Sialic Acid—Modified Nanoparticles—New Approaches in the Glioma Management—Perspective Review
Abstract
:1. Introduction
2. Sialome as a Potential Target in Therapy of Glioma and Other Human Cancers
3. Theranostic Aspect of the Use of Nanoparticles (NPs) in Glioma
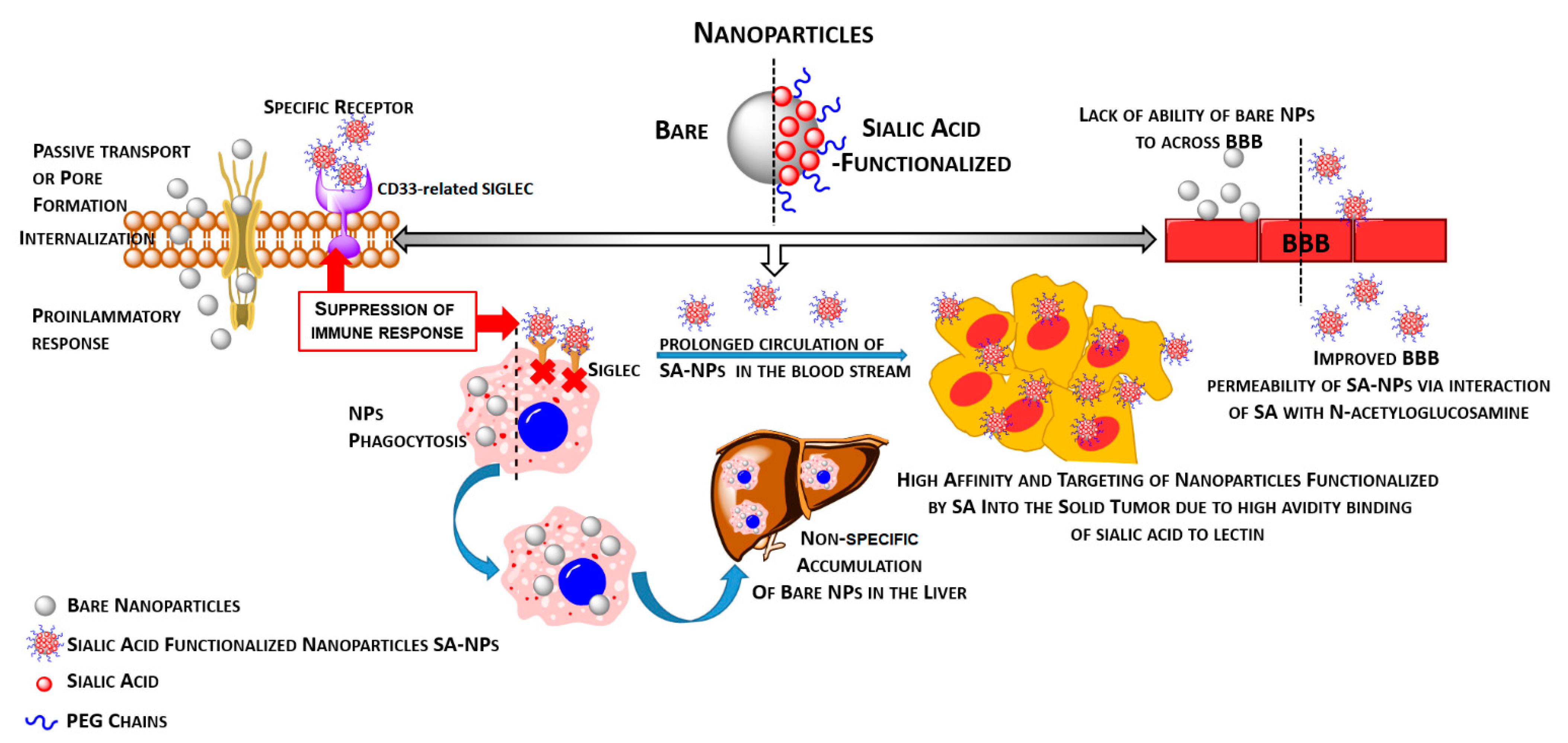
3.1. Functionalization of Nanoparticles by Sialic Acid or Their Analogues Provides an Effective Way to Modulate Immune Response as Well as the Ability to Cross the Blood–Brain Barrier
3.2. Nanoparticle-Based Therapy and Sialic Acid–Siglec Interplay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Seow, P.; Wong, J.H.D.; Ahmad-Annuar, A.; Mahajan, A.; Abdullah, N.A.; Ramli, N. Quantitative magnetic resonance imaging and radiogenomic biomarkers for glioma characterisation: A systematic review. Br. J. Radiol. 2018, 91, 20170930. [Google Scholar] [CrossRef] [PubMed]
- Rushing, E.J. WHO Classification of Tumors of the Nervous System: Preview of the Upcoming, 5th ed.; MEMO—Magazine of European Medical Oncology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosma, I.; Reijneveld, J.C.; Douw, L.; Vos, M.J.; Postma, T.J.; Aaronson, N.K.; Muller, M.; Vandertop, W.P.; Slotman, B.J.; Taphoorn, M.J.; et al. Health-related quality of life of long-term high-grade glioma survivors. Neuro Oncol. 2009, 11, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, J.; Wei, J.; Liu, X.; Cho, W.C. Immune checkpoint blockade as a potential therapeutic target in non-small cell lung cancer. Expert Opin. Biol. Ther. 2016, 16, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.L.; Garzon-Muvdi, T.; Lim, M. Biomarkers and Immunotherapeutic Targets in Glioblastoma. World Neurosurg. 2017, 102, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Perus, L.J.M.; Walsh, L.A. Microenvironmental Heterogeneity in Brain Malignancies. Front. Immunol. 2019, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, R.; Tanaka, T.; Miyake, K.; Yoshida, K.; Sasaki, H. Bevacizumab for malignant gliomas: Current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017, 34, 62–77. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Westhoff, M.A.; Kast, R.E.; Wirtz, C.R.; Halatsch, M.E. Erlotinib in glioblastoma: Lost in translation? Anticancer Agents Med. Chem. 2011, 11, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.Z.; McDonnell, M.D.; Fornaciari, E.; Bagherian, N.S.; Scheer, K.G.; Samuel, M.S.; Yaghoobi, M.; Ormsby, R.J.; Poonnoose, S.; Tumes, D.J.; et al. A deep convolutional neural network for segmentation of whole-slide pathology images identifies novel tumour cell-perivascular niche interactions that are associated with poor survival in glioblastoma. Br. J. Cancer 2021, 1–14. [Google Scholar] [CrossRef]
- Shembrey, C.; Huntington, N.D.; Hollande, F. Impact of Tumor and Immunological Heterogeneity on the Anti-Cancer Immune Response. Cancers 2019, 11, 1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef]
- Al-Kharboosh, R.; ReFaey, K.; Lara-Velazquez, M.; Grewal, S.S.; Imitola, J.; Quiñones-Hinojosa, A. Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Vidyarthi, A.; Agnihotri, T.; Khan, N.; Singh, S.; Tewari, M.K.; Radotra, B.D.; Chatterjee, D.; Agrewala, J.N. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol. Immunother. 2019, 68, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdizadeh, S.; Bayatipoor, H.; Pashangzadeh, S.; Jafarpour, R.; Shojaei, Z.; Motallebnezhad, M. Immune checkpoints and cancer development: Therapeutic implications and future directions. Pathol. Res. Pract. 2021, 223, 153485. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Zhang, K.N.; Wang, Z.; Hu, H.M.; Wang, Z.L.; Huang, R.Y.; Jiang, H.Y.; Zhai, Y.; Feng, Y.M.; Chang, Y.H.; et al. Siglecs, Novel Immunotherapy Targets, Potentially Enhance The Effectiveness of Existing Immune Checkpoint Inhibitors in Glioma Immunotherapy. Oncol. Targets Ther. 2019, 12, 10263–10273. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef] [Green Version]
- Adams, O.J.; Stanczak, M.A.; von Gunten, S.; Läubli, H. Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology 2018, 28, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef] [Green Version]
- Fraschilla, I.; Pillai, S. Viewing Siglecs through the lens of tumor immunology. Immunol. Rev. 2017, 276, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Van de Wall, S.; Santegoets, K.C.M.; van Houtum, E.J.H.; Büll, C.; Adema, G.J. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends Immunol. 2020, 41, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Nam, S.; Koo, J.S.; Kim, S.; Yang, M.; Jeong, D.I.; Hwang, C.; Park, J.; Cho, H.J. Possible contribution of sialic acid to the enhanced tumor targeting efficiency of nanoparticles engineered with doxorubicin. Sci. Rep. 2020, 10, 19738. [Google Scholar] [CrossRef]
- Rhodes, K.E.; Fawcett, J.W. Chondroitin sulphate proteoglycans: Preventing plasticity or protecting the CNS? J. Anat. 2004, 204, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Bandtlow, C.E.; Zimmermann, D.R. Proteoglycans in the developing brain: New conceptual insights for old proteins. Physiol. Rev. 2000, 80, 1267–1290. [Google Scholar] [CrossRef]
- Galuska, C.E.; Dambon, J.A.; Kühnle, A.; Bornhöfft, K.F.; Prem, G.; Zlatina, K.; Lütteke, T.; Galuska, S.P. Artificial Polysialic Acid Chains as Sialidase-Resistant Molecular-Anchors to Accumulate Particles on Neutrophil Extracellular Traps. Front. Immunol. 2017, 8, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Maarouf, A.; Petridis, A.K.; Rutishauser, U. Use of polysialic acid in repair of the central nervous system. Proc. Natl. Acad. Sci. USA 2006, 103, 16989–16994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef]
- Zhang, T.; She, Z.; Huang, Z.; Li, J.; Luo, X.; Deng, Y. Application of sialic acid/polysialic acid in the drug delivery systems. Asian J. Pharm. Sci. 2014, 9, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Aarnoudse, C.A.; Garcia Vallejo, J.J.; Saeland, E.; van Kooyk, Y. Recognition of tumor glycans by antigen-presenting cells. Curr. Opin. Immunol. 2006, 18, 105–111. [Google Scholar] [CrossRef]
- Amoureux, M.C.; Coulibaly, B.; Chinot, O.; Loundou, A.; Metellus, P.; Rougon, G.; Figarella-Branger, D. Polysialic acid neural cell adhesion molecule (PSA-NCAM) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC Cancer 2010, 10, 91. [Google Scholar] [CrossRef]
- Petridis, A.K.; Wedderkopp, H.; Hugo, H.H.; Mehdorn, H.M. Polysialic acid overexpression in malignant astrocytomas. Acta Neurochir. 2009, 151, 601–604. [Google Scholar] [CrossRef]
- Kannagi, R. Carbohydrate antigen sialyl Lewis a—Its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med. J. 2007, 30, 189–209. [Google Scholar]
- Vajaria, B.N.; Patel, K.R.; Begum, R.; Patel, P.S. Sialylation: An Avenue to Target Cancer Cells. Pathol. Oncol. Res. 2016, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Boltje, T.J.; Wassink, M.; de Graaf, A.M.; van Delft, F.L.; den Brok, M.H.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [Green Version]
- Lübbers, J.; Rodríguez, E.; van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büll, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; van der Vlag, J.; Heise, T.; et al. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell-Mediated Tumor Immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, G.; Weigle, B.; Crocker, P.R. Siglec and anti-Siglec therapies. Curr. Opin. Chem. Biol. 2021, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.P.; Dobie, C.; Szabo, R.; Hallam, L.; Ranson, M.; Yu, H.; Skropeta, D. Design, synthesis and evaluation of carbamate-linked uridyl-based inhibitors of human ST6Gal I. Bioorg. Med. Chem. 2020, 28, 115561. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Mantuano, N.; Natoli, M.; Zippelius, A.; Läubli, H. Tumor-associated carbohydrates and immunomodulatory lectins as targets for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001222. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Rainger, G.E.; Bradfield, P.F.; Nash, G.B.; Simmons, D.L. Cell adhesion: More than just glue (review). Mol. Membr. Biol. 1998, 15, 167–176. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Hong, R.; Zhao, H.; Wei, G.; Wu, W.; Xu, H.; Cui, J.; Zhang, Y.; Chang, A.H.; et al. A retrospective comparison of CD19 single and CD19/CD22 bispecific targeted chimeric antigen receptor T cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J. 2020, 10, 105. [Google Scholar] [CrossRef]
- Gbadamosi, M.; Meshinchi, S.; Lamba, J.K. Gemtuzumab ozogamicin for treatment of newly diagnosed CD33-positive acute myeloid leukemia. Future Oncol. 2018, 14, 3199–3213. [Google Scholar] [CrossRef] [PubMed]
- Lenza, M.P.; Atxabal, U.; Oyenarte, I.; Jiménez-Barbero, J.; Ereño-Orbea, J. Current Status on Therapeutic Molecules Targeting Siglec Receptors. Cells 2020, 9, 2691. [Google Scholar] [CrossRef]
- Kovalovsky, D.; Yoon, J.H.; Cyr, M.G.; Simon, S.; Voynova, E.; Rader, C.; Wiestner, A.; Alejo, J.; Pittaluga, S.; Gress, R.E. Siglec-6 is a target for chimeric antigen receptor T-cell treatment of chronic lymphocytic leukemia. Leukemia 2021, 1–11. [Google Scholar] [CrossRef]
- Läubli, H.; Kawanishi, K.; George Vazhappilly, C.; Matar, R.; Merheb, M.; Siddiqui, S.S. Tools to study and target the Siglec-sialic acid axis in cancer. FEBS J. 2020. [Google Scholar] [CrossRef]
- Angata, T.; Kerr, S.C.; Greaves, D.R.; Varki, N.M.; Crocker, P.R.; Varki, A. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J. Biol. Chem. 2002, 277, 24466–24474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Neumann, H. Alleviation of neurotoxicity by microglial human Siglec-11. J. Neurosci. 2010, 30, 3482–3488. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K. Siglec receptors and hiding plaques in Alzheimer’s disease. J. Mol. Med. 2009, 87, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, K.C.M.; Gielen, P.R.; Büll, C.; Schulte, B.M.; Kers-Rebel, E.D.; Küsters, B.; Bossman, S.A.J.F.; Ter Laan, M.; Wesseling, P.; Adema, G.J. Expression profiling of immune inhibitory Siglecs and their ligands in patients with glioma. Cancer Immunol. Immunother. 2019, 68, 937–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopatz, J.; Beutner, C.; Welle, K.; Bodea, L.G.; Reinhardt, J.; Claude, J.; Linnartz-Gerlach, B.; Neumann, H. Siglec-h on activated microglia for recognition and engulfment of glioma cells. Glia 2013, 61, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Wielgat, P.; Czarnomysy, R.; Trofimiuk, E.; Car, H. The sialoglycan-Siglec-E checkpoint axis in dexamethasone-induced immune subversion in glioma-microglia transwell co-culture system. Immunol. Res. 2019, 67, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Wielgat, P.; Trofimiuk, E.; Czarnomysy, R.; Braszko, J.J.; Car, H. Sialic acids as cellular markers of immunomodulatory action of dexamethasone on glioma cells of different immunogenicity. Mol. Cell. Biochem. 2019, 455, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Yu, M.; Guo, L.; Zhang, B.; Liu, S.; Zhang, W.; Zhou, B.; Yan, J.; Ma, Q.; Yang, Z.; et al. Tumor Derived SIGLEC Family Genes May Play Roles in Tumor Genesis, Progression, and Immune Microenvironment Regulation. Front. Oncol. 2020, 10, 586820. [Google Scholar] [CrossRef]
- Angata, T. Siglecs that Associate with DAP12. Adv. Exp. Med. Biol. 2020, 1204, 215–230. [Google Scholar] [PubMed]
- Deyell, M.; Garris, C.S.; Laughney, A.M. Cancer metastasis as a non-healing wound. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Michael, J.S.; Lee, B.S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Int. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.K. Use of nanoparticles for drug delivery in glioblastoma multiforme. Expert Rev. Neurother. 2007, 7, 363–372. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Zhang, L. Nanoparticle-Based Modulation of the Immune System. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 305–326. [Google Scholar] [CrossRef]
- Kim, Y.H.; Min, K.H.; Wang, Z.; Kim, J.; Jacobson, O.; Huang, P.; Zhu, G.; Liu, Y.; Yung, B.; Niu, G.; et al. Development of Sialic Acid-coated Nanoparticles for Targeting Cancer and Efficient Evasion of the Immune System. Theranostics 2017, 7, 962–973. [Google Scholar] [CrossRef]
- Alessandrini, F.; Vennemann, A.; Gschwendtner, S.; Neumann, A.U.; Rothballer, M.; Seher, T.; Wimmer, M.; Kublik, S.; Traidl-Hoffmann, C.; Schloter, M.; et al. Pro-Inflammatory versus Immunomodulatory Effects of Silver Nanoparticles in the Lung: The Critical Role of Dose, Size and Surface Modification. Nanomaterials 2017, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51–63. [Google Scholar]
- Bhat, A.; Edwards, L.W.; Fu, X.; Badman, D.L.; Huo, S.; Jin, A.J.; Lu, Q. Effects of gold nanoparticles on lipid packing and membrane pore formation. Appl. Phys. Lett. 2016, 109, 263106. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Car, H.; Sadowska, A.; Wątek, M.; Krętowski, R.; Cechowska-Pasko, M.; Wilczewska, A.Z.; Mystkowska, J.; Kasacka, I.; Torres, A.; et al. Pharmacokinetics and Anticancer Activity of Folic Acid-Functionalized Magnetic Nanoparticles. J. Biomed. Nanotech. 2017, 13, 665–677. [Google Scholar] [CrossRef]
- Dacoba, T.G.; Olivera, A.; Torres, D.; Crecente-Campo, J.; Alonso, M.J. Modulating the immune system through nanotechnology. Semin. Immunol. 2017, 34, 78–102. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, W.W.; Xu, D.G. Stimuli-responsive nanoscale drug delivery systems for cancer therapy. J. Drug Target. 2018, 27, 423–433. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Luo, X.; Ning, X.; Luo, J.; Guo, J.; Liu, Q.; Ling, G.; Zhou, N. Selenium nanoparticles reduce glucose metabolism and promote apoptosis of glioma cells through reactive oxygen species-dependent manner. Neuroreport 2020, 31, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Zheng, S.Y.; Zhang, Y.B.; Yu, B.; Zheng, W.; Yang, F.; Chen, T. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles. Colloids Surf. B Biointerfaces 2011, 83, 183–187. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Calvo, P.; Gouritin, B.; Chacun, H.; Desmaële, D.; D’Angelo, J.; Noel, J.P.; Georgin, D.; Fattal, E.; Andreux, J.P.; Couvreur, P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm. Res. 2001, 18, 1157–1166. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bors, L.A.; Erdo, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.C.; Wang, L.J.; Rajesh, R. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: Colocalization of ALDH and CD44. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Vergoni, A.V.; Ruozi, B.; Bondioli, L.; Badiali, L.; Rivasi, F.; Costantino, L.; Forni, F.; Vandelli, M.A. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: In vivo pharmacological evidence and biodistribution. J. Control Release 2010, 145, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Spence, S.; Greene, M.K.; Fay, F.; Hams, E.; Saunders, S.P.; Hamid, U.; Fitzgerald, M.; Beck, J.; Bains, B.K.; Smyth, P.; et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 2015, 7, 303ra140. [Google Scholar] [CrossRef] [Green Version]
- Yeini, E.; Ofek, P.; Pozzi, S.; Albeck, N.; Ben-Shushan, D.; Tiram, G.; Golan, S.; Kleiner, R.; Sheinin, R.; Israeli Dangoor, S.; et al. P-selectin axis plays a key role in microglia immunophenotype and glioblastoma progression. Nat. Commun. 2021, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.S.; Warner, R.L.; Lowe, J.B.; Smith, P.L.; Suzuki, Y.; Miyasaka, M.; Yamaguchi, S.; Ohta, Y.; Tsukada, Y.; Kiso, M.; et al. In vitro and in vivo selectin-blocking activities of sulfated lipids and sulfated sialyl compounds. Int. Immunol. 1998, 10, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Haddad, H.F.; Burke, J.A.; Scott, E.A.; Ameer, G.A. Clinical Relevance of Pre-Existing and Treatment-Induced Anti-Poly(Ethylene Glycol) Antibodies. Regen. Eng. Transl. Med. 2021, 1–11. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayakawa, T.; Khedri, Z.; Schwarz, F.; Landig, C.; Liang, S.Y.; Yu, H.; Chen, X.; Fujito, N.T.; Satta, Y.; Varki, A.; et al. Coevolution of Siglec-11 and Siglec-16 via gene conversion in primates. BMC Ecol. Evol. 2017, 17, 228. [Google Scholar] [CrossRef]
- Shahraz, A.; Kopatz, J.; Mathy, R.; Kappler, J.; Winter, D.; Kapoor, S.; Schütza, V.; Scheper, T.; Gieselmann, V.; Neumann, H. Anti-inflammatory activity of low molecular weight polysialic acid on human macrophages. Sci. Rep. 2015, 5, 16800. [Google Scholar] [CrossRef] [Green Version]
- Karlstetter, M.; Kopatz, J.; Aslanidis, A.; Shahraz, A.; Caramoy, A.; Linnartz-Gerlach, B.; Lin, Y.; Lückoff, A.; Fauser, S.; Düker, K.; et al. Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol. Med. 2017, 9, 154–166. [Google Scholar] [CrossRef]
- Ali, S.R.; Fong, J.J.; Carlin, A.F.; Busch, T.D.; Linden, R.; Angata, T.; Areschoug, T.; Parast, M.; Varki, N.; Murray, J.; et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 2014, 211, 1231–1242. [Google Scholar] [CrossRef]
- Munkley, J.; Scott, E. Targeting Aberrant Sialylation to Treat Cancer. Medicines 2019, 6, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemersma, M.; Sandrock, J.; Boltje, T.J.; Büll, C.; Heise, T.; Ashikov, A.; Adema, G.J.; van Bokhoven, H.; Lefeber, D.J. Disease mutations in CMP-sialic acid transporter SLC35A1 result in abnormal α-dystroglycan O-mannosylation, independent from sialic acid. Hum. Mol. Genet. 2015, 24, 2241–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estadella, I.; Pedrós-Gámez, O.; Colomer-Molera, M.; Bosch, M.; Sorkin, A.; Felipe, A. Endocytosis: A Turnover Mechanism Controlling Ion Channel Function. Cells 2020, 9, 1833. [Google Scholar] [CrossRef]
- Cortes, J.E.; de Lima, M.; Dombret, H.; Estey, E.H.; Giralt, S.A.; Montesinos, P.; Röllig, C.; Venditti, A.; Wang, E.S. Prevention, recognition, and management of adverse events associated with gemtuzumab ozogamicin use in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Paulson, J.C. CD22 Ligands on a Natural N-Glycan Scaffold Efficiently Deliver Toxins to B-Lymphoma Cells. J. Am. Chem. Soc. 2017, 139, 12450–12458. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hazama, S.; Suzuki, N.; Xu, M.; Nakagami, Y.; Fujiwara, N.; Tsunedomi, R.; Yoshida, S.; Tomochika, S.; Matsukuma, S.; et al. Siglec-7 is a predictive biomarker for the efficacy of cancer vaccination against metastatic colorectal cancer. Oncol. Lett. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielgat, P.; Niemirowicz-Laskowska, K.; Wilczewska, A.Z.; Car, H. Sialic Acid—Modified Nanoparticles—New Approaches in the Glioma Management—Perspective Review. Int. J. Mol. Sci. 2021, 22, 7494. https://doi.org/10.3390/ijms22147494
Wielgat P, Niemirowicz-Laskowska K, Wilczewska AZ, Car H. Sialic Acid—Modified Nanoparticles—New Approaches in the Glioma Management—Perspective Review. International Journal of Molecular Sciences. 2021; 22(14):7494. https://doi.org/10.3390/ijms22147494
Chicago/Turabian StyleWielgat, Przemyslaw, Katarzyna Niemirowicz-Laskowska, Agnieszka Z. Wilczewska, and Halina Car. 2021. "Sialic Acid—Modified Nanoparticles—New Approaches in the Glioma Management—Perspective Review" International Journal of Molecular Sciences 22, no. 14: 7494. https://doi.org/10.3390/ijms22147494
APA StyleWielgat, P., Niemirowicz-Laskowska, K., Wilczewska, A. Z., & Car, H. (2021). Sialic Acid—Modified Nanoparticles—New Approaches in the Glioma Management—Perspective Review. International Journal of Molecular Sciences, 22(14), 7494. https://doi.org/10.3390/ijms22147494





