PAR2-Induced Tissue Factor Synthesis by Primary Cultures of Human Kidney Tubular Epithelial Cells Is Modified by Glucose Availability
Abstract
1. Introduction
2. Results
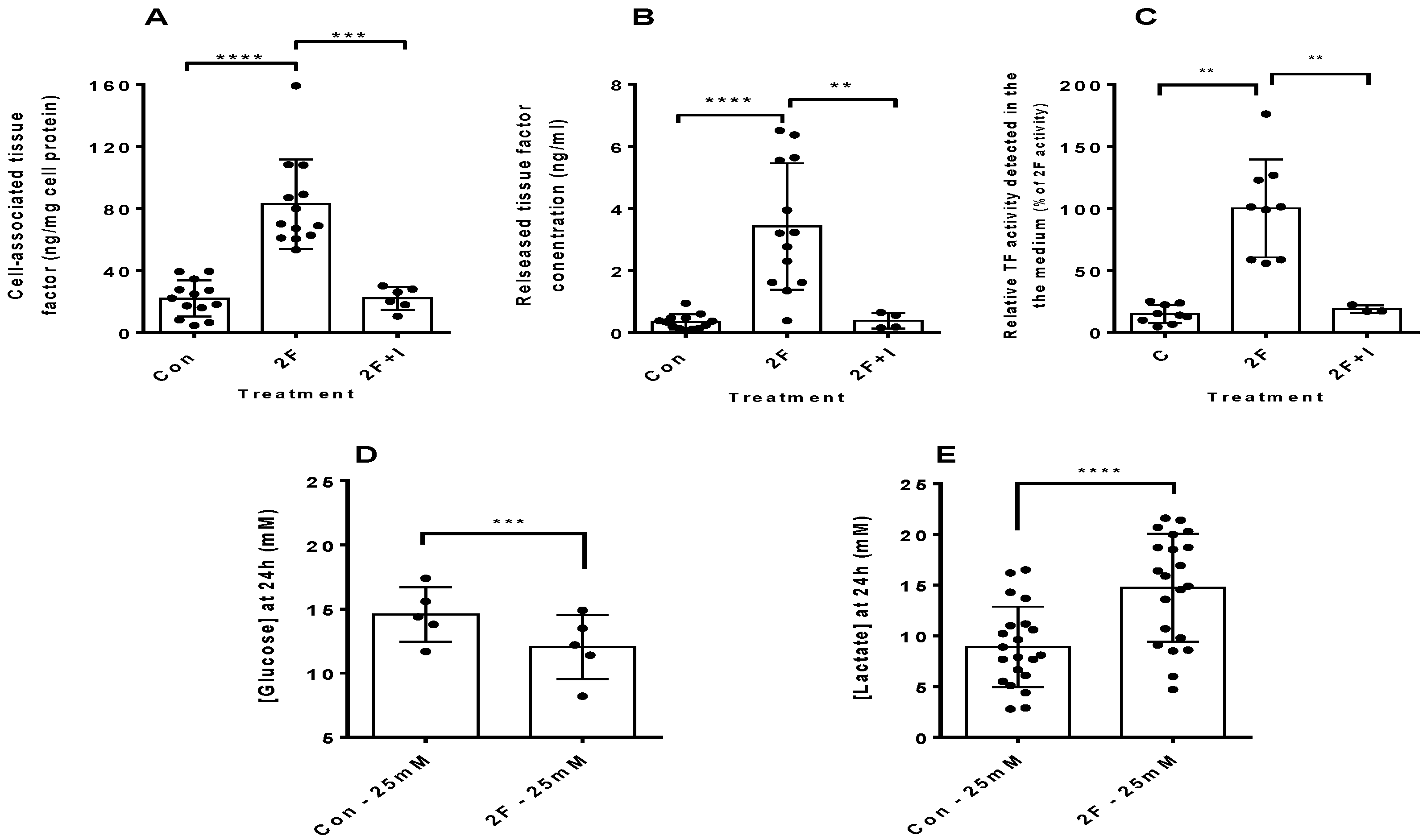
2.1. PAR2 Activation Induces Synthesis of Tissue Factor by HTECs
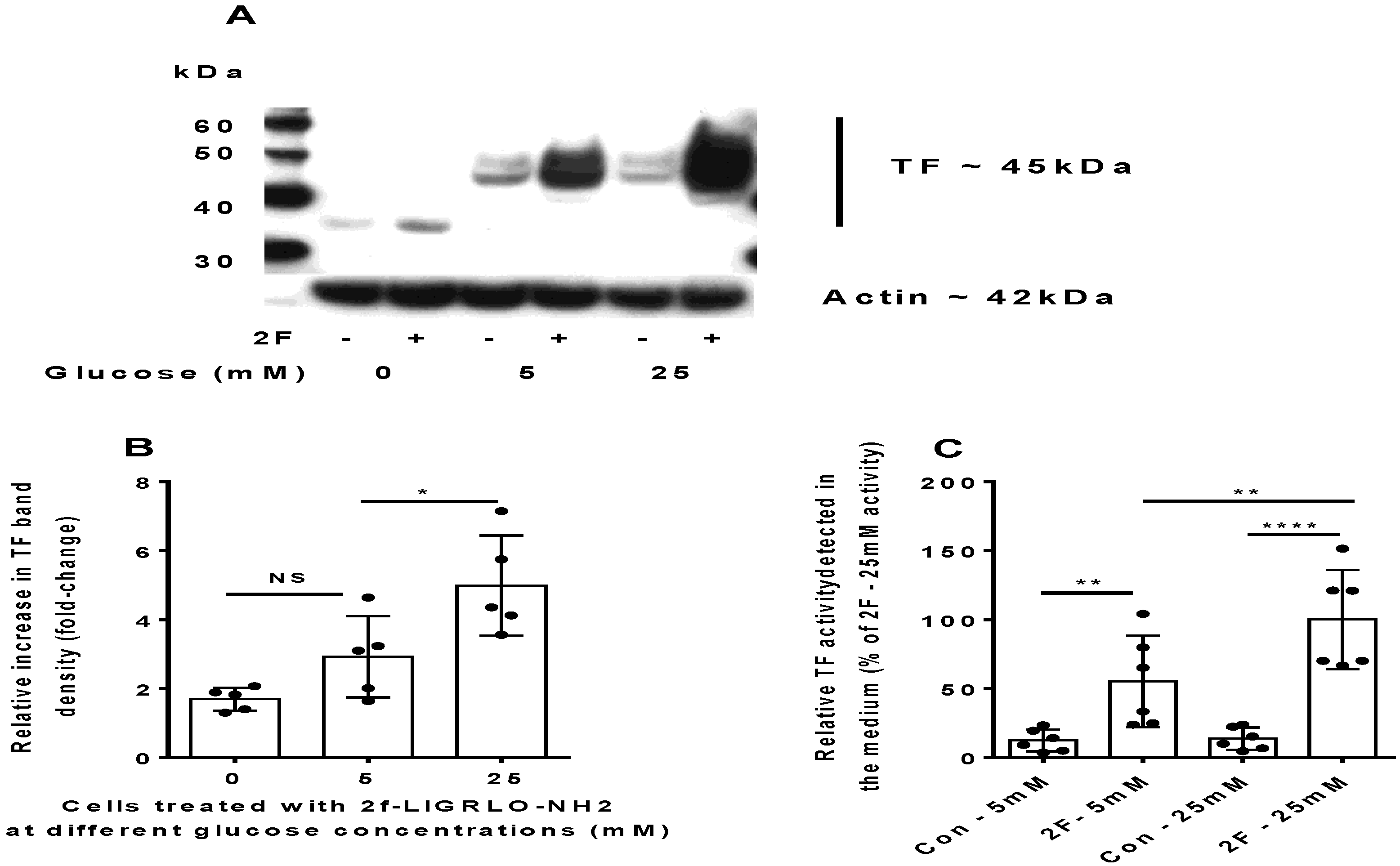
2.2. PAR2-Induced TF Synthesis by HTECs Is Upregulated by Glucose
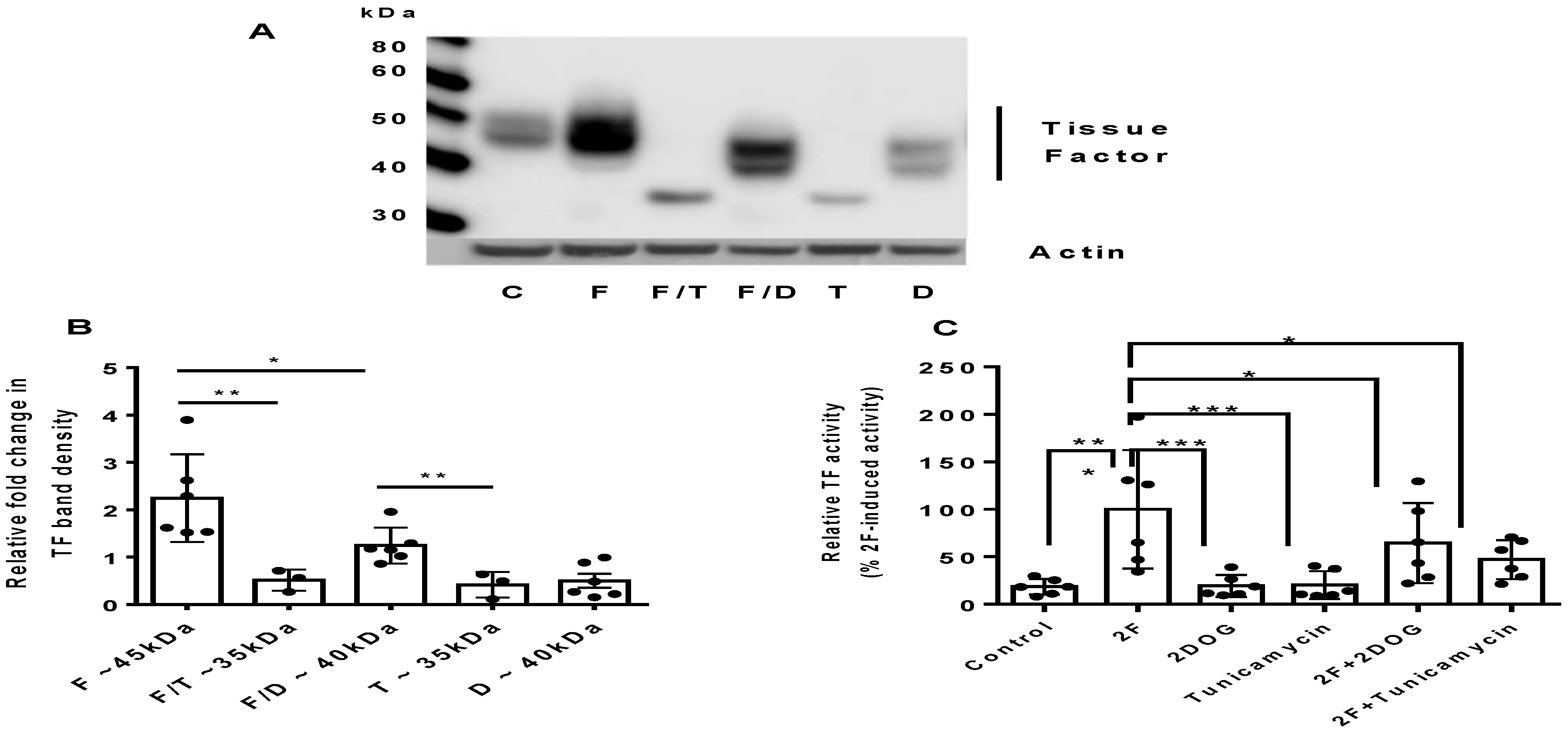
2.3. PAR2-Induced TF Synthesis Is Glucose Dependent
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Tubule Cell Isolation and Cell Culture
4.3. Cell Treatments
4.4. Western Blot Analysis
4.5. Tissue Factor ELISA
4.6. Tissue Factor Activity Assay
4.7. Glucose and Lactate Concentrations
4.8. Statistical Analysis
5. Conclusions
6. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perkovic, V.; Agarwal, R.; Fioretto, P.; Hemmelgarn, B.R.; Levin, A.; Thomas, M.C.; Wanner, C.; Kasiske, B.L.; Wheeler, D.C.; Groop, P.-H.; et al. Management of patients with diabetes and CKD: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 90, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.W.; Brown, K.E.; Min, T. Chronic kidney disease in type 2 diabetes: Implications for managing glycaemic control, cardiovascular and renal risk. Diabetes Obes. Meta. 2020, 22 (Suppl. 1), 32–45. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef]
- United States Renal Data Systems. 2018 Annual Data Report. USRDS Coordinating Centre, Ann Arbor. 2018. Available online: https://www.usrds.org/adr.aspx (accessed on 1 July 2021).
- Ceriello, A. Coagulation activation in diabetes mellitus: The role of hyperglycaemia and therapeutic prospects. Diabetologia 1993, 36, 1119–1125. [Google Scholar] [CrossRef]
- Stegenga, M.E.; van der Crabben, S.N.; Levi, M.; de Vos, A.F.; Tanck, M.W.; Sauerwein, H.P.; Van Der Poll, T. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes 2006, 55, 1807–1812. [Google Scholar] [CrossRef]
- Wang, Y.; Pratt, J.R.; Hartley, B.; Evans, B.; Zhang, L.; Sacks, S.H. Expression of tissue type plasminogen activator and type 1 plasminogen activator inhibitor, and persistent fibrin deposition in chronic renal allograft failure. Kidney Int. 1997, 52, 371–377. [Google Scholar] [CrossRef][Green Version]
- Kincaid-Smith, P. Coagulation and renal disease. Kidney Int. 1972, 2, 183–190. [Google Scholar] [CrossRef]
- Huang, M.J.; Wei, R.B.; Wang, Y.; Su, T.Y.; Di, P.; Li, Q.P.; Yang, X.; Li, P.; Chen, X.-M. Blood coagulation system in patients with chronic kidney disease: A prospective observational study. BMJ Open 2017, 7, e014294. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.H.; Ajjan, R.A. Coagulation and fibrinolysis in diabetes. Diabetes Vas. Dis. Res. 2010, 7, 260–273. [Google Scholar] [CrossRef]
- Nogami, K.; Muraki, I.; Imano, H.; Iso, H. Risk of disseminated intravascular coagulation in patients with type 2 diabetes mellitus: Retrospective cohort study. BMJ Open 2017, 7, e013894. [Google Scholar] [CrossRef] [PubMed]
- Butenas, S. Comparison of natural and recombinant tissue factor proteins: New insights. Biol. Chem. 2013, 394, 819–829. [Google Scholar] [CrossRef]
- Cimmino, G.; Cirillo, P. Tissue factor: Newer concepts in thrombosis and its role beyond thrombosis and hemostasis. Cardiovasc. Diagn. Ther. 2018, 8, 581–593. [Google Scholar] [CrossRef]
- Kothari, H.; Pendurthi, U.R.; Rao, L.V. Tissue factor purified from different cellular sources and non-glycosylated tissue factor show similar procoagulant activity. J. Throm. Haemost. JTH 2013, 11, 2066–2068. [Google Scholar] [CrossRef] [PubMed]
- Butenas, S. Tissue factor structure and function. Scientifica 2012, 2012, 964862. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Throm. Vas. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef]
- Adams, M.N.; Ramachandran, R.; Yau, M.K.; Suen, J.Y.; Fairlie, D.P.; Hollenberg, M.D.; Hooper, J.D. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 2011, 130, 248–282. [Google Scholar] [CrossRef]
- Chung, H.; Ramachandran, R.; Hollenberg, M.D.; Muruve, D.A. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. J. Biol. Chem. 2013, 288, 37319–37331. [Google Scholar] [CrossRef]
- Vesey, D.A.; Hooper, J.D.; Gobe, G.C.; Johnson, D.W. Potential physiological and pathophysiological roles for protease-activated receptor-2 in the kidney. Nephrology 2007, 12, 36–43. [Google Scholar] [CrossRef]
- Barry, G.D.; Suen, J.Y.; Le, G.T.; Cotterell, A.; Reid, R.C.; Fairlie, D.P. Novel agonists and antagonists for human protease activated receptor 2. J. Med. Chem. 2010, 53, 7428–7440. [Google Scholar] [CrossRef]
- Grandaliano, G.; Pontrelli, P.; Cerullo, G.; Monno, R.; Ranieri, E.; Ursi, M.; Loverre, A.; Gesualdo, L.; Schena, F.P. Protease-activated receptor-2 expression in IgA nephropathy: A potential role in the pathogenesis of interstitial fibrosis. J. Am. Soc. Nephrol. JASN 2003, 14, 2072–2083. [Google Scholar] [CrossRef]
- Han, Y.; Tian, L.; Ma, F.; Tesch, G.; Vesey, D.A.; Gobe, G.C.; Lohman, R.-J.; Morais, C.; Suen, J.Y.; Fairlie, D.; et al. Pharmacological inhibition of protease-activated receptor-2 reduces crescent formation in rat nephrotoxic serum nephritis. Clin. Expe. Pharmacol. Physiol. 2019, 46, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Erlich, J.; Fearns, C.; Mathison, J.; Ulevitch, R.J.; Mackman, N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect. Immun. 1999, 67, 2540–2546. [Google Scholar] [CrossRef]
- Mackman, N.; Sawdey, M.S.; Keeton, M.R.; Loskutoff, D.J. Murine tissue factor gene expression in vivo. Tissue and cell specificity and regulation by lipopolysaccharide. Am. J. Pathol. 1993, 143, 76–84. [Google Scholar]
- Erlich, J.H.; Holdsworth, S.R.; Tipping, P.G. Tissue factor initiates glomerular fibrin deposition and promotes major histocompatibility complex class II expression in crescentic glomerulonephritis. Am. J. Pathol. 1997, 150, 873–880. [Google Scholar] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Uribarri, J. Advanced glycation end product accumulation: A new enemy to target in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2014, 23, 547–554. [Google Scholar] [CrossRef]
- Jenkin, K.A.; McAinch, A.J.; Zhang, Y.; Kelly, D.J.; Hryciw, D.H. Elevated cannabinoid receptor 1 and G protein-coupled receptor 55 expression in proximal tubule cells and whole kidney exposed to diabetic conditions. Clin. Exp. Pharmacol. Physiol. 2015, 42, 256–262. [Google Scholar] [CrossRef]
- Allen, D.A.; Harwood, S.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003, 17, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Mather, A.; Pollock, C. Glucose handling by the kidney. Kidney Int. 2011, 79, S1–S6. [Google Scholar] [CrossRef]
- Jones, S.C.; Saunders, H.J.; Pollock, C.A. High glucose increases growth and collagen synthesis in cultured human tubulointerstitial cells. Diabet. Med. 1999, 16, 932–938. [Google Scholar] [CrossRef]
- Jones, S.C.; Saunders, H.J.; Qi, W.; Pollock, C.A. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia 1999, 42, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Oe, Y.; Sato, E.; Sekimoto, A.; Sato, H.; Ito, S.; Takahashi, N. Protease-activated receptor 2 exacerbates cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal. Physiol. 2019, 316, F654–F659. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Oe, Y.; Fushima, T.; Sato, E.; Sato, H.; Ito, S.; Takahashi, N. Protease-activated receptor 2 exacerbates adenine-induced renal tubulointerstitial injury in mice. Biochem. Biophys. Res. Commun. 2017, 483, 547–552. [Google Scholar] [CrossRef]
- Moussa, L.; Apostolopoulos, J.; Davenport, P.; Tchongue, J.; Tipping, P.G. Protease-activated receptor-2 augments experimental crescentic glomerulonephritis. Am. J. Pathol. 2007, 171, 800–808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oe, Y.; Hayashi, S.; Fushima, T.; Sato, E.; Kisu, K.; Sato, H.; Ito, S.; Takahashi, N. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1525–1533. [Google Scholar] [CrossRef]
- Vesey, D.A.; Suen, J.Y.; Seow, V.; Lohman, R.J.; Liu, L.; Gobe, G.C.; Johnson, D.W.; Fairlie, D. PAR2-induced inflammatory responses in human kidney tubular epithelial cells. Am. J. Physiol. Renal. Physiol. 2013, 304, F737–F750. [Google Scholar] [CrossRef]
- Vesey, D.A.; Kruger, W.A.; Poronnik, P.; Gobe, G.C.; Johnson, D.W. Proinflammatory and proliferative responses of human proximal tubule cells to PAR-2 activation. Am. J. Physiol. Renal. Physiol. 2007, 293, F1441–F1449. [Google Scholar] [CrossRef][Green Version]
- Vesey, D.A.; Cheung, C.W.; Kruger, W.A.; Poronnik, P.; Gobe, G.; Johnson, D.W. Thrombin stimulates proinflammatory and proliferative responses in primary cultures of human proximal tubule cells. Kidney Int. 2005, 67, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Suen, J.Y.; Cotterell, A.; Lohman, R.J.; Lim, J.; Han, A.; Yau, M.K.; Liu, L.; Cooper, M.; Vesey, D.; Fairlie, D.P. Pathway-selective antagonism of proteinase activated receptor 2. Br. J. Pharmacol. 2014, 171, 4112–4124. [Google Scholar] [CrossRef]
- Iyer, A.; Humphries, T.L.R.; Owens, E.P.; Zhao, K.N.; Masci, P.P.; Johnson, D.W.; Nikolic-Paterson, D.; Gobe, G.C.; Fairlie, D.P.; Vesey, D.A. PAR2 Activation on Human Kidney Tubular Epithelial Cells Induces Tissue Factor Synthesis, That Enhances Blood Clotting. Front. Physiol. 2021, 12, 615428. [Google Scholar] [CrossRef]
- Ettelaie, C.; Collier, M.E.; Featherby, S.; Benelhaj, N.E.; Greenman, J.; Maraveyas, A. Analysis of the potential of cancer cell lines to release tissue factor-containing microvesicles: Correlation with tissue factor and PAR2 expression. Thromb. J. 2016, 14, 2. [Google Scholar] [CrossRef]
- Nystedt, S.; Ramakrishnan, V.; Sundelin, J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. 1996, 271, 14910–14915. [Google Scholar] [CrossRef]
- Ritchie, E.; Saka, M.; Mackenzie, C.; Drummond, R.; Wheeler-Jones, C.; Kanke, T.; Plevin, R. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br. J. Pharmacol. 2007, 150, 1044–1054. [Google Scholar] [CrossRef]
- Boeri, D.; Almus, F.E.; Maiello, M.; Cagliero, E.; Rao, L.V.; Lorenzi, M. Modification of tissue-factor mRNA and protein response to thrombin and interleukin 1 by high glucose in cultured human endothelial cells. Diabetes 1989, 38, 212–218. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef]
- Wick, A.N.; Drury, D.R.; Nakada, H.I.; Wolfe, J.B. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 1957, 224, 963–969. [Google Scholar] [CrossRef]
- Compton, S.J.; Renaux, B.; Wijesuriya, S.J.; Hollenberg, M.D. Glycosylation and the activation of proteinase-activated receptor 2 (PAR(2)) by human mast cell tryptase. Br. J. Pharmacol. 2001, 134, 705–718. [Google Scholar] [CrossRef]
- Yau, M.K.; Liu, L.; Suen, J.Y.; Lim, J.; Lohman, R.J.; Jiang, Y.; Cotterell, A.J.; Barry, G.D.; Mak, J.; Vesey, D. PAR2 Modulators Derived from GB88. ACS Med. Chem. Lett. 2016, 7, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yau, M.K.; Lim, J.; Wu, K.C.; Xu, W.; Suen, J.Y.; Fairlie, D.P. A Potent Antagonist of Protease-Activated Receptor 2 That Inhibits Multiple Signaling Functions in Human Cancer Cells. J. Pharmacol. Exp. Ther. 2018, 364, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Vesey, D.A.; Qi, W.; Chen, X.; Pollock, C.A.; Johnson, D.W. Isolation and primary culture of human proximal tubule cells. Methods Mol. Biol. 2009, 466, 19–24. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humphries, T.L.R.; Shen, K.; Iyer, A.; Johnson, D.W.; Gobe, G.C.; Nikolic-Paterson, D.; Fairlie, D.P.; Vesey, D.A. PAR2-Induced Tissue Factor Synthesis by Primary Cultures of Human Kidney Tubular Epithelial Cells Is Modified by Glucose Availability. Int. J. Mol. Sci. 2021, 22, 7532. https://doi.org/10.3390/ijms22147532
Humphries TLR, Shen K, Iyer A, Johnson DW, Gobe GC, Nikolic-Paterson D, Fairlie DP, Vesey DA. PAR2-Induced Tissue Factor Synthesis by Primary Cultures of Human Kidney Tubular Epithelial Cells Is Modified by Glucose Availability. International Journal of Molecular Sciences. 2021; 22(14):7532. https://doi.org/10.3390/ijms22147532
Chicago/Turabian StyleHumphries, Tyrone L. R., Kunyu Shen, Abishek Iyer, David W. Johnson, Glenda C. Gobe, David Nikolic-Paterson, David P. Fairlie, and David A. Vesey. 2021. "PAR2-Induced Tissue Factor Synthesis by Primary Cultures of Human Kidney Tubular Epithelial Cells Is Modified by Glucose Availability" International Journal of Molecular Sciences 22, no. 14: 7532. https://doi.org/10.3390/ijms22147532
APA StyleHumphries, T. L. R., Shen, K., Iyer, A., Johnson, D. W., Gobe, G. C., Nikolic-Paterson, D., Fairlie, D. P., & Vesey, D. A. (2021). PAR2-Induced Tissue Factor Synthesis by Primary Cultures of Human Kidney Tubular Epithelial Cells Is Modified by Glucose Availability. International Journal of Molecular Sciences, 22(14), 7532. https://doi.org/10.3390/ijms22147532







