Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain
Abstract
:1. Introduction
2. Results
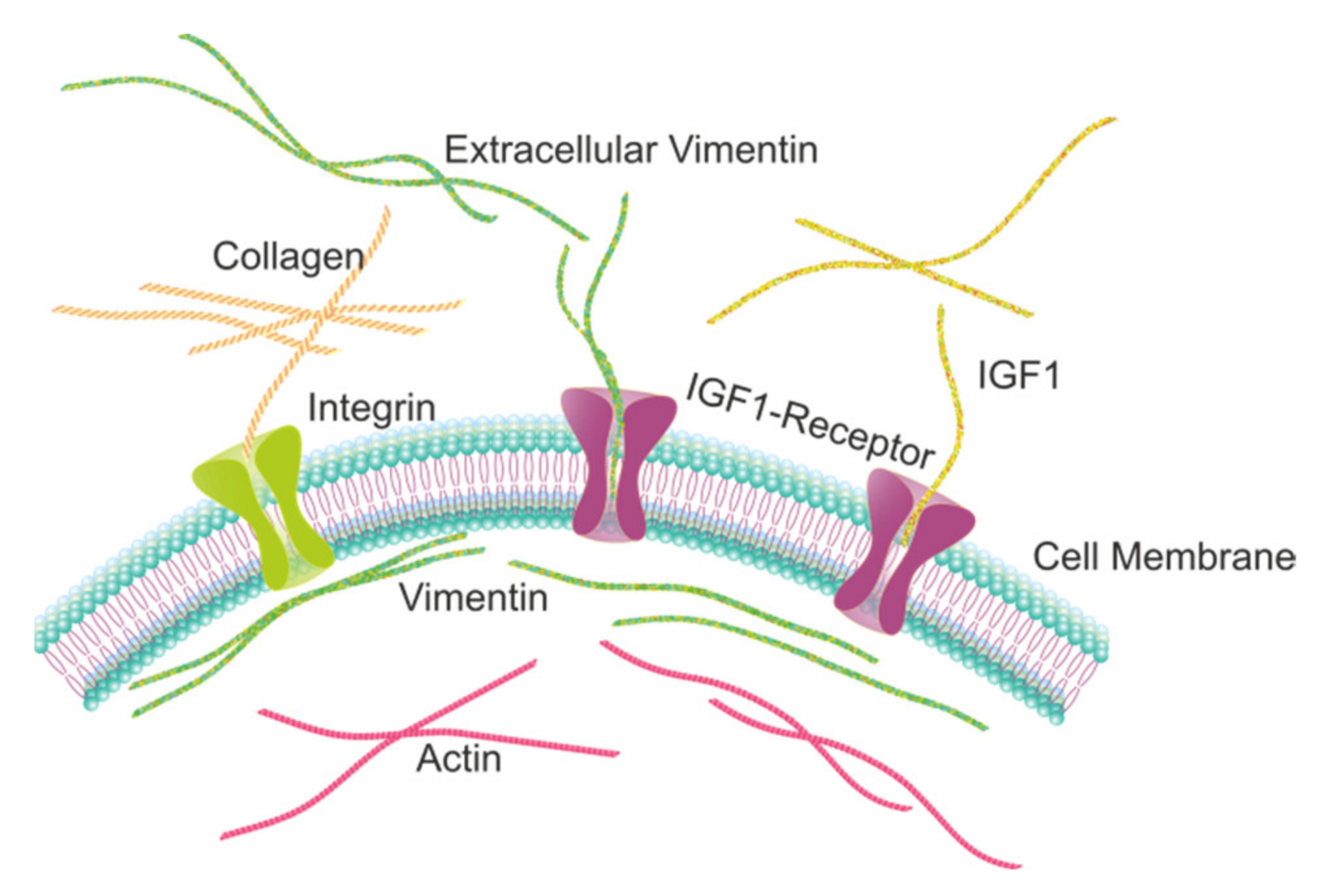
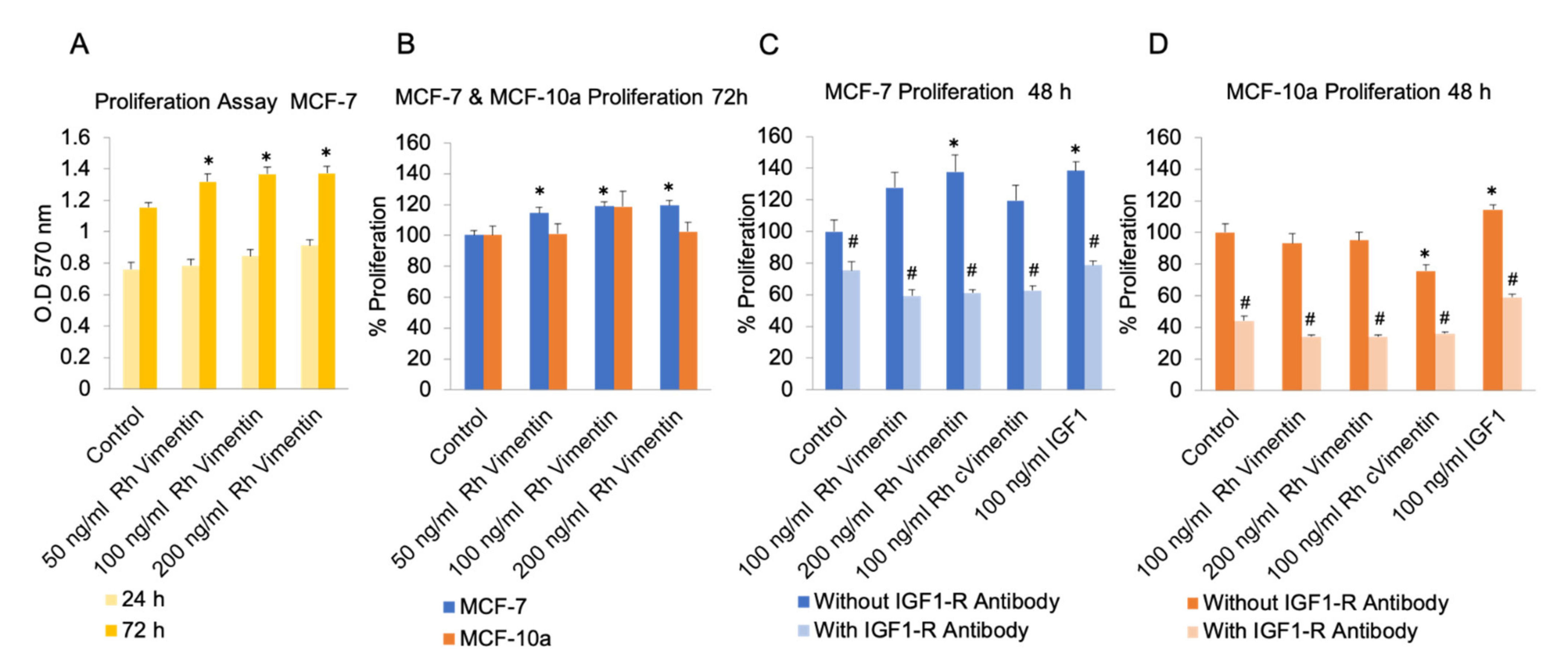
2.1. Extracellular Vimentin Promotes Proliferation in MCF-7 Cells through Activation of IGF-1R
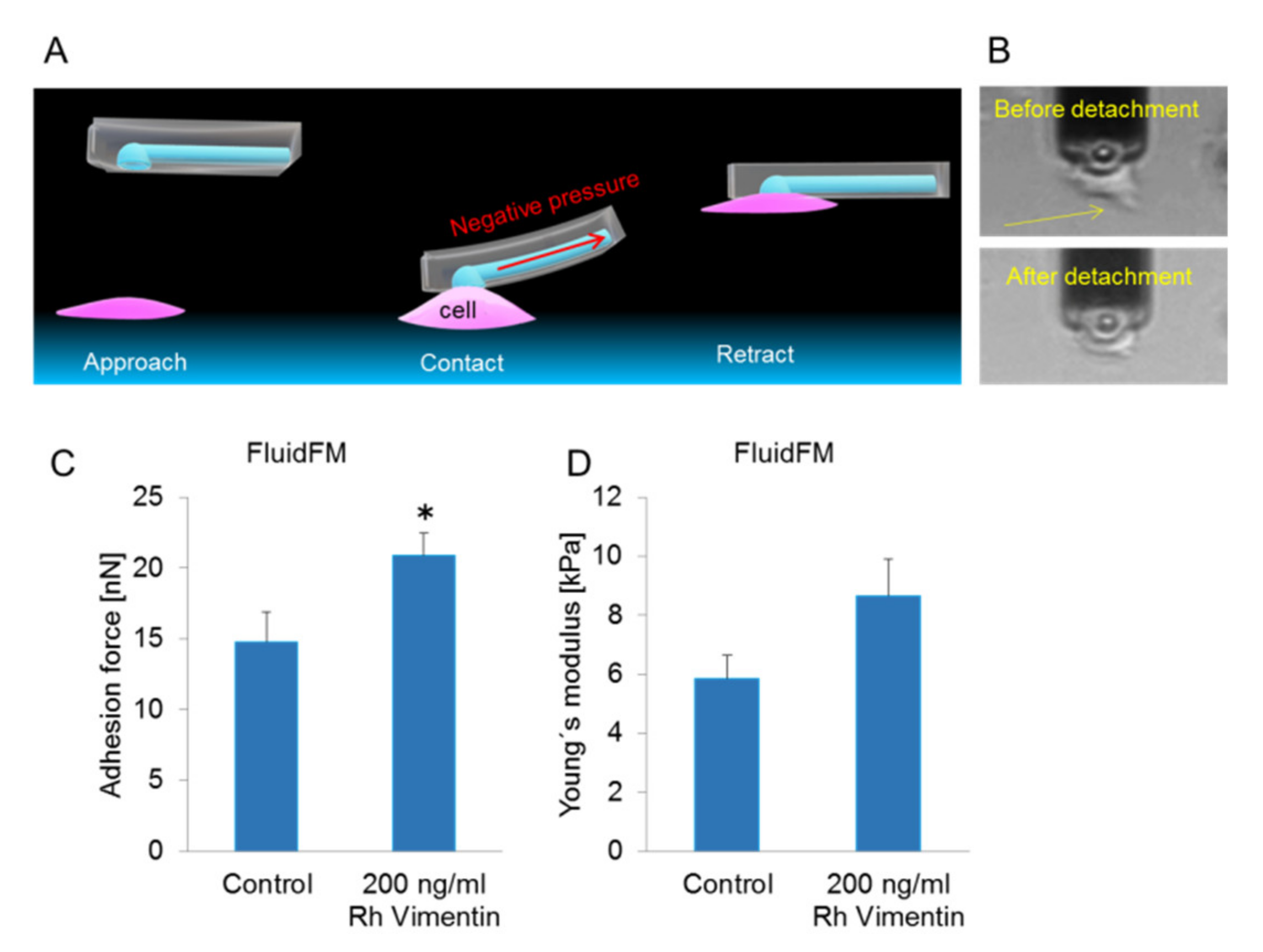
2.2. Extracellular Vimentin Promotes Stronger Adherence to the Underlying Substrate for MCF-7 Cells
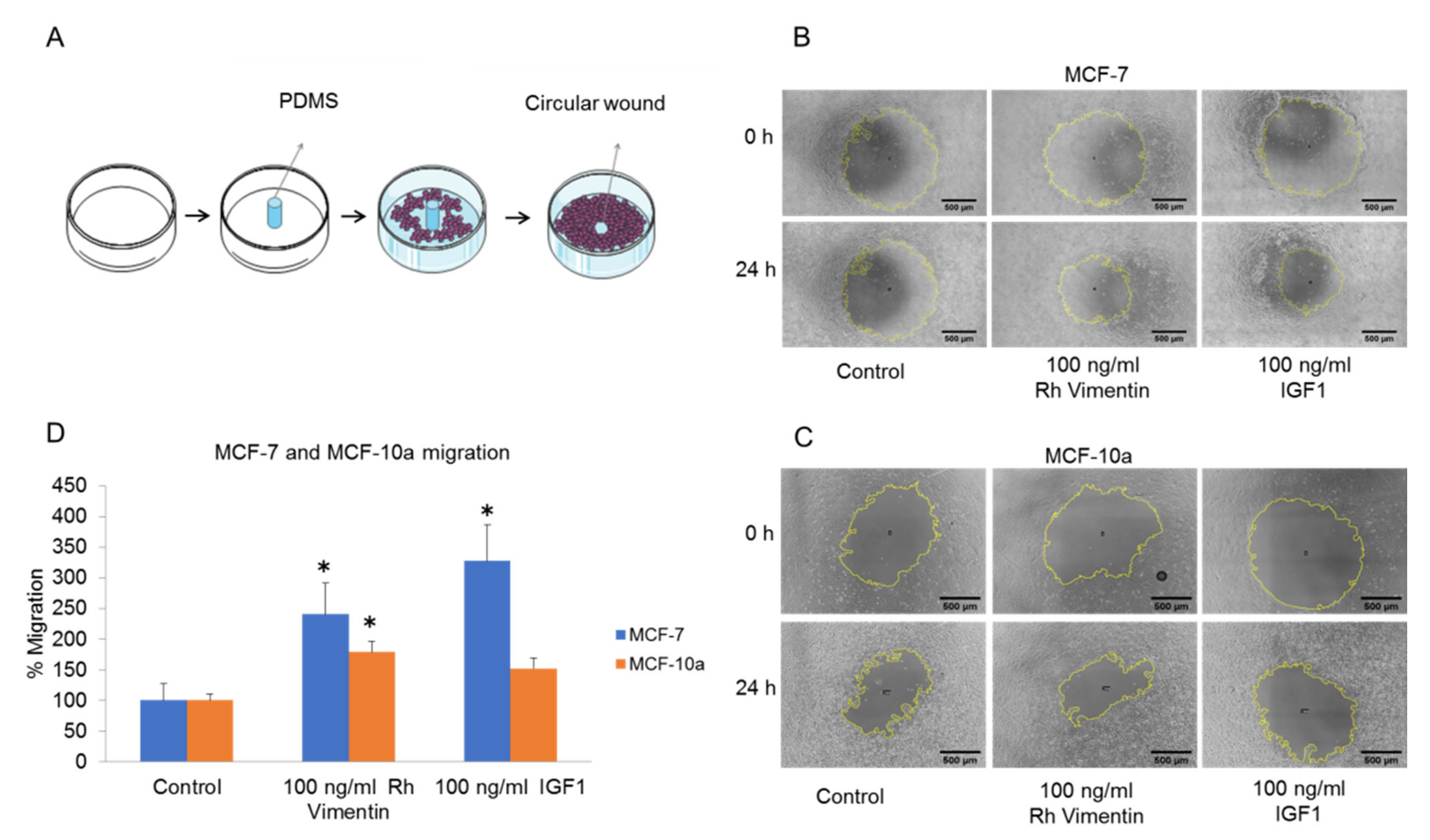
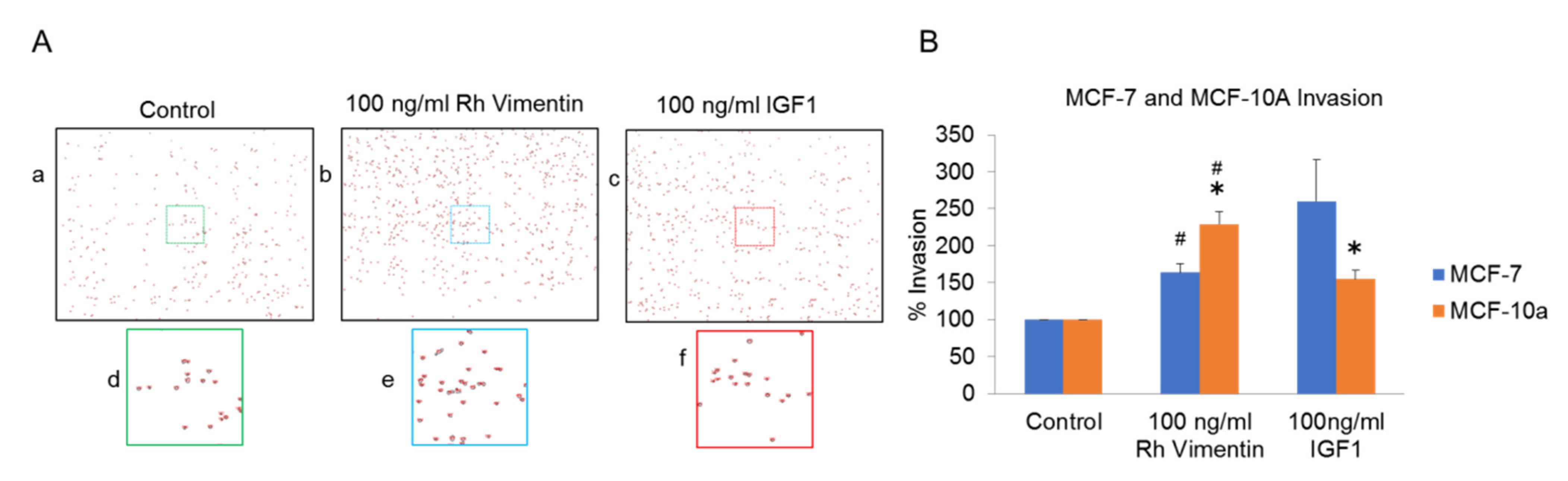
2.3. Extracellular Vimentin Induces Migration of MCF-10a and MCF-7 Cells
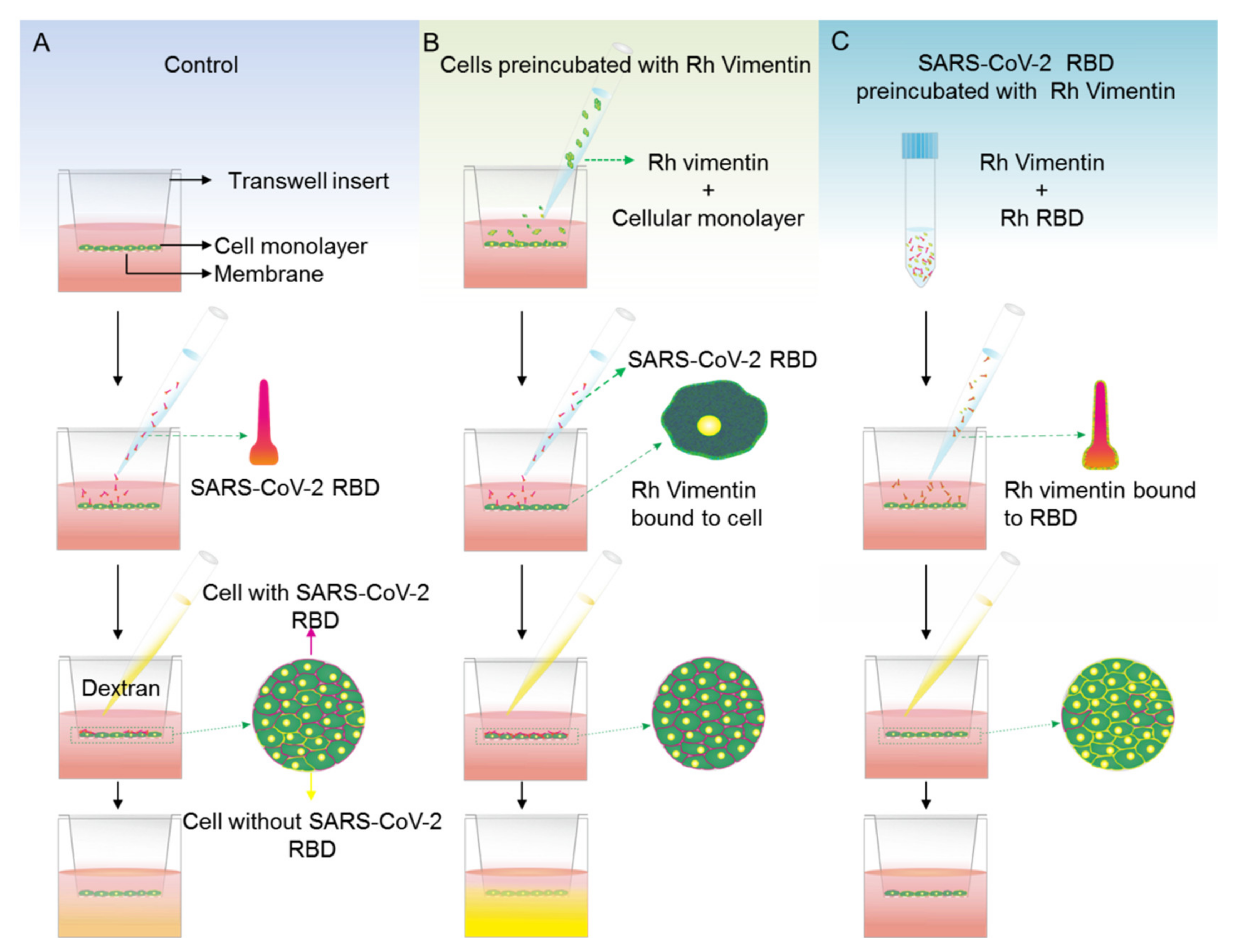
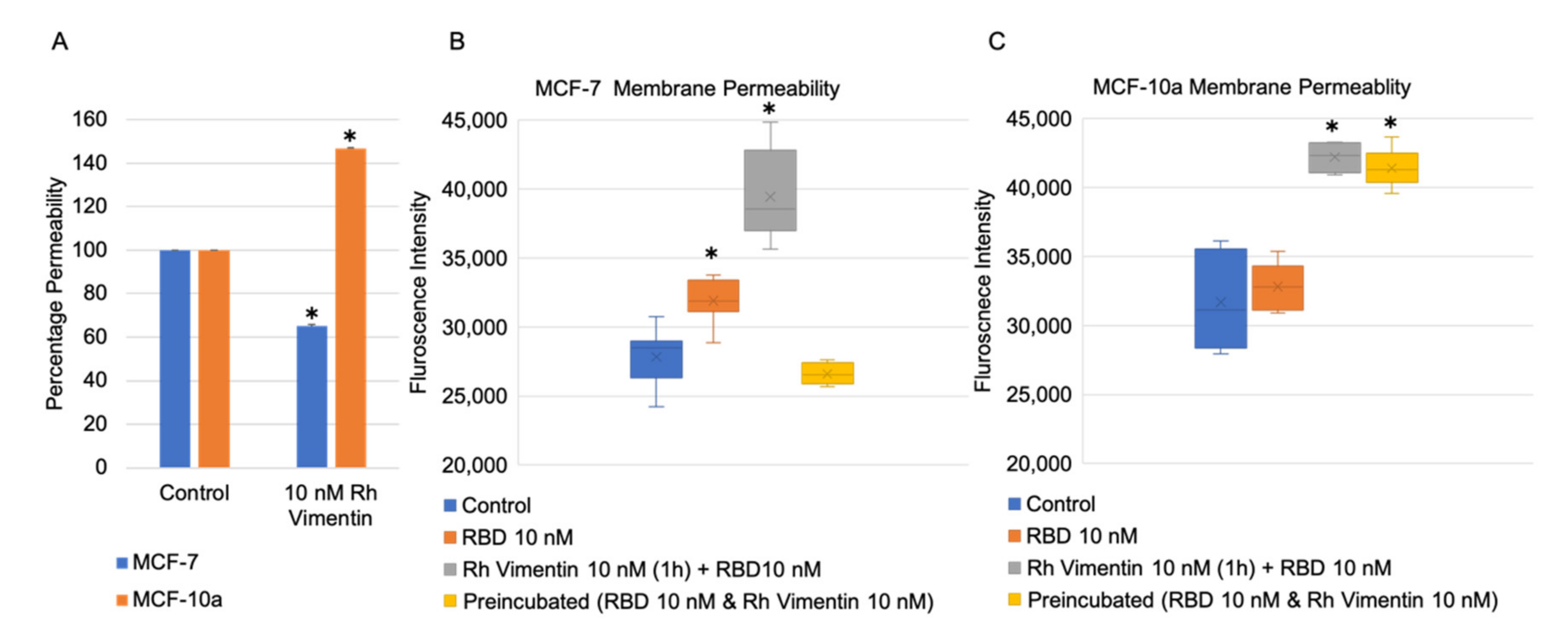
2.4. Extracellular Vimentin Effects on MCF-10a and MCF-7 Cell Monolayer Permeabilities and Alterations of Cell Monolayer Integrity Caused by the Receptor Binding Domain of SARS-CoV-2 Spike Protein
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Proliferation Assay
4.3. Migration Assay
4.4. Transwell Migration Assay
4.5. Single-Cell Force Spectroscopy
4.6. Permeabilty Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| BSA | Bovine serum albumin |
| CSV | Cell-surface vimentin |
| CTCs | Circulating tumor cells |
| FluidFM | Fluidic force microscopy |
| IGF-1 | Insulin-like growth factor-1 |
| IGF-1R | Insulin-like growth factor-1 receptor |
| MCF-7 cells | Human breast cancer epithelial cell line |
| MCF-10a | Non-tumorigenic epithelial cell line |
| MTT | 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PDMS | Polydimethylsiloxane |
| RBD | Receptor binding domain |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
References
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, F.; Peterson, M.K.; Caldeira Araújo, H.; Lautenschläger, F.; Gad, A.K.B. Vimentin diversity in health and disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef]
- Frescas, D.; Roux, C.M.; Aygun-Sunar, S.; Gleiberman, A.S.; Krasnov, P.; Kurnasov, O.V.; Strom, E.; Virtuoso, L.P.; Wrobel, M.; Osterman, A.L.; et al. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl. Acad. Sci. USA 2017, 114, E1668–E1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, T.; Seeholzer, S.H.; Mak, A.; Spruce, L.; Ischiropoulos, H. Quantitative Mass Spectrometry-based Proteomics Reveals the Dynamic Range of Primary Mouse Astrocyte Protein Secretion. J. Proteome Res. 2010, 9, 2764–2774. [Google Scholar] [CrossRef]
- Kaplan, M.J. Role of neutrophils in systemic autoimmune diseases. Arthritis Res. Ther. 2013, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boilard, E.; Bourgoin, S.G.; Bernatchez, C.; Surette, M.E. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood 2003, 102, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Moisan, E.; Girard, D. Cell surface expression of intermediate filament proteins vimentin and lamin B1in human neutrophil spontaneous apoptosis. J. Leukoc. Biol. 2005, 79, 489–498. [Google Scholar] [CrossRef]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, H.; Hyangsoon, N.; Hong, S.; Kong, L.-Y.; Gabrusiewicz, K.; Xia, X.; Heimberger, A.B.; Ling-Yuan, K. Discovery of cell surface vimentin targeting mAb for direct disruption of GBM tumor initiating cells. Oncotarget 2016, 7, 72021–72032. [Google Scholar] [CrossRef] [Green Version]
- Satelli, A.; Mitra, A.; Cutrera, J.J.; Devarie, M.; Xia, X.; Ingram, D.R.; Dibra, D.; Somaiah, N.; Torres, K.E.; Ravi, V.; et al. Universal Marker and Detection Tool for Human Sarcoma Circulating Tumor Cells. Cancer Res. 2014, 74, 1645–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satelli, A.; Mitra, A.; Brownlee, Z.; Xia, X.; Bellister, S.; Overman, M.J.; Kopetz, S.; Ellis, L.M.; Meng, Q.H.; Li, S. Epithelial–Mesenchymal Transitioned Circulating Tumor Cells Capture for Detecting Tumor Progression. Clin. Cancer Res. 2015, 21, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; Satelli, A.; Xia, X.; Cutrera, J.; Mishra, L.; Li, S. Cell-surface Vimentin: A mislocalized protein for isolating csVimentin+CD133− novel stem-like hepatocellular carcinoma cells expressing EMT markers. Int. J. Cancer 2015, 137, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zou, L.; Yang, Y.; Yuan, J.; Hu, Z.; Liu, H.; Peng, H.; Shang, W.; Zhang, X.; Zhu, J.; et al. Superficial vimentin mediates DENV-2 infection of vascular endothelial cells. Sci. Rep. 2016, 6, 38372. [Google Scholar] [CrossRef]
- Ghosh, P.; Halvorsen, E.M.; Ammendolia, D.A.; Mor-Vaknin, N.; O’Riordan, M.; Brumell, J.H.; Markovitz, D.M.; Higgins, D.E. Invasion of the Brain by Listeria monocytogenes Is Mediated by InlF and Host Cell Vimentin. mBio 2018, 9, e00160-18. [Google Scholar] [CrossRef] [Green Version]
- Bryant, A.E.; Bayer, C.R.; Huntington, J.D.; Stevens, D.L. Group A Streptococcal Myonecrosis: Increased Vimentin Expression after Skeletal-Muscle Injury Mediates the Binding ofStreptococcus pyogenes. J. Infect. Dis. 2006, 193, 1685–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, G.; Graham, L.M.; Lang, D.M.; Blumenthal, M.; Marušič, M.B.; Katz, A.A. Vimentin Modulates Infectious Internalization of Human Papillomavirus 16 Pseudovirions. J. Virol. 2017, 91, 00307–00317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.T.C.; Chien, S.C.; Chen, I.Y.; Lai, C.T.; Tsay, Y.G.; Chang, S.C.; Chang, M.F. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016, 23, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suprewicz, Ł.; Swoger, M.; Gupta, S.; Piktel, E.; Byfield, F.J.; Iwamoto, D.V.; Germann, D.A.; Reszeć, J.; Marcińczyk, N.; Janmey, P.; et al. Vimentin binds to SARS-CoV-2 spike protein and antibodies targeting extracellular vimentin block in vitro uptake of SARS-CoV-2 virus-like particles. bioRxiv 2021. [Google Scholar] [CrossRef]
- Thiagarajan, P.S.; Yakubenko, V.P.; Elsori, D.H.; Yadav, S.P.; Willard, B.; Tan, C.D.; Rodriguez, E.R.; Febbraio, M.; Cathcart, M.K. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc. Res. 2013, 99, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Barnes, P.F.; Porgador, A.; Roy, S.; Wu, S.; Nanda, J.S.; Griffith, D.E.; Girard, W.M.; Rawal, N.; Shetty, S.; et al. Vimentin Expressed onMycobacterium tuberculosis-Infected Human Monocytes Is Involved in Binding to the NKp46 Receptor. J. Immunol. 2006, 177, 6192–6198. [Google Scholar] [CrossRef] [Green Version]
- Shigyo, M.; Kuboyama, T.; Sawai, Y.; Tada-Umezaki, M.; Tohda, C. Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci. Rep. 2015, 5, 12055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.J.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-like growth factor-I regulation of immune function: A potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milano, F.; Campbell, A.P.; Guthrie, K.A.; Kuypers, J.; Englund, J.A.; Corey, L.; Boeckh, M. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 2010, 115, 2088–2094. [Google Scholar] [CrossRef] [Green Version]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Solomon-Zemler, R.; Sarfstein, R.; Werner, H. Nuclear insulin-like growth factor-1 receptor (IGF1R) displays proliferative and regulatory activities in non-malignant cells. PLoS ONE 2017, 12, e0185164. [Google Scholar] [CrossRef] [Green Version]
- Guillaume-Gentil, O.; Potthoff, E.; Ossola, D.; Franz, C.; Zambelli, T.; Vorholt, J.A. Force-controlled manipulation of single cells: From AFM to FluidFM. Trends Biotechnol. 2014, 32, 381–388. [Google Scholar] [CrossRef]
- Buchert, M.; Turksen, K.; Hollande, F. Methods to Examine Tight Junction Physiology in Cancer Stem Cells: TEER, Paracellular Permeability, and Dilution Potential Measurements. Stem Cell Rev. Rep. 2012, 8, 1030–1034. [Google Scholar] [CrossRef]
- McCaffrey, L.M.; Macara, I.G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011, 21, 727–735. [Google Scholar] [CrossRef]
- Gray, R.; Cheung, K.J.; Ewald, A.J. Cellular mechanisms regulating epithelial morphogenesis and cancer invasion. Curr. Opin. Cell Biol. 2010, 22, 640–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patteson, A.E.; Vahabikashi, A.; Goldman, R.D.; Janmey, P.A. Mechanical and Non-Mechanical Functions of Filamentous and Non-Filamentous Vimentin. BioEssays 2020, 42, 2000078. [Google Scholar] [CrossRef]
- Kim, S.; Cho, W.; Kim, I.; Lee, S.-H.; Oh, G.T.; Park, Y.M. Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J. Mol. Med. 2020, 98, 973–983. [Google Scholar] [CrossRef]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Cui, Y.; Roberts, C.; Lu, M.; Wilhelmsson, U.; Pekny, M.; Chopp, M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 2014, 62, 2022–2033. [Google Scholar] [CrossRef]
- Shigyo, M.; Tohda, C. Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci. Rep. 2016, 6, 28293. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef]
- Satelli, A.; Hu, J.; Xia, X.; Li, S. Potential Function of Exogenous Vimentin on the Activation of Wnt Signaling Pathway in Cancer Cells. J. Cancer 2016, 7, 1824–1832. [Google Scholar] [CrossRef] [Green Version]
- Komura, K.; Ise, H.; Akaike, T. Dynamic behaviors of vimentin induced by interaction with GlcNAc molecules. Glycobiology 2012, 22, 1741–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezi, S.; Todi, L.; Orsi, E.; Angeloni, A.; Mancini, P. Involvement of the Src-cortactin pathway in migration induced by IGF-1 and EGF in human breast cancer cells. Int. J. Oncol. 2012, 41, 2128–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Maziveyi, M.; Alahari, S.K. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget 2017, 8, 48471–48487. [Google Scholar] [CrossRef] [Green Version]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet 1998, 351, 1393–1396. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Holly, J.M.; Pollak, M.N.; Hankinson, S.E. Circulating Levels of Insulin-like Growth Factors, their Binding Proteins, and Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, Z.; de Blacquière, G.E.; Westley, B.R.; May, F.E. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: Implications for therapy. Neoplasia 2011, 13, 504–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, J.; Le Roith, D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: New insights into their synergistic effects. Mol. Pathol. 2001, 54, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Poon, R.T.P.; Lee, N.P.; Yeung, C.; Chan, K.L.; Ng, I.O.-L.; Day, P.J.R.; Luk, J.M. Proteomics of Hepatocellular Carcinoma: Serum Vimentin As a Surrogate Marker for Small Tumors (≤2 cm). J. Proteome Res. 2010, 9, 1923–1930. [Google Scholar] [CrossRef]
- Bukhari, S.; Mokhdomi, T.A.; Chikan, N.; Amin, A.; Qazi, H.; Wani, S.H.; Wafai, A.H.; Tyub, S.; Mustafa, F.; Mir, M.S.; et al. Affinity proteomics led identification of vimentin as a potential biomarker in colon cancers: Insights from serological screening and computational modelling. Mol. BioSyst. 2014, 11, 159–169. [Google Scholar] [CrossRef]
- Leoncikas, V.; Wu, H.; Ward, L.T.; Kierzek, A.; Plant, N.J. Generation of 2000 breast cancer metabolic landscapes reveals a poor prognosis group with active serotonin production. Sci. Rep. 2016, 6, 19771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Steendam, K.; Tilleman, K.; De Ceuleneer, M.; De Keyser, F.; Elewaut, D.; Deforce, D. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 2010, 12, R132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ospelt, C.; Bang, H.; Feist, E.; Camici, G.; Keller, S.; Detert, J.; Krämer, A.; Gay, S.; Ghannam, K.; Burmester, G.R. Carbamylation of vimentin is inducible by smoking and represents an independent autoantigen in rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1176–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalla, D.G.; Jung, P.; Bischoff, M.; Lautenschläger, F. Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain. Int. J. Mol. Sci. 2021, 22, 7469. https://doi.org/10.3390/ijms22147469
Thalla DG, Jung P, Bischoff M, Lautenschläger F. Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain. International Journal of Molecular Sciences. 2021; 22(14):7469. https://doi.org/10.3390/ijms22147469
Chicago/Turabian StyleThalla, Divyendu Goud, Philipp Jung, Markus Bischoff, and Franziska Lautenschläger. 2021. "Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain" International Journal of Molecular Sciences 22, no. 14: 7469. https://doi.org/10.3390/ijms22147469
APA StyleThalla, D. G., Jung, P., Bischoff, M., & Lautenschläger, F. (2021). Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain. International Journal of Molecular Sciences, 22(14), 7469. https://doi.org/10.3390/ijms22147469






