magnaporthe Oryzae Interaction in Resistant and Susceptible Cultivars of Rice under Panicle Blast Infection Using a Time-Course Transcriptome Analysis. Genes 2021, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bordeos, A.; Madamba, M.R.S.; Baraoidan, M.; Ramos, M.; Wang, G.L.; Leach, J.E.; Leung, H. Rice Lesion Mimic Mutants with Enhanced Resistance to Diseases. Mol. Genet. Genom. 2008, 279, 605–619. [Google Scholar] [CrossRef]
- Huang, Q.N.; Yang, Y.; Shi, Y.F.; Chen, J.; Wu, J.L. Spotted-Leaf Mutants of Rice (Oryza Sativa). Rice Sci. 2010, 17, 247–256. [Google Scholar] [CrossRef]
- Moeder, W.; Yoshioka, K. Lesion Mimic Mutants. Plant. Signal. Behav. 2008, 3, 764–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Jiang, W.; Lee, J.; Park, B.; Choi, M.S.; Piao, R.; Woo, M.O.; Roh, J.H.; Han, L.; Paek, N.C.; et al. SPL28 Encodes a Clathrin-Associated Adaptor Protein Complex 1, Medium Subunit Μ1 (AP1M1) and Is Responsible for Spotted Leaf and Early Senescence in Rice (Oryza Sativa). New Phytol. 2010, 185, 258–274. [Google Scholar] [CrossRef]
- Shirsekar, G.S.; Vega-Sanchez, M.E.; Bordeos, A.; Baraoidan, M.; Swisshelm, A.; Fan, J.; Park, C.H.; Leung, H.; Wang, G.L. Identification and Characterization of Suppressor Mutants of Spl11-Mediated Cell Death in Rice. Mol. Plant Microbe Interact. 2014, 27, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lei, C.; Wang, J.; Ma, J.; Tang, S.; Wang, C.; Zhao, K.; Tian, P.; Zhang, H.; Qi, C.; et al. SPL33, Encoding an EEF1A-like Protein, Negatively Regulates Cell Death and Defense Responses in Rice. J. Exp. Bot. 2017, 68, 899–913. [Google Scholar] [CrossRef]
- Feng, B.H.; Yang, Y.; Shi, Y.F.; Shen, H.C.; Wang, H.M.; Huang, Q.N.; Xu, X.; Lü, X.G.; Wu, J.L. Characterization and Genetic Analysis of a Novel Rice Spotted-Leaf Mutant HM47 with Broad-Spectrum Resistance to Xanthomonas Oryzae Pv. Oryzae. J. Integr. Plant Biol. 2013, 55, 473–483. [Google Scholar] [CrossRef]
- Shen, H.C.; Shi, Y.F.; Feng, B.H.; Wang, H.M.; Xu, X.; Huang, Q.N.; Lü, X.G.; Wu, J.L. Identification and Genetic Analysis of a Novel Rice Spotted-Leaf Mutant with Broad-Spectrum Resistance to X Anthomonas Oryzae Pv. Oryzae. J. Integr. Agric. 2014, 13, 713–721. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, T.; Sathe, A.P.; He, Y.; Zhang, X.B.; Wu, J.L. Identification of a Novel Semi-Dominant Spotted-Leaf Mutant with Enhanced Resistance to Xanthomonas Oryzae Pv. Oryzae in Rice. Int. J. Mol. Sci. 2018, 19, 3766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhuo, D.; Zhang, F.; Huang, L.; Wang, W.; Xu, J.; Cruz, C.V.; Li, Z.; Zhou, Y. Xa39, a Novel Dominant Gene Conferring Broad-Spectrum Resistance to Xanthomonas Oryzae Pv. Oryzae in Rice. Plant Pathol. 2015, 64, 568–575. [Google Scholar] [CrossRef]
- Chen, X.; Hao, L.; Pan, J.; Zheng, X.; Jiang, G.; Jin, Y.; Gu, Z.; Qian, Q.; Zhai, W.; Ma, B. SPL5, a Cell Death and Defense-Related Gene, Encodes a Putative Splicing Factor 3b Subunit 3 (SF3b3) in Rice. Mol. Breed. 2012, 30, 939–949. [Google Scholar] [CrossRef]
- Ruan, B.; Hua, Z.; Zhao, J.; Zhang, B.; Ren, D.; Liu, C.; Yang, S.; Zhang, A.; Jiang, H.; Yu, H.; et al. OsACL-A2 Negatively Regulates Cell Death and Disease Resistance in Rice. Plant Biotechnol. J. 2019, 17, 1344–1356. [Google Scholar] [CrossRef]
- Song, G.; Kwon, C.; Kim, S.; Shim, Y.; Lim, C. The Rice SPOTTED LEAF4 ( SPL4 ) Encodes a Plant Spastin That Inhibits ROS Accumulation in Leaf Development and Functions in Leaf Senescence. Front. Plant Sci. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Tang, J.; Lin, A.; Zhang, F.; Fang, J. RLIN1, Encoding a Putative Coproporphyrinogen III Oxidase, Is Involved in Lesion Initiation in Rice. J. Genet. Genom. 2011, 38, 29–37. [Google Scholar] [CrossRef]
- Wang, S.; Lim, J.; Kim, S.; Cho, S.; Yoo, S.; Koh, H.; Sakuraba, Y.; Paek, N. Mutation of SPOTTED LEAF3 (SPL3) Impairs Abscisic Acid- Responsive Signalling and Delays Leaf Senescence in Rice. J. Exp. Bot. 2015, 66, 7045–7059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liao, H.; Chern, M.; Yin, J.; Chen, Y.; Wang, J.; Zhu, X.; Chen, Z.; Yuan, C.; Zhao, W.; et al. Loss of Function of a Rice TPR-Domain RNA-Binding Protein Confers Broad-Spectrum Disease Resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 3174–3179. [Google Scholar] [CrossRef] [Green Version]
- Fekih, R.; Tamiru, M.; Kanzaki, H.; Abe, A. The Rice (Oryza Sativa L.) LESION MIMIC RESEMBLING, Which Encodes an AAA—Type ATPase, Is Implicated in Defense Response. Mol. Genet. Genom. 2015, 290, 611–622. [Google Scholar] [CrossRef]
- Sathe, A.P.; Su, X.; Chen, Z.; Chen, T.; Wei, X.; Tang, S.; Zhang, X.; Wu, J.L. Identification and Characterization of a Spotted-Leaf Mutant Spl40 with Enhanced Bacterial Blight Resistance in Rice. Rice 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, R.; Hirabayashi, H.; Kaji, R.; Nishizawa, Y.; Yoshimura, A.; Satoh, H.; Ogawa, T.; Okamoto, M. Isolation and Characterization of Rice Lesion-Mimic Mutants with Enhanced Resistance to Rice Blast and Bacterial Blight. Plant Sci. 2002, 163, 345–353. [Google Scholar] [CrossRef]
- Hammond-kosack, K.E.; Jones, J.D.G. Resistance Gene-Dependent Plant Defense Responses. Plant Cell 1996, 8, 1773–1791. [Google Scholar]
- Meguro, M.; Ito, H.; Takabayashi, A.; Tanaka, R.; Tanaka, A. Identification of the 7-Hydroxymethyl Chlorophyll a Reductase of the Chlorophyll Cycle in Arabidopsis. Plant Cell 2011, 23, 3442–3453. [Google Scholar] [CrossRef] [Green Version]
- Sakuraba, Y.; Kim, Y.S.; Yoo, S.C.; Hörtensteiner, S.; Paek, N.C. 7-Hydroxymethyl Chlorophyll a Reductase Functions in Metabolic Channeling of Chlorophyll Breakdown Intermediates during Leaf Senescence. Biochem. Biophys. Res. Commun. 2013, 430, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Piao, W.; Han, S.; Sakuraba, Y.; Paek, N. Molecules and Cells Rice 7-Hydroxymethyl Chlorophyll a Reductase Is Involved in the Promotion of Chlorophyll Degradation and Modulates Cell Death Signaling. Mol. Cells 2017, 40, 773–786. [Google Scholar] [PubMed] [Green Version]
- Zhao, X.; Jia, T.; Hu, X. HCAR Is a Limitation Factor for Chlorophyll Cycle and Chlorophyll B Degradation in Chlorophyll-B-Overproducing Plants. Biomolecules 2020, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Rana, N.; Singh, A.; Dhiman, P.; Mandlik, R.; Sonah, H.; Deshmukh, R.; Sharma, T.R. Evolutionary Understanding of Metacaspase Genes in Cultivated and Wild Oryza Species and Its Role in Disease Resistance Mechanism in Rice. Genes 2020, 11, 1412. [Google Scholar] [CrossRef]
- Rani, M.H.; Liu, Q.; Yu, N.; Zhang, Y.; Wang, B.; Cao, Y.; Zhang, Y.; Islam, M.A.; Zegeye, W.A.; Cao, L.; et al. ES5 Is Involved in the Regulation of Phosphatidylserine Synthesis and Impacts on Early Senescence in Rice (Oryza Sativa L.). Plant Mol. Biol. 2020, 102, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Mizobuchi, R.; Hirabayashi, H.; Kaji, R.; Nishizawa, Y.; Satoh, H.; Ogawa, T.; Okamoto, M. Differential Expression of Disease Resistance in Rice Lesion-Mimic Mutants. Plant Cell Rep. 2002, 21, 390–396. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z.; Sathe, A.P.; Zhang, Z.H.; Li, L.; Shang, H.H.; Tang, S.; Zhang, X.; Wu, J.L. Characterization of a Novel Gain-of-Function Spotted-Leaf Mutant with Enhanced Disease Resistance in Rice. Rice Sci. 2019, 26, 372–383. [Google Scholar]
- Zhang, Y.; Liu, Q.; Zhang, Y.; Chen, Y.; Yu, N.; Cao, Y.; Zhan, X.; Cheng, S.; Cao, L. LMM24 Encodes Receptor-like Cytoplasmic Kinase 109, Which Regulates Cell Death and Defense Responses in Rice. Int. J. Mol. Sci. 2019, 20, 3242. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lu, Y.; Shi, Y.; Wu, L.; Xu, Y.; Huang, F.; Guo, X.; Zhang, Y.; Fan, J.; Zhao, J.; et al. Multiple Rice MicroRNAs Are Involved in Immunity against the Blast Fungus Magnaporthe Oryzae. Plant Physiol. 2014, 164, 1077–1092. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Sharma, S. Reactive Oxygen Species, Oxidative Stress and ROS Scavenging System in Plants. J. Chem. Pharm. Res. 2016, 8, 595–604. [Google Scholar]
- He, Y.; Li, L.; Zhang, Z.; Wu, J.L. Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza Sativa L.). Int. J. Mol. Sci. 2018, 19, 140. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.G.; Shi, Y.F.; Xu, X.; Wei, Y.L.; Wang, H.M.; Zhang, X.B.; Wu, J.L. Oryza Sativa Chloroplast Signal Recognition Particle 43 (OscpSRP43) Is Required for Chloroplast Development and Photosynthesis. PLoS ONE 2015, 10, e0143249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Feng, B.; Wang, H.; Xu, X.; Shi, Y.; He, Y.; Chen, Z.; Sathe, A.P.; Shi, L.; Wu, J. A Substitution Mutation in OsPELOTA Confers Bacterial Blight Resistance by Activating the Salicylic Acid Pathway. J. Integr. Plant Biol. 2018, 60, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaoki, D.; Seo, S.; Yamada, S.; Kano, A.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Jasmonic Acid and Salicylic Acid Activate a Common Defense System in Rice. Plant Signal. Behav. 2013, 8, 8–10. [Google Scholar] [CrossRef]
- Al-barzinji, I.M. Spectrophotometric Method Using Different Solvents and SPAD Chlorophyll Meter for Determination Some Photosynthesis Pigments of Bean and Cowpea Plants. Int. J. Eng. Technol. Manag. Appl. Sci. 2015, 3, 108–113. [Google Scholar]
- Huang, L.; Sun, Q.; Qin, F.; Li, C.; Zhao, Y.; Zhou, D.X. Down-Regulation of a SILENT INFORMATION REGULATOR2-Related Histone Deacetylase Gene, OsSRT1, Induces DNA Fragmentation and Cell Death in Rice. Plant Physiol. 2007, 144, 1508–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Toki, S.; Hara, N.; Ono, K.; Onodera, H.; Tagiri, A.; Oka, S.; Tanaka, H. Early Infection of Scutellum Tissue with Agrobacterium Allows High-Speed Transformation of Rice. Plant J. 2006, 47, 969–976. [Google Scholar] [CrossRef]
- Kauffman, H.E. An improved technique for evaluating resistance of rice varieties to Xanthomonas Oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampire, M.G.; Sanglou, R.K.; Wang, H.; Kazeem, B.B.; Wu, J.-l.; Zhang, X. A Novel Allele Encoding 7-Hydroxymethyl Chlorophyll a Reductase Confers Bacterial Blight Resistance in Rice. Int. J. Mol. Sci. 2021, 22, 7585. https://doi.org/10.3390/ijms22147585
Kampire MG, Sanglou RK, Wang H, Kazeem BB, Wu J-l, Zhang X. A Novel Allele Encoding 7-Hydroxymethyl Chlorophyll a Reductase Confers Bacterial Blight Resistance in Rice. International Journal of Molecular Sciences. 2021; 22(14):7585. https://doi.org/10.3390/ijms22147585
Chicago/Turabian StyleKampire, Marie Gorette, Ringki Kuinamei Sanglou, Huimei Wang, Bello Babatunde Kazeem, Jian-li Wu, and Xiaobo Zhang. 2021. "A Novel Allele Encoding 7-Hydroxymethyl Chlorophyll a Reductase Confers Bacterial Blight Resistance in Rice" International Journal of Molecular Sciences 22, no. 14: 7585. https://doi.org/10.3390/ijms22147585
APA StyleKampire, M. G., Sanglou, R. K., Wang, H., Kazeem, B. B., Wu, J.-l., & Zhang, X. (2021). A Novel Allele Encoding 7-Hydroxymethyl Chlorophyll a Reductase Confers Bacterial Blight Resistance in Rice. International Journal of Molecular Sciences, 22(14), 7585. https://doi.org/10.3390/ijms22147585





