Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow
Abstract
:1. Introduction
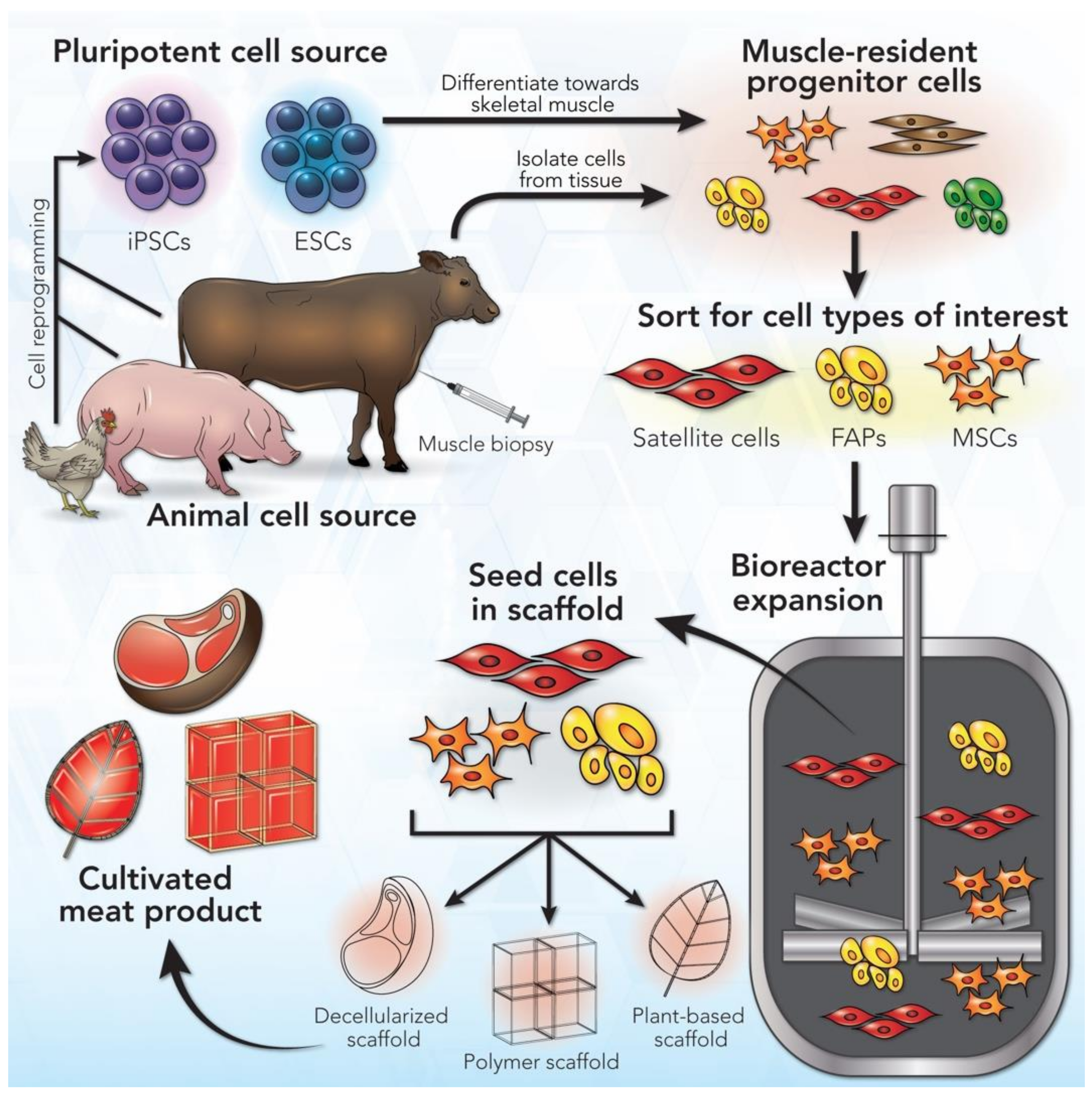
2. A General Workflow for Cultivated Meat Production
3. Cell Sources for Cultivated Meat Production
3.1. Cell Types
3.2. Cellular Considerations for Scale-Up
3.3. Culture Medium Considerations
3.4. Bioreactor Considerations
3.5. Biological Scaffold Considerations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.I.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22. [Google Scholar] [CrossRef]
- Scollan, N.D.; Greenwood, P.L.; Newbold, C.J.; Ruiz, D.R.Y.; Shingfield, K.J.; Wallace, R.J.; Hocquette, J.-F. Future research priorities for animal production in a changing world. Anim. Prod. Sci. 2011, 51, 1–5. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; FAO: Rome, Italy, 2013; p. 139. [Google Scholar]
- Bellarby, J.; Tirado, R.; Leip, A.; Weiss, F.; Lesschen, J.P.; Smith, P. Livestock greenhouse gas emissions and mitigation potential in Europe. Glob. Chang. Biol. 2012, 19, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sinke, P.; Odegard, I. LCA of Cultivated Meat Future Predictions for Different Scenarios. Available online: https://cedelft.eu/publications/rapport-lca-of-cultivated-meat-future-projections-for-different-scenarios/ (accessed on 28 February 2021).
- United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 25 April 2020).
- Mattick, C.S.; Landis, A.E.; Allenby, B.R.; Genovese, N.J. Anticipatory Life Cycle Analysis of In Vitro Biomass Cultivation for Cultured Meat Production in the United States. Environ. Sci. Technol. 2015, 49, 11941–11949. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; De Mattos, M.J.T. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G. The importance of animal influenza for human disease. Vaccine 2002, 20, S16–S20. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Stephens, N.; Sexton, A.E.; Driessen, C. Making Sense of Making Meat: Key Moments in the First 20 Years of Tissue Engineering Muscle to Make Food. Front. Sustain. Food Syst. 2019, 3, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, D.; Tseng, T.W.; Swartz, E. The Business of Cultured Meat. Trends Biotechnol. 2020, 38, 573–577. [Google Scholar] [CrossRef]
- Arshad, M.S.; Javed, M.; Sohaib, M.; Saeed, F.; Imran, A.; Amjad, Z. Tissue engineering approaches to develop cultured meat from cells: A mini review. Cogent Food Agric. 2017, 3. [Google Scholar] [CrossRef]
- Edelman, P.; McFarland, D.; Mironov, V.; Matheny, J. Commentary: In Vitro-Cultured Meat Production. Tissue Eng. 2005, 11, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Benjaminson, M.; Gilchriest, J.; Lorenz, M. In vitro edible muscle protein production system (mpps): Stage 1, fish. Acta Astronaut. 2002, 51, 879–889. [Google Scholar] [CrossRef]
- Post, M.J. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef]
- González, A.; Koltrowitz, S. The $280,000 Lab-Grown Burger Could Be a More Palatable $10 in Two Years. Available online: https://www.reuters.com/article/us-food-tech-labmeat/the-280000-lab-grown-burger-could-be-a-more-palatable-10-in-two-years-idUSKCN1U41W8 (accessed on 25 April 2020).
- Dodson, M.; Martin, E.; Brannon, M.; Mathison, B.; McFarland, D. Optimization of bovine satellite cell-derived myotube formation in vitro. Tissue Cell 1987, 19, 159–166. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z.; Quinn, L.S.; Nameroff, M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev. Biol. 1987, 119, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Dodson, M.V.; McFarland, D.C.; Martin, E.L.; Brannon, M.A. Isolation of satellite cells from ovine skeletal muscles. J. Tissue Cult. Methods 1986, 10, 233–237. [Google Scholar] [CrossRef]
- Powell, R.L.; Dodson, M.V.; Cloud, J.G. Cultivation and differentiation of satellite cells from skeletal muscle of the rainbow trout Salmo gairdneri. J. Exp. Zool. 1989, 250, 333–338. [Google Scholar] [CrossRef]
- Doumit, M.E.; Merkel, R.A. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell 1992, 24, 253–262. [Google Scholar] [CrossRef]
- Hanga, M.P.; Ali, J.; Moutsatsou, P.; De La Raga, F.A.; Hewitt, C.; Nienow, A.; Wall, I. Bioprocess development for scalable production of cultivated meat. Biotechnol. Bioeng. 2020, 117, 3029–3039. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2004, 319, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hanga, M.P.; de la Raga, F.A.; Moutsatsou, P.; Hewitt, C.J.; Nienow, A.W.; Wall, I. Scale-up of an intensified bioprocess for the expansion of bovine adipose-derived stem cells (bASCs) in stirred tank bioreactors. Biotechnol. Bioeng. 2021. [Google Scholar] [CrossRef]
- Khatri, M.; O’Brien, T.D.; Sharma, J.M. Isolation and Differentiation of Chicken Mesenchymal Stem Cells From Bone Marrow. Stem Cells Dev. 2009, 18, 1485–1492. [Google Scholar] [CrossRef]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Veter. Res. 2012, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Bosch, P.; Pratt, S.L.; Stice, S.L. Isolation, Characterization, Gene Modification, and Nuclear Reprogramming of Porcine Mesenchymal Stem Cells1. Biol. Reprod. 2006, 74, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Das, A.K.; Yang, Q.-Y.; Zhu, M.-J.; Du, M. Zfp423 Promotes Adipogenic Differentiation of Bovine Stromal Vascular Cells. PLoS ONE 2012, 7, e47496. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Hu, X.; Liu, L.; Xing, Y.; Zhou, Z.; Liang, X.; Yang, Q.; Jin, S.; Bao, J.; Gao, H.; et al. bta-miR-23a involves in adipogenesis of progenitor cells derived from fetal bovine skeletal muscle. Sci. Rep. 2017, 7, 43716. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Chu, W.; Liu, K.; Tian, Y.; Jiang, Z.; Chen, J. Muscle Conditional Medium Reduces Intramuscular Adipocyte Differentiation and Lipid Accumulation through Regulating Insulin Signaling. Int. J. Mol. Sci. 2017, 18, 1799. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Wang, F.; Liu, L. Isolation and Culture of Bovine Embryonic Stem Cells. Adv. Struct. Saf. Stud. 2013, 1074, 111–123. [Google Scholar] [CrossRef]
- Lavial, F.; Pain, B. Chicken embryonic stem cells as a non-mammalian embryonic stem cell model. Dev. Growth Differ. 2009, 52, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Vilarino, M.; Soto, D.A.; Bogliotti, Y.S.; Yu, L.; Zhang, Y.; Wang, C.; Paulson, E.; Zhong, C.; Jin, M.; Belmonte, J.C.I.; et al. Derivation of sheep embryonic stem cells under optimized conditions. Reproduction 2020, 160, 761–772. [Google Scholar] [CrossRef]
- Hong, Y.; Schartl, M.; Kursad, T. Isolation and Differentiation of Medaka Embryonic Stem Cells. In Embryonic Stem Cell Protocols; Springer: Berlin/Heidelberg, Germany, 2006; Volume 329, pp. 3–16. [Google Scholar]
- Holen, E.; Kausland, A.; Skjærven, K. Embryonic stem cells isolated from Atlantic cod (Gadus morhua) and the developmental expression of a stage-specific transcription factor ac-Pou2. Fish Physiol. Biochem. 2010, 36, 1029–1039. [Google Scholar] [CrossRef]
- Li, M.; Zhang, D.; Hou, Y.; Jiao, L.; Zheng, X.; Wang, W.-H. Isolation and culture of embryonic stem cells from porcine blastocysts. Mol. Reprod. Dev. 2003, 65, 429–434. [Google Scholar] [CrossRef]
- Talluri, T.R.; Kumar, D.; Glage, S.; Garrels, W.; Ivics, Z.; Debowski, K.; Behr, R.; Niemann, H.; Kues, W.A. Derivation and Characterization of Bovine Induced Pluripotent Stem Cells by Transposon-Mediated Reprogramming. Cell. Reprogram. 2015, 17, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.M.; Park, Y.H.; Lim, J.M.; Jung, H.; Han, J.Y. Technical note: Induction of pluripotent stem cell-like cells from chicken feather follicle cells1. J. Anim. Sci. 2017, 95, 3479–3486. [Google Scholar] [CrossRef]
- Rosselló, R.A.; Chen, C.-C.; Dai, R.; Howard, J.T.; Hochgeschwender, U.; Jarvis, E.D. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. eLife 2013, 2, e00036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Balehosur, D.; Murray, B.; Kelly, J.M.; Sumer, H.; Verma, P.J. Generation and characterization of reprogrammed sheep induced pluripotent stem cells. Theriogenology 2012, 77, 338–346.e1. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, Y.; Duan, A.; Cheng, D.; Zhang, S.; Wang, H. Optimization of Culture Conditions for Maintaining Porcine Induced Pluripotent Stem Cells. DNA Cell Biol. 2014, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Warriss, P.D.; Rhodes, D.N. Haemoglobin concentrations in beef. J. Sci. Food Agric. 1977, 28, 931–934. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Li, B.-J.; Li, P.-H.; Huang, R.-H.; Sun, W.-X.; Wang, H.; Li, Q.-F.; Chen, J.; Wu, W.-J.; Liu, H.-L. Isolation, Culture and Identification of Porcine Skeletal Muscle Satellite Cells. Asian-Australas. J. Anim. Sci. 2015, 28, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Swennen, G.N.M.; Messmer, T.; Gagliardi, M.; Molin, D.G.M.; Li, C.; Zhou, G.; Post, M.J. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, M.; Eisner, C.; Rossi, F. Fibro/Adipogenic Progenitors (FAPs): Isolation by FACS and Culture. Methods Mol. Biol. 2017, 1556, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.-I.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musina, R.A.; Bekchanova, E.S.; Belyavskii, A.V.; Sukhikh, G.T. Differentiation potential of mesenchymal stem cells of different origin. Bull. Exp. Biol. Med. 2006, 141, 147–151. [Google Scholar] [CrossRef]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef]
- A Redondo, P.; Pavlou, M.; Loizidou, M.; Cheema, U. Elements of the niche for adult stem cell expansion. J. Tissue Eng. 2017, 8, 2041731417725464. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Jiwlawat, S.; Lynch, E.; Glaser, J.; Smit-Oistad, I.; Jeffrey, J.; Van Dyke, J.M.; Suzuki, M. Differentiation and sarcomere formation in skeletal myocytes directly prepared from human induced pluripotent stem cells using a sphere-based culture. Differentiation 2017, 96, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Jiwlawat, N.; Lynch, E.; Jeffrey, J.; Van Dyke, J.M.; Suzuki, M. Current Progress and Challenges for Skeletal Muscle Differentiation from Human Pluripotent Stem Cells Using Transgene-Free Approaches. Stem Cells Int. 2018, 2018, 6241681. [Google Scholar] [CrossRef]
- Al Tanoury, Z.; Rao, J.; Tassy, O.; Gobert, B.; Gapon, S.; Garnier, J.-M.; Wagner, E.; Hick, A.; Hall, A.; Gussoni, E.; et al. Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage in vitro. Development 2020, 147. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Gronthos, S.; Bartold, M.P. Differentiation of iPSC to Mesenchymal Stem-Like Cells and Their Characterization. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2014; Volume 1357, pp. 353–374. [Google Scholar]
- Guénantin, A.-C.; Briand, N.; Capel, E.; Dumont, F.; Morichon, R.; Provost, C.; Stillitano, F.; Jeziorowska, D.; Siffroi, J.-P.; Hajjar, R.J.; et al. Functional Human Beige Adipocytes from Induced Pluripotent Stem Cells. Diabetes 2017, 66, 1470–1478. [Google Scholar] [CrossRef] [Green Version]
- Ahfeldt, T.; Schinzel, R.T.; Lee, Y.-K.; Hendrickson, D.; Kaplan, A.; Lum, D.H.; Camahort, R.; Xia, F.; Shay, J.; Rhee, E.P.; et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat. Cell Biol. 2012, 14, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Bar-Nur, O.; Gerli, M.F.M.; Di Stefano, B.; Almada, A.E.; Galvin, A.; Coffey, A.; Huebner, A.J.; Feige, P.; Verheul, C.; Cheung, P.; et al. Direct Reprogramming of Mouse Fibroblasts into Functional Skeletal Muscle Progenitors. Stem Cell Rep. 2018, 10, 1505–1521. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Kii, I.; Shimizu, N.; Tanaka, H.; Takeda, S. Direct reprogramming of fibroblasts into skeletal muscle progenitor cells by transcription factors enriched in undifferentiated subpopulation of satellite cells. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ding, S. Maintaining the Stemness of Satellite Cells during Long-Term Culture; Maastricht University, Proefschrift Maken: Maastricht, The Netherlands, 2019. [Google Scholar]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Bornemann, A.; Schmalbruch, H. Immunocytochemistry of M-cadherin in mature and regenerating rat muscle. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1994, 239, 119–125. [Google Scholar] [CrossRef]
- Cornelison, D.; Filla, M.S.; Stanley, H.M.; Rapraeger, A.C.; Olwin, B.B. Syndecan-3 and Syndecan-4 Specifically Mark Skeletal Muscle Satellite Cells and Are Implicated in Satellite Cell Maintenance and Muscle Regeneration. Dev. Biol. 2001, 239, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.K.; Hall, J.K.; Troy, A.A.; Cornelison, D.; Majka, S.M.; Olwin, B.B. Syndecan-4-Expressing Muscle Progenitor Cells in the SP Engraft as Satellite Cells during Muscle Regeneration. Cell Stem Cell 2009, 4, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Tapscott, S.J.; Davis, R.L.; Thayer, M.J.; Cheng, P.F.; Weintraub, H.; Lassar, A.B. MyoD1: A nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 1988, 242, 405–411. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Judson, R.N.; Low, M.; Eisner, C.; Rossi, F.M. Isolation, Culture, and Differentiation of Fibro/Adipogenic Progenitors (FAPs) from Skeletal Muscle. Adv. Struct. Saf. Stud. 2017, 1668, 93–103. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Wu, J.; Vilarino, M.; Okamura, D.; Soto, D.A.; Zhong, C.; Sakurai, M.; Sampaio, R.; Suzuki, K.; Belmonte, J.C.I.; et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2090–2095. [Google Scholar] [CrossRef] [Green Version]
- Mackey, L.C.; Annab, L.A.; Yang, J.; Rao, B.; E Kissling, G.; Schurman, S.H.; Dixon, D.; Archer, T.K. Epigenetic Enzymes, Age, and Ancestry Regulate the Efficiency of Human iPSC Reprogramming. Stem Cells 2018, 36, 1697–1708. [Google Scholar] [CrossRef] [Green Version]
- Kyttälä, A.; Moraghebi, R.; Valensisi, C.; Kettunen, J.; Andrus, C.; Pasumarthy, K.K.; Nakanishi, M.; Nishimura, K.; Ohtaka, M.; Weltner, J.; et al. Genetic Variability Overrides the Impact of Parental Cell Type and Determines iPSC Differentiation Potential. Stem Cell Rep. 2016, 6, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Judson, R.N.; Quarta, M.; Oudhoff, M.J.; Soliman, H.; Yi, L.; Chang, C.K.; Loi, G.; Werff, R.V.; Cait, A.; Hamer, M.; et al. Inhibition of Methyltransferase Setd7 Allows the In Vitro Expansion of Myogenic Stem Cells with Improved Therapeutic Potential. Cell Stem Cell 2018, 22, 177.e7–190.e7. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.T.; Aydogdu, T.; Sala, D.; Malecova, B.; Gatto, S.; Puri, P.L.; Latella, L.; Sacco, A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 2014, 20, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Li, L.; Yu, X.; Zhang, R.; Yan, S.; Zeng, Z.; Shu, Y.; Zhao, C.; Wu, X.; Lei, J.; et al. CRISPR/Cas9-mediated reversibly immortalized mouse bone marrow stromal stem cells (BMSCs) retain multipotent features of mesenchymal stem cells (MSCs). Oncotarget 2017, 8, 111847–111865. [Google Scholar] [CrossRef]
- Robin, J.; Wright, W.E.; Zou, Y.; Cossette, S.C.; Lawlor, M.W.; Gussoni, E. Isolation and Immortalization of Patient-derived Cell Lines from Muscle Biopsy for Disease Modeling. J. Vis. Exp. 2015, 52307. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.; Diol, N.R.; E Propson, N.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Mimura, S.; Kimura, N.; Hirata, M.; Tateyama, D.; Hayashida, M.; Umezawa, A.; Kohara, A.; Nikawa, H.; Okamoto, T.; Furue, M.K. Growth factor-defined culture medium for human mesenchymal stem cells. Int. J. Dev. Biol. 2011, 55, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Rumsey, J.W.; Bhargava, N.; Gregory, C.; Riedel, L.; Kang, J.F.; Hickman, J.J. Developing a novel serum-free cell culture model of skeletal muscle differentiation by systematically studying the role of different growth factors in myotube formation. Vitr. Cell. Dev. Biol. Anim. 2009, 45, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Baker, M. Reproducibility: Respect your cells! Nat. Cell Biol. 2016, 537, 433–435. [Google Scholar] [CrossRef]
- Park, Y.H.; Gong, S.P.; Kim, H.Y.; Kim, G.A.; Choi, J.H.; Ahn, J.Y.; Lim, J.M. Development of a serum-free defined system employing growth factors for preantral follicle culture. Mol. Reprod. Dev. 2013, 80, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.; Fernandes, T.G.; Cordeiro, C.S.M.; Boucher, S.; Kuninger, D.; Vemuri, M.C.; Diogo, M.M.; Cabral, J.M. Defined Essential 8™ Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS ONE 2016, 11, e0151264. [Google Scholar] [CrossRef]
- Kolkmann, A.M.; Post, M.J.; Rutjens, M.A.M.; Van Essen, A.L.M.; Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 2020, 72, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, A.J.; Mirliani, A.B.; White, E.C.; Yuen, J.S.K.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, D.; Mai, Q.; Li, T.; Huang, J.; Ding, C.; Jia, M.; Zhou, C.; Xu, Y. Comparison of a xeno-free and serum-free culture system for human embryonic stem cells with conventional culture systems. Stem Cell Res. Ther. 2016, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Miki, H.; Takagi, M. Design of serum-free medium for suspension culture of CHO cells on the basis of general commercial media. Cytotechnology 2014, 67, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specht, L. An Analysis of Culture Medium Costs and Production Volumes for Cultivated Meat; GFI: Washington, DC, USA, 2020; p. 14. [Google Scholar]
- Pérez, C.M.R.; Alvarez, Z.; Chen, F.; Aytun, T.; Stupp, S.I. Mimicking the Bioactivity of Fibroblast Growth Factor-2 Using Supramolecular Nanoribbons. ACS Biomater. Sci. Eng. 2017, 3, 2166–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, T.; Yuasa, N.; Ota, H.; Habu, M.; Kawano, M.; Nakayama, F.; Nishihara, S. Highly sulfated hyaluronic acid maintains human induced pluripotent stem cells under feeder-free and bFGF-free conditions. Biochem. Biophys. Res. Commun. 2019, 518, 506–512. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, K.; Tesseur, I.; Wyss-Coray, T. Small Molecule TGF-beta Mimetics as Potential Neuroprotective Factors. Curr. Alzheimer Res. 2005, 2, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Bolis, S.; Vassalli, G.; Barile, L. Flow Cytometric Analysis of Extracellular Vesicles from Cell-conditioned Media. J. Vis. Exp. 2019, e59128. [Google Scholar] [CrossRef] [Green Version]
- Henningsen, J.; Rigbolt, K.T.G.; Blagoev, B.; Pedersen, B.K.; Kratchmarova, I. Dynamics of the Skeletal Muscle Secretome during Myoblast Differentiation. Mol. Cell. Proteom. 2010, 9, 2482–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spier, M.; Vandenberghe, L.; Medeiros, A.; Soccol, C. Application of different types of bioreactors in bioprocesses. In Bioreactors: Design, Properties, and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; Volume 1, pp. 53–87. [Google Scholar]
- Liu, S. Batch Reactor. In Bioprocess Engineering, 2nd ed.; Liu, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–178. [Google Scholar]
- Yamanè, T.; Shimizu, S. Fed-batch techniques in microbial processes. In Proceedings of the Bioprocess Parameter Control; Springer: Berlin/Heidelberg, Germany, 2005; pp. 147–194. [Google Scholar]
- Lindskog, E.K. The Upstream Process: Principal Modes of Operation. In Biopharmaceutical Processing; Jagschies, G., Lindskog, E., Łącki, K., Galliher, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 625–635. [Google Scholar]
- Allan, S.J.; De Bank, P.; Ellis, M. Bioprocess Design Considerations for Cultured Meat Production with a Focus on the Expansion Bioreactor. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Meyer, H.-P.; Minas, W.; Schmidhalter, D. Industrial-Scale Fermentation. In Industrial Biotechnology; Wiley: Hoboken, NJ, USA, 2016; pp. 1–53. [Google Scholar]
- Radtke, A.L.; Herbst-Kralovetz, M.M. Culturing and Applications of Rotating Wall Vessel Bioreactor Derived 3D Epithelial Cell Models. J. Vis. Exp. 2012, 3868, e3868. [Google Scholar] [CrossRef] [Green Version]
- Hammond, T.G.; Hammond, J.M. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bettahalli, N.; Steg, H.; Wessling, M.; Stamatialis, D. Development of poly(l-lactic acid) hollow fiber membranes for artificial vasculature in tissue engineering scaffolds. J. Membr. Sci. 2011, 371, 117–126. [Google Scholar] [CrossRef]
- Bettahalli, N.M.S.; Vicente, J.; Moroni, L.; Higuera, G.A.; van Blitterswijk, C.; Wessling, M.; Stamatialis, D.F. Integration of hollow fiber membranes improves nutrient supply in three-dimensional tissue constructs. Acta Biomater. 2011, 7, 3312–3324. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ito, A.; Jitsunobu, H.; Yamaguchi, K.; Kawabe, Y.; Mizumoto, H.; Kamihira, M. Hollow Fiber Bioreactor Perfusion Culture System for Magnetic Force-Based Skeletal Muscle Tissue Engineering. J. Chem. Eng. Jpn. 2012, 45, 348–354. [Google Scholar] [CrossRef]
- Baba, K.; Sankai, Y. Development of biomimetic system for scale up of cell spheroids - building blocks for cell transplantation. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 1611–1616. [Google Scholar]
- LaBarge, M.A.; Blau, H.M. Skeletal Muscle Stem Cells. In Handbook of Stem Cells, 2nd ed.; Lanza, R., Atala, A., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 631–640. [Google Scholar]
- Hosoyama, T.; McGivern, J.V.; Van Dyke, J.M.; Ebert, A.D.; Suzuki, M. Derivation of Myogenic Progenitors Directly from Human Pluripotent Stem Cells Using a Sphere-Based Culture. Stem Cells Transl. Med. 2014, 3, 564–574. [Google Scholar] [CrossRef]
- Cerino, G.; Gaudiello, E.; Grussenmeyer, T.; Melly, L.; Massai, D.; Banfi, A.; Martin, I.; Eckstein, F.; Grapow, M.; Marsano, A. Three dimensional multi-cellular muscle-like tissue engineering in perfusion-based bioreactors. Biotechnol. Bioeng. 2016, 113, 226–236. [Google Scholar] [CrossRef]
- Williams, C.; Wick, T.M. Endothelial Cell–Smooth Muscle Cell Co-Culture in a Perfusion Bioreactor System. Ann. Biomed. Eng. 2005, 33, 920–928. [Google Scholar] [CrossRef]
- Rebelo, S.P.; Costa, R.; Silva, M.M.; Marcelino, P.; Brito, C.; Alves, P.M.; Montes, A.C.M.B.A. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: Improved functionality in long-term bioreactor cultures. J. Tissue Eng. Regen. Med. 2015, 11, 2034–2045. [Google Scholar] [CrossRef]
- Datar, I.; Betti, M. Possibilities for an in vitro meat production system. Innov. Food Sci. Emerg. Technol. 2010, 11, 13–22. [Google Scholar] [CrossRef]
- Specht, E.A.; Welch, D.R.; Clayton, E.M.R.; Lagally, C.D. Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry. Biochem. Eng. J. 2018, 132, 161–168. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells 2015, 7, 728–744. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, present, and future of microcarrier-based tissue engineering. J. Orthop. Transl. 2015, 3, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.D.; Elicson, J.M.; Murphy, W.L. Microcarriers with Synthetic Hydrogel Surfaces for Stem Cell Expansion. Adv. Health Mater. 2017, 16, 1700072. [Google Scholar] [CrossRef]
- Verbruggen, S.; Luining, D.; Van Essen, A.; Post, M.J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2017, 70, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.R.; Orellana, M.D.; Mizukami, A.; Fernandes, T.R.; Fontes, A.M.; Suazo, C.A.T.; Oliveira, V.D.C.; Covas, D.; Swiech, K. Growth and functional harvesting of human mesenchymal stromal cells cultured on a microcarrier-based system. Biotechnol. Prog. 2014, 30, 889–895. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Oner, F.; Dhert, W. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef]
- Saunders, R.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef] [Green Version]
- Qazi, T.H.; Mooney, D.; Duda, G.N.; Geissler, S. Niche-mimicking interactions in peptide-functionalized 3D hydrogels amplify mesenchymal stromal cell paracrine effects. Biomaterials 2020, 230, 119639. [Google Scholar] [CrossRef] [PubMed]
- Seyedmahmoud, R.; Saltik, B.C.; Barros, N.; Nasiri, R.; Banton, E.; Shamloo, A.; Ashammakhi, N.; Dokmeci, M.R.; Ahadian, S. Saltik, Çelebi- Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines 2019, 10, 679. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.; Matsumoto, S.; Le, H.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Health Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef] [Green Version]
| Cell Source | Relevant Cell Types | Location of Cell Type In Vivo | Method to Obtain | Proliferative Capacity | Differentiation Potential | Cell Type Markers | Isolated from Relevant Species |
|---|---|---|---|---|---|---|---|
| Adult stem cells | Muscle satellite cells | Beneath basement membrane of skeletal myotubes | Muscle biopsy | Limited | Skeletal myotubes | Pax7 M-cadherin Syndecan-4 CXCR4 α-7 integrin VCAM-1 CD56 | Bovine [18] Galline [19] Ovine [20] Piscine [21] Porcine [22] |
| Mesenchymal stem/stromal cells (MSCs) | Numerous locations (ex. bone marrow, umbilical cord, skeletal muscle, adipose tissue) | Tissue biopsy | Limited | Adipocytes Chondrocytes Fibroblasts | CD105 CD73 CD90 Sca-1 PDGFRα | Bovine [23,24,25] Galline [26] Ovine [27] Porcine [28] | |
| Fibro-adipogenic progenitors (FAPs) | Interstitial space of skeletal muscle | Muscle biopsy | Limited | Adipocytes Fibroblasts | Sca-1 PDGFRα | Bovine [29,30] Porcine [31,32] | |
| Pluripotent stem cells | Embryonic stem cells (ESCs) | Inner cell mass of blastocyst | Isolate from inner cell mass | Indefinite | Any cell type | Oct4 Sox2 Nanog c-Myc Klf4 | Bovine [33] Galline [34] Ovine [35] Piscine [36,37] Porcine [38] |
| Induced pluripotent stem cells (iPSCs) | N/A | Somatic cell reprogramming - Overexpression of pluripotent transcription factors - Small-molecule-mediated reprogramming | Indefinite | Any cell type | Oct4 Sox2 Nanog c-Myc Klf4 | Bovine [39] Galline [40,41] Ovine [42] Porcine [43] |
| Production Component | Advancements/Benefits | Limitations |
|---|---|---|
| Cell source | + Pluripotent and adult stem cell sources applicable + Isolation and sorting protocols established for agriculturally relevant species | - Cost and ease of obtaining cell type is inversely proportional to the proliferative capacity and potential of the cell type - Limited expansion capability in vitro for adult stem cells - Low iPSC reprogramming yield and possible phenotypic side effects from reprogramming - Ethical sourcing of ESCs |
| Culture medium | + Well-developed expansion and differentiation medium for relevant cell types + Development of several xeno-free medium formulations | - Xeno-free medium is still not as effective as medium with serum. - Key growth factors needed are expensive |
| Bioreactor | + Several media introduction and recycling options + Permits dynamic cell culture + Improves cell expansion and differentiation + Allows significantly larger cell quantities to be cultured | - Further scale-up needed - Energy expensive - Some dynamic culture methods may damage cells |
| Scaffold | + Provides anchorage to enable and/or improve cell differentiation + Enables tailored cell distribution and localization + Microcarriers may improve taste and texture of the final meat product + 3D bioprinting enables tailored architecture and material distribution | - Nutrient and oxygen diffusion limited at larger scaffold sizes - Requirements for biocompatibility and edibility limit biomaterial options |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. https://doi.org/10.3390/ijms22147513
Reiss J, Robertson S, Suzuki M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. International Journal of Molecular Sciences. 2021; 22(14):7513. https://doi.org/10.3390/ijms22147513
Chicago/Turabian StyleReiss, Jacob, Samantha Robertson, and Masatoshi Suzuki. 2021. "Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow" International Journal of Molecular Sciences 22, no. 14: 7513. https://doi.org/10.3390/ijms22147513
APA StyleReiss, J., Robertson, S., & Suzuki, M. (2021). Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. International Journal of Molecular Sciences, 22(14), 7513. https://doi.org/10.3390/ijms22147513






