Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage
Abstract
:1. Introduction
2. Results
2.1. Experiment I
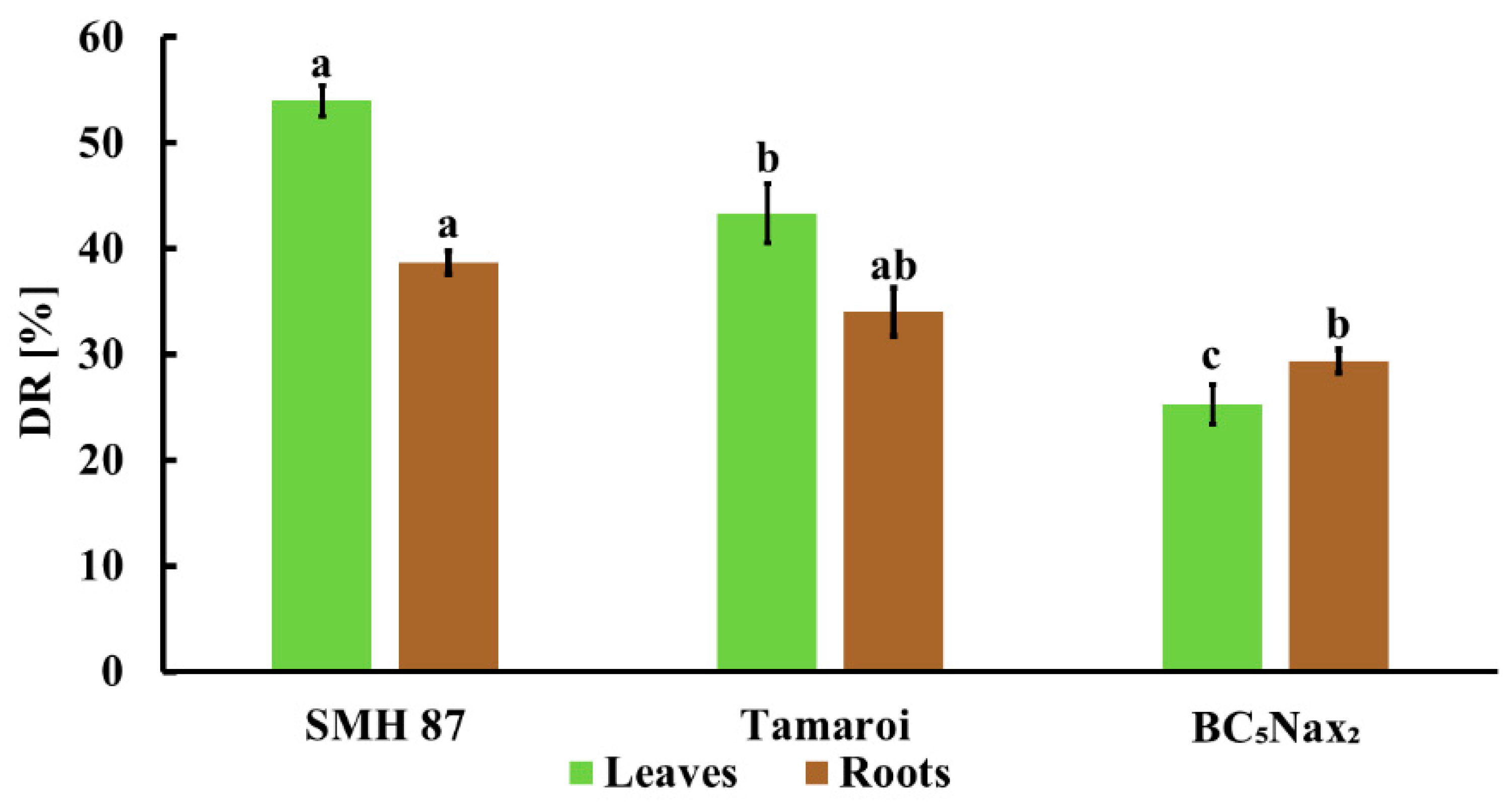
2.1.1. Disease Rating (DR) and Fresh Weight (FW) Loss
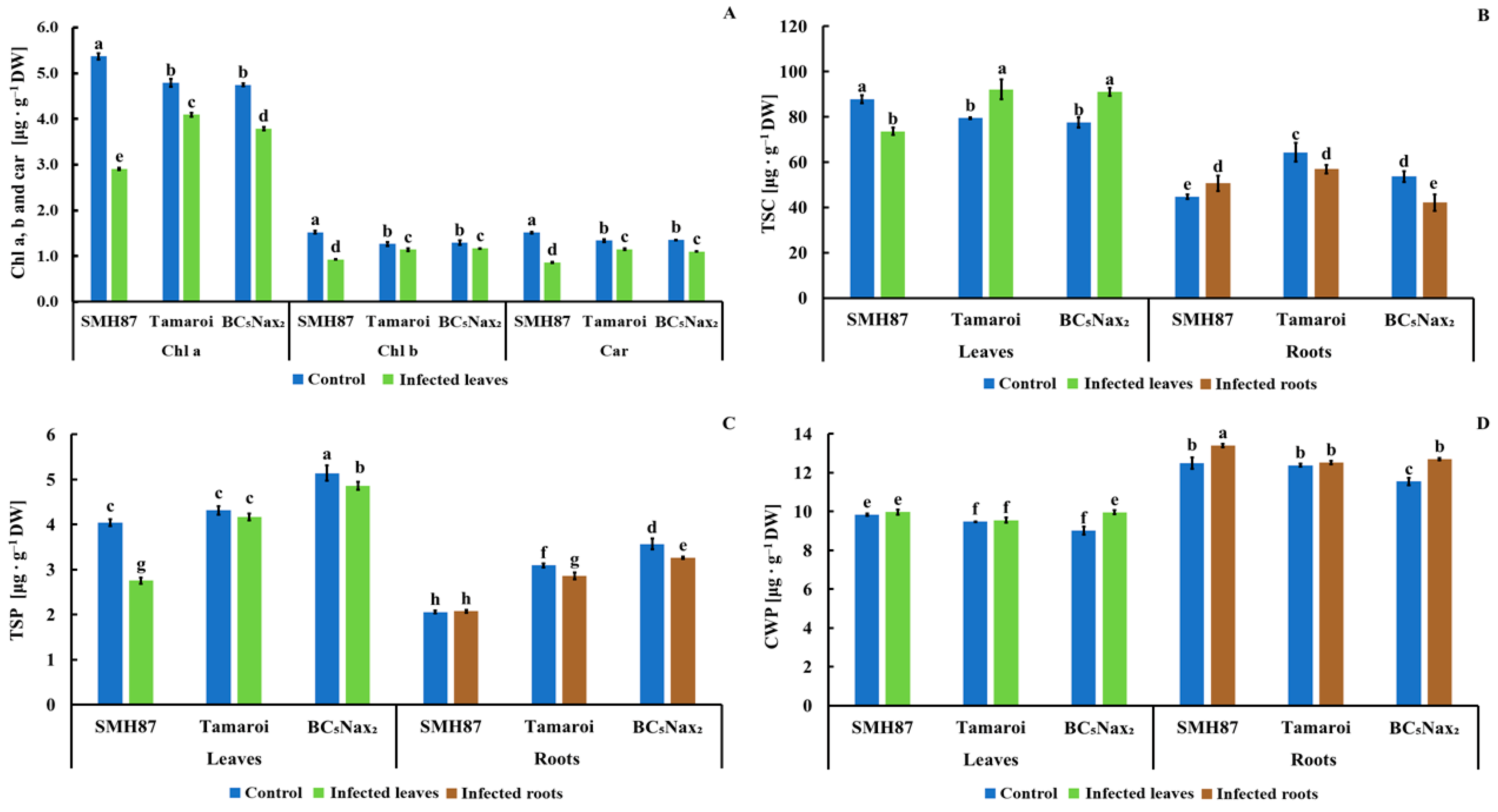
2.1.2. Chlorophyll a, b, and Carotenoid (Chla, b, and car) Content
2.1.3. Total Soluble Carbohydrates (TSC)
2.1.4. Total Soluble Phenolics (TSP)
2.1.5. Cell Wall-Bound Phenolics (CWP)
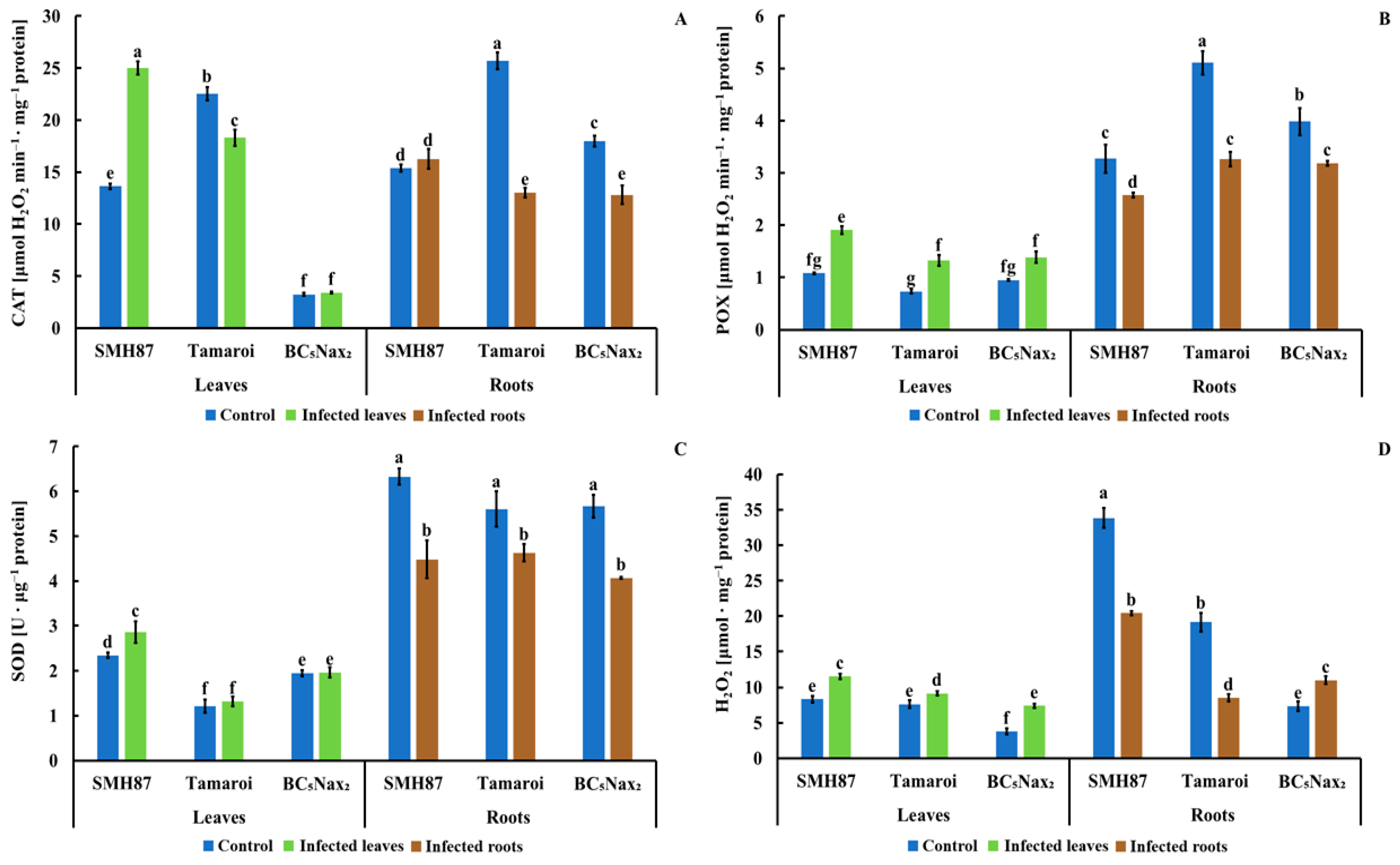
2.1.6. Catalase (CAT) Activity
2.1.7. Peroxidases (POXs) Activity
2.1.8. Superoxide Dismutase (SOD) Activity
2.1.9. Hydrogen Peroxide (H2O2) Content
2.2. Correlation Analysis
2.3. Experiment II
2.3.1. Fusarium Head Blight Index (FHBi) and Yield Parameters
2.3.2. Mycotoxin Content
2.3.3. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.1.1. Preparation of Fusarium culmorum Isolate
4.1.2. Experiment I—Box Test Assay
4.1.3. Experiment II—Open Foil Tunnel
4.2. Analyses
4.2.1. Disease Rating (DR) and Loss of Fresh Weight (FW)
4.2.2. Chlorophyll (a, b) and Carotenoid (Car) Content
4.2.3. Determination of Total Water-Soluble Carbohydrates (TSC)
4.2.4. Determination of Total Phenolic Compounds (TPC)
4.2.5. Determination of Cell Wall-Bound Phenolics (CWP)
4.2.6. Activity of Enzymatic Antioxidants
4.2.7. Hydrogen Peroxide Content
4.2.8. Spore Suspension Preparation and Spike Inoculation Procedure
4.2.9. UHPLC-MS/MS Estimation of Mycotoxin Accumulation
4.2.10. Yield Components
4.3. Statistical Analyses
5. Conclusions
- Fusarium culmorum infection significantly reduces the content of active photosynthetic pigments and the weight of leaves and roots.
- The infected cv. ‘Tamaroi’ and BC5Nax2 plants recognized as more resistant to F. culmorum than SMH87, accumulated increased amounts of sugar in the leaves, which correlated with an increased number of phenolic compounds.
- Phenolic compounds participate in H2O2 decomposition in durum wheat plants infected by F. culmorum.
- The study confirmed the important role of H2O2 in increasing the content of phenolic compounds that are then incorporated into cell walls of plants infected with F. culmorum.
- Nivalenol and deoxynivalenol secreted by F. cumlorum significantly reduce the yield of durum wheat.
- Early evaluation of durum wheat spikes infection done seven days after inoculation with F. culmorum spores may help predict the potential degree of DON and NIV accumulation in the grain.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Nicholson, P. Community Ecology of Fungal Pathogens Causing Wheat Head Blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef]
- Salgado, J.D.; Madden, L.; Paul, P.A. Efficacy and Economics of Integrating In-Field and Harvesting Strategies to Manage Fusarium Head Blight of Wheat. Plant Dis. 2014, 98, 1407–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Trail, F. For Blighted Waves of Grain: Fusarium graminearum in the Postgenomics Era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Mycotoxins Plant Dis. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Foroud, N.A.; Eudes, F. Trichothecenes in Cereal Grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef] [Green Version]
- Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1126/2007 of 28 September 2007 Amending Regulation (EC) No 1881/2006 Setting Maxi-mum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32007R1126 (accessed on 2 February 2021).
- Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006H0576 (accessed on 2 February 2021).
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D.; Stępień, Ł. Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum. Toxins 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Tarkowski, Ł.P.; Van De Poel, B.; Höfte, M.; Ende, W.V.D. Sweet Immunity: Inulin Boosts Resistance of Lettuce (Lactuca sativa) against Grey Mold (Botrytis cinerea) in an Ethylene-Dependent Manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef] [Green Version]
- Streuter, N.; Moerschbacher, B.; Fischer, Y.; Noll, U.; Reisener, H. Fructose-2,6-Bisphosphate in Wheat Leaves Infected with Stem Rust. J. Plant Physiol. 1989, 134, 254–257. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Laroche, A.; Yoshida, M. Low temperature-wheat-fungal interactions: A carbohydrate connection. Physiol. Plant. 1999, 106, 437–444. [Google Scholar] [CrossRef]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-induced lupine defense against Fusarium oxysporum: Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic Compounds and Their Role in Disease Resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Nicholson, P.; Doohan, F. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2009, 185, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- De Gara, L.; Locato, V.; Dipierro, S.; de Pinto, M.C. Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Respir. Physiol. Neurobiol. 2010, 173, S13–S19. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Zurbriggen, M.D.; Carrillo, N.; Tognetti, V.B.; Melzer, M.; Peisker, M.; Hause, B.; Hajirezaei, M.-R. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J. 2009, 60, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Barna, B.; Fodor, J.; Harrach, B.; Pogany, M.; Király, Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Beszterda, M.; Goliński, P. Nonenzymatic Antioxidants in Plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 201–234. [Google Scholar]
- Yang, T.; Poovaiah, B.W. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madadkhah, E.; Lotfi, M.; Nabipour, A.; Rahmanpour, S.; Banihashemi, Z.; Shoorooei, M. Enzymatic activities in roots of melon genotypes infected with Fusarium oxysporum f. sp. melonis race 1. Sci. Hortic. 2012, 135, 171–176. [Google Scholar] [CrossRef]
- Wang, W.; Xia, M.X.; Chen, J.; Yuan, R.; Deng, F.N.; Shen, F. Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochemistry 2016, 81, 465–480. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, S.; Liu, S.; Zhang, Z.; Gui, H.; Duan, J.; Chen, Q. Characteristics of chlorophyll metabolism and chlorophyll fluorescence in the silvered leaf of summer squash. Acta Hortic. Sinica. 2009, 36, 879–884. [Google Scholar]
- Alwathnani, H.A. Biological control of fusarium wilt of tomato by antagonist fungi and cyanobacteria. Afr. J. Biotechnol. 2012, 11, 1100–1105. [Google Scholar] [CrossRef]
- Warzecha, T.; Skrzypek, E.; Sutkowska, A. Effect of Fusarium culmorum infection on selected physiological and biochemical parameters of barley (Hordeum vulgare L.) DH lines. Physiol. Mol. Plant Pathol. 2015, 89, 62–69. [Google Scholar] [CrossRef]
- Wagacha, J.; Muthomi, J. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop. Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Chekali, S.; Gargouri, S.; Paulitz, T.; Nicol, J.M.; Rezgui, M.; Nasraoui, B. Effects of Fusarium culmorum and water stress on durum wheat in Tunisia. Crop. Prot. 2011, 30, 718–725. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Zwart, A.B.; Hare, R.A.; Rathjen, A.J.; Munns, R. Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils. Funct. Plant Biol. 2012, 39, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, J.; Kopeć, P.; Płażek, A.; Gondek, K.; Szczerba, A.; Hornyák, M.; Dubert, F. Cadmium accumulation in the grain of durum wheat is associated with salinity resistance degree. Plant Soil Environ. 2020, 66, 257–263. [Google Scholar] [CrossRef]
- Pastuszak, J.; Kopeć, P.; Płażek, A.; Gondek, K.; Szczerba, A.; Hornyák, M.; Dubert, F. Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance. Open Chem. 2020, 18, 1230–1241. [Google Scholar] [CrossRef]
- Lops, R.; Pascale, M.; Pancaldi, D.; Visconti, A. Infezionifungine e presenza di deossinivalenolo in cariossidi di frumento-prodotte in diverse regioniitaliane. Inf. Fitopatol. 1998, 48, 60–66. [Google Scholar]
- Miedaner, T.; Longin, C.F.H. Genetic variation for resistance to Fusarium head blight in winter durum material. Crop. Pasture Sci. 2014, 65, 46–51. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Grey, W.E.; Mathre, D.E. Evaluation of spring barleys for reaction to Fusarium culmorum seedling blight and root rot. Can. J. Plant Sci. 1988, 68, 23–30. [Google Scholar] [CrossRef]
- Wojciechowski, S.; Chelkowski, J.; Ponitka, A.; Ślusarkiewicz-Jarzina, A. Evaluation of Spring and Winter Wheat Reaction to Fusarium culmorum and Fusarium avenaceum. J. Phytopathol. 1997, 145, 99–103. [Google Scholar] [CrossRef]
- Warzecha, T.; Zieliński, A.; Skrzypek, E.; Wójtowicz, T.; Moś, M. Effect of mechanical damage on vigor, physiological parameters, and susceptibility of oat (Avena sativa) to Fusarium culmorum infection. Phytoparasitica 2012, 40, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Malalasekera, R.; Sanderson, F.; Colhoun, J. Fusarium diseases of cereals: IX. Penetration and invasion of wheat seedlings by Fusarium culmorum and F. nivale. Trans. Br. Mycol. Soc. 1973, 60, 453–462.IN7. [Google Scholar] [CrossRef]
- Knudsen, I.M.B.; Hockenhull, J.; Jensen, D. Biocontrol of seedling diseases of barley and wheat caused by Fusarium culmorum and Bipolaris sorokiniana: effects of selected fungal antagonists on growth and yield components. Plant Pathol. 1995, 44, 467–477. [Google Scholar] [CrossRef]
- Płażek, A. Relationship between soluble carbohydrate level and tolerance of meadow fescue callus to Bipolaris sorokiniana (Sacc.) Shoem. and Drechslera dictyoides (Drechsl.) Shoem. metabolites. Acta Physiol. Plant. 1998, 20, 347–351. [Google Scholar] [CrossRef]
- Herbers, K.; Takahata, Y.; Melzer, M.; Mock, H.-P.; Hajirezaei, M.; Sonnewald, U. Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol. Plant Pathol. 2000, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pociecha, E.; Płażek, A.; Janowiak, F.; Dubert, F.; Kolasińska, I.; Irla, M. Factors contributing to enhanced pink snow mould resistance of winter rye (Secale cereale L.)–Pivotal role of crowns. Physiol. Mol. Plant Pathol. 2013, 81, 54–63. [Google Scholar] [CrossRef]
- Eveland, A.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bani, M.; Pérez-De-Luque, A.; Rubiales, D.; Rispail, N. Physical and Chemical Barriers in Root Tissues Contribute to Quantitative Resistance to Fusarium oxysporum f. sp. pisi in Pea. Front. Plant Sci. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Formela-Luboińska, M.; Remlein-Starosta, D.; Waśkiewicz, A.; Karolewski, Z.; Bocianowski, J.; Stępień, Ł.; Labudda, M.; Jeandet, P.; Morkunas, I. The Role of Saccharides in the Mechanisms of Pathogenicity of Fusarium oxysporum f. sp. lupini in Yellow Lupine (Lupinus luteus L.). Int. J. Mol. Sci. 2020, 21, 7258. [Google Scholar] [CrossRef]
- Goodman, R.N.; Király, Z.; Wood, K.R. The Biochemistry and Physiology of Plant Disease; University of Missouri Press: Columbia, MO, USA, 1986. [Google Scholar]
- Płażek, A.; Hura, K.; Żur, I. Reaction of winter oilseed rape callus to different concentrations of elicitors: Pectinase or chitosan. Acta Physiol. Plant. 2003, 25, 83–89. [Google Scholar] [CrossRef]
- Płażek, A.; Hura, K.; Żur, I. Influence of chitosan, pectinase and fungal metabolites on activation of phenylopropanoid pathway and antioxidant activity in oilseed rape callus. Acta Physiol. Plant. 2005, 27, 95–102. [Google Scholar]
- Hura, K.; Hura, T.; Dziurka, K.; Dziurka, M. Biochemical defense mechanisms induced in winter oilseed rape seedlings with different susceptibility to infection with Leptosphaeria maculans. Physiol. Mol. Plant Pathol. 2014, 87, 42–50. [Google Scholar] [CrossRef]
- Derckel, J.-P.; Audran, J.-C.; Haye, B.; Lambert, B.; Legendre, L. Characterization, induction by wounding and salicylic acid, and activity against Botrytis cinerea of chitinases and β-1,3-glucanases of ripening grape berries. Physiol. Plant. 1998, 104, 56–64. [Google Scholar] [CrossRef]
- Arora, N.K.; Kim, M.J.; Kang, S.C.; Maheshwari, D.K. Role of chitinase and β-1,3-glucanase activities produced by a fluorescent pseudomonad and in vitro inhibition of Phytophthora capsici and Rhizoctonia solani. Can. J. Microbiol. 2007, 53, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Smith, C. Tansley Review No. 86 Accumulation of phytoalexins: Defence mechanism and stimulus response system. New Phytol. 1996, 132, 1–45. [Google Scholar] [CrossRef]
- Bizuneh, G.K. The chemical diversity and biological activities of phytoalexins. Adv. Tradit. Med. 2021, 21, 31–43. [Google Scholar] [CrossRef]
- Hakulinen, J.; Sorjonen, S.; Julkunen-Tiitto, R. Leaf phenolics of three willow clones differing in resistance to Melampsora rust infection. Physiol. Plant. 1999, 105, 662–669. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.O.; Taylor, C.B. Plant-Microbe Interactions: Life and Death at the Interface. Plant Cell 1996, 8, 1651–1668. [Google Scholar] [CrossRef]
- Datta, J.; Lal, N. Temporal and spatial changes in phenolic compounds in response to Fusarium wilt in chickpea and pigeon pea. Cell. Mol. Boil. 2012, 58, 96–102. [Google Scholar]
- Noman, A.; Aqeel, M.; Qari, S.H.; Al Surhanee, A.A.; Yasin, G.; Alamri, S.; Hashem, M.; Al-Saadi, A.M. Plant hypersensitive response vs pathogen ingression: Death of few gives life to others. Microb. Pathog. 2020, 145, 104224. [Google Scholar] [CrossRef]
- Badawi, G.H.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kawano, N.; Tanaka, K.; Tanaka, K. Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci. 2004, 166, 919–928. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced Reactive Oxygen Species Scavenging by Overproduction of Superoxide Dismutase and Catalase Delays Postharvest Physiological Deterioration of Cassava Storage Roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef] [Green Version]
- Helepciuc, F.E.; Mitoi, M.E.; Manole-Paunescu, A.; Aldea, F.; Brezeanu, A.; Cornea, C.P. Induction of plant antioxidant system by interaction with beneficial and/or pathogenic microorganisms. Rom. Biotech. Lett. 2014, 19, 9366–9375. [Google Scholar]
- Torres, M.A.; Jones, J.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Morkunas, I.; Gmerek, J. The possible involvement of peroxidase in defense of yellow lupine embryo axes against Fusarium oxysporum. J. Plant Physiol. 2007, 164, 185–194. [Google Scholar] [CrossRef]
- Mandal, S.; Mitra, A.; Mallick, N. Biochemical characterization of oxidative burst during interaction between Solanum lycopersicum and Fusarium oxysporum f. sp. lycopersici. Physiol. Mol. Plant Pathol. 2008, 72, 56–61. [Google Scholar] [CrossRef]
- Plazek, A.; Zur, I. Cold-induced plant resistance to necrotrophic pathogens and antioxidant enzyme activities and cell membrane permeability. Plant Sci. 2003, 164, 1019–1028. [Google Scholar] [CrossRef]
- Bushnell, W.R.; Hazen, E.; Pritsch, C. Histology and physiology of Fusarium head blight. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2003; pp. 44–83. [Google Scholar]
- Gale, L.R.; Harrison, S.A.; Ward, T.J.; O’Donnell, K.; Milus, E.A.; Gale, S.W.; Kistler, H. Nivalenol-Type Populations of Fusarium graminearum and F. asiaticum Are Prevalent on Wheat in Southern Louisiana. Phytopatholigy 2011, 101, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2004, 85, 191–198. [Google Scholar] [CrossRef]
- Nowicki, T. Vomitoxin And Fusarium Damaged Kernels–Is There A Relationship in Canadian Wheat? In Proceedings of the 2nd Canadian Workshop on Fusarium Head Blight, Ottawa, ON, Canada, 3–5 November 2001. [Google Scholar]
- Pascale, M.; Visconti, A.; Chelkowski, J. Ear Rot Susceptibility and Mycotoxin Contamination of Maize Hybrids Inoculated with Fusarium Species Under Field Conditions. Eur. J. Plant Pathol. 2002, 108, 645–651. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H. Pathogenicity and In Planta Mycotoxin Accumulation Among Members of the Fusarium graminearum Species Complex on Wheat and Rice. Phytopathology 2005, 95, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Y.; Sun, H.-Y.; Li, W.; Xia, Y.-L.; Deng, Y.-Y.; Zhang, A.-X.; Chen, H.-G. Fitness of three chemotypes of Fusarium graminearum species complex in major winter wheat-producing areas of China. PLoS ONE 2017, 12, e0174040. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, A.E.; Plattner, R.D. Diverse traits for pathogen fitness inGibberella zeae. Can. J. Plant Pathol. 2003, 25, 21–27. [Google Scholar] [CrossRef]
- Llorens, A.; Mateo, R.; Hinojo, M.; Valle-Algarra, F.; Jiménez, M. Influence of environmental factors on the biosynthesis of type B trichothecenes by isolates of Fusarium spp. from Spanish crops. Int. J. Food Microbiol. 2004, 94, 43–54. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Góral, T.; Ochodzki, P.; Walentyn-Góral, D.; Kwiatek, M.; Majka, M.; Kurleto, D. Resistance of winter triticale breeding lines to Fusarium head blight. Bull. Plant Breed. Acclim. Inst. 2014, 271, 29–43. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Univ. Calif. Agric. Exp. Stn. Circ. 1938, 347, 29–32. [Google Scholar]
- Warzecha, T.; Skrzypek, E.; Adamski, T.; Surma, M.; Kaczmarek, Z.; Sutkowska, A. Chlorophyll a Fluorescence Parameters of Hulled and Hull-less Barley (Hordeum vulgare L.) DH Lines Inoculated with Fusarium culmorum. Plant Pathol. J. 2019, 35, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Czyczyło-Mysza, I.; Tyrka, M.; Marcińska, I.; Skrzypek, E.; Karbarz, M.; Dziurka, M.; Hura, T.; Quarrie, S. Quantitative trait loci for leaf chlorophyll fluorescence parameters, chlorophyll and carotenoid contents in relation to biomass and yield in bread wheat and their chromosome deletion bin assignments. Mol. Breed. 2013, 32, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Dubois, M.Y.; A Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F.G. A Colorimetric Method for the Determination of Sugars. Nat. Cell Biol. 1951, 168, 167. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, M. Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia ‘Ronina’ and ‘Rupert’ in vitro cultures under different lighting environments. Sci. Hortic. 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. Different allocation of carbohydrates and phenolics in dehydrated leaves of triticale. J. Plant Physiol. 2016, 202, 1–9. [Google Scholar] [CrossRef]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Luck, H. Methoden der enzymatischenanalyse. In Verlag Chemie, 1st ed.; Bergmeyer, H.U.: Weinheim, Germany, 1962. [Google Scholar]
- Gudyś, K.; Guzy-Wrobelska, J.; Janiak, A.; Dziurka, M.A.; Ostrowska, A.; Hura, K.; Jurczyk, B.; Żmuda, K.; Grzybkowska, D.; Śróbka, J.; et al. Prioritization of Candidate Genes in QTL Regions for Physiological and Biochemical Traits Underlying Drought Response in Barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Szechynska-Hebda, M.; Skrzypek, E.; Dąbrowska, G.; Wędzony, M.; Van Lammeren, A. The effect of endogenous hydrogen peroxide induced by cold treatment in the improvement of tissue regeneration efficiency. Acta Physiol. Plant. 2011, 34, 547–560. [Google Scholar] [CrossRef]
- Wojtania, A.; Skrzypek, E.; Gabryszewska, E. Morphological and Biochemical Responses to Gibberellic Acid in Magnolia × ‘Spectrum’ in Vitro. Acta Biol. Cracoviensias. Bot. 2016, 58, 103–111. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mohanty, J.; Jaffe, J.S.; Schulman, E.S.; Raible, D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 1997, 202, 133–141. [Google Scholar] [CrossRef]
- ThermoFisher Protocol of Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp22188.pdf (accessed on 30 June 2021).
- Warzecha, T.; Adamski, T.; Kaczmarek, Z.; Surma, M.; Goliński, P.; Perkowski, J.; Chełkowski, J.; Wiśniewska, H.; Krystkowiak, K.; Kuczynska, A. Susceptibility of hulled and hulless barley doubled haploids to Fusarium culmorum head blight. Cereal Res. Commun. 2010, 38, 220–232. [Google Scholar] [CrossRef]
- Dziurka, M.; Maksymowicz, A.; Ostrowska, A.; Biesaga-Kościelniak, J. The Interaction Effect of Drought and Exogenous Application of Zearalenone on the Physiological, Biochemical Parameters and Yield of Legumes. J. Plant Growth Regul. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Klötzel, M.; Lauber, U.; Humpf, H.-U. A new solid phase extraction clean-up method for the determination of 12 type A and B trichothecenes in cereals and cereal-based food by LC-MS/MS. Mol. Nutr. Food Res. 2006, 50, 261–269. [Google Scholar] [CrossRef] [PubMed]
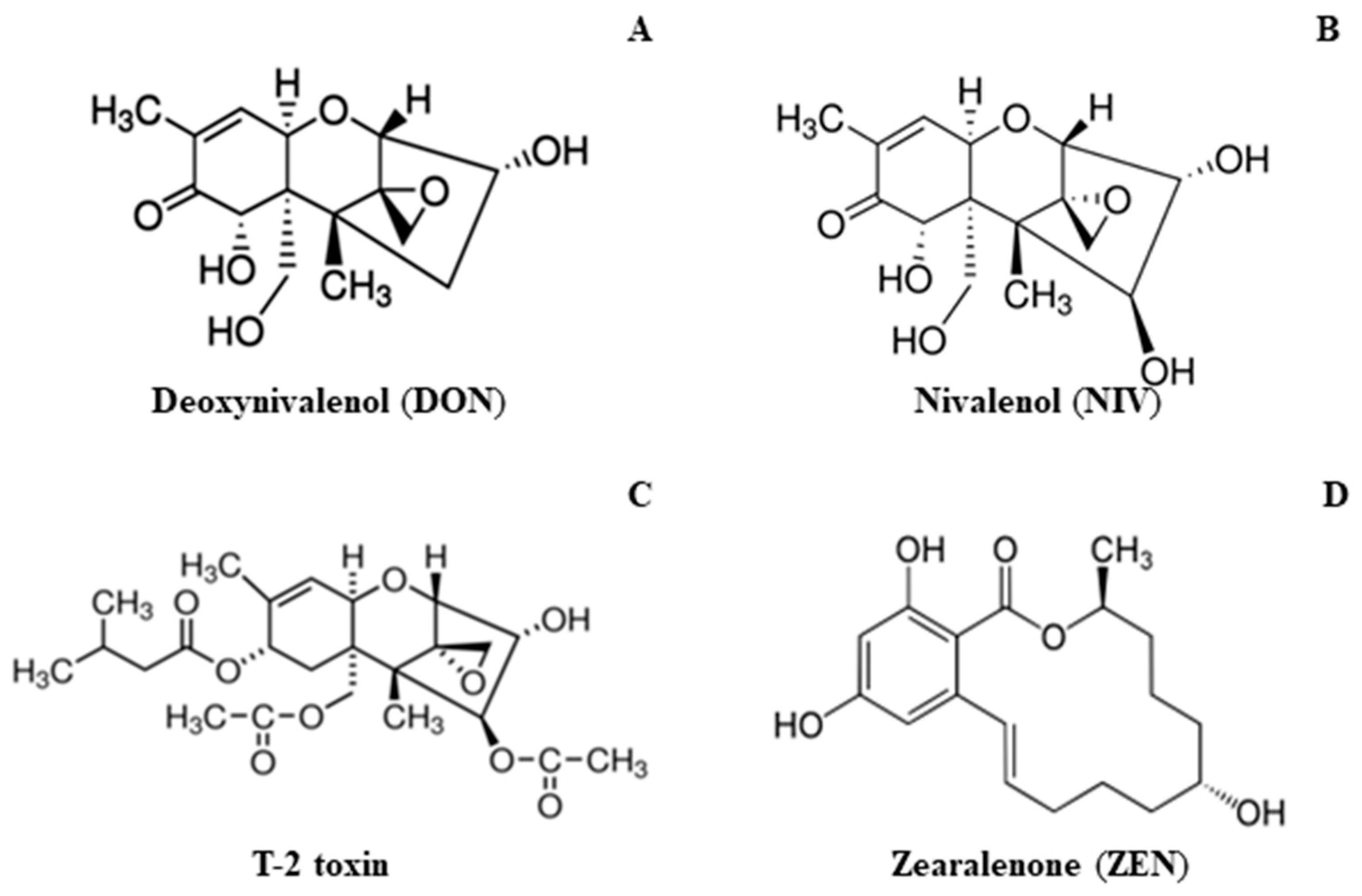

| Variable | Chl a | Chl b | Car | FW of Leaves | FW of Roots |
|---|---|---|---|---|---|
| DR in leaves | −0.859 | −0.804 | −0.870 | −0.715 | −0.755 |
| DR in roots | −0.891 | −0.811 | −0.898 | −0.672 | −0.821 |
| Variable | H2O2 Content | |
|---|---|---|
| Leaves | Roots | |
| CAT in leaves | 0.710 | ns |
| CAT in roots | ns | ns |
| POX in leaves | 0.688 | ns |
| POX in roots | ns | ns |
| SOD in leaves | ns | ns |
| SOD in roots | ns | 0.613 |
| TPC in leaves | −0.863 | ns |
| TPC in roots | −0.763 | −0.658 |
| CWP in leaves | 0.679 | ns |
| CWP in roots | 0.861 | ns |
| Accession | Treatment | FHBi [%] | Amount of Grain Per Spike | Grain Mass Per Spike (g) | Mass of One piece of Grain (g) | MTS (g) | |
|---|---|---|---|---|---|---|---|
| DAI 7 | DAI 14 | ||||||
| SMH87 | Control | - | - | 12.3 ± 1.4 b | 0.328 ± 0.048 b | 0.050 ± 0.003 b | 36.437 ± 0.319 b |
| Inoculum | 10.1 ± 1.8 a | 34.7 ± 4.1 a | 2.0 ± 0.4 d | 0.055 ± 0.016 d | 0.012 ± 0.003 d | 15.603 ± 0.348 d | |
| Tamaroi | Control | - | - | 16.8 ± 1.2 a | 0.459 ± 0.047 a | 0.049 ± 0.005 b | 37.674 ± 1.077 b |
| Inoculum | 4.8 ± 0.9 b | 8.3 ± 1.1 b | 5.9 ± 1.0 d | 0.099 ± 0.019 d | 0.016 ± 0.004 d | 16.200 ± 1.853 d | |
| BC5Nax2 | Control | - | - | 13.6 ± 1.2 b | 0.355 ± 0.042 b | 0.061 ± 0.004 a | 51.763 ± 0.602 a |
| Inoculum | 3.0 ± 0.7 b | 10.7 ± 1.9 b | 10.9 ± 1.1 c | 0.209 ± 0.023 c | 0.028 ± 0.004 c | 28.209 ± 1.905 c | |
| Accession | Treatment | NIV | DON 1 | ZEN 2 | T-2 |
|---|---|---|---|---|---|
| SMH87 | Control | 0.098 ± 0.008 d | 0.093 ± 0.002 c | 0.008 ± 0.001 e | 0.101 ± 0.003 e |
| Inoculum | 1.785 ± 0.073 b | 5.763 ± 0.137 b | 0.002 ± 0.001 e | 0.166 ± 0.013 c | |
| Tamaroi | Control | 0.414 ± 0.021 c | 0.037 ± 0.003 c | 0.004 ± 0.001 d | 0.136 ± 0.006 d |
| Inoculum | 2.474 ± 0.109 a | 8.052 ± 0.373 a | 0.031 ± 0.001 b | 0.317 ± 0.010 a | |
| BC₅Nax₂ | Control | 0.406 ± 0.026 c | 0.047 ± 0.002 c | 0.022 ± 0.002 c | 0.126 ± 0.004 d |
| Inoculum | 1.765 ± 0.018 b | 6.208 ± 0.318 b | 0.138 ± 0.002 a | 0.221 ± 0.005 b |
| Variable | FHBi DAI 7 | FHBi DAI 14 | NIV | DON 1 | ZEN 2 | T–2 |
|---|---|---|---|---|---|---|
| Number of grain per spike | −0.591 | −0.575 | −0.503 | −0.549 | ns | ns |
| Mass of grain per spike [g] | −0.589 | −0.576 | −0.566 | −0.612 | ns | ns |
| Mass of a single grain [g] | −0.754 | −0.743 | −0.863 | −0.864 | −0.714 | ns |
| MTS [g] | −0.741 | −0.848 | −0.551 | −0.589 | ns | ns |
| Variable | NIV | DON 1 | ZEN 2 | T-2 Toxin |
|---|---|---|---|---|
| FHBi DAI 7 | 0.731 | 0.733 | 0.484 | ns |
| FHBi DAI 14 | 0.630 | 0.632 | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastuszak, J.; Szczerba, A.; Dziurka, M.; Hornyák, M.; Kopeć, P.; Szklarczyk, M.; Płażek, A. Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. Int. J. Mol. Sci. 2021, 22, 7433. https://doi.org/10.3390/ijms22147433
Pastuszak J, Szczerba A, Dziurka M, Hornyák M, Kopeć P, Szklarczyk M, Płażek A. Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. International Journal of Molecular Sciences. 2021; 22(14):7433. https://doi.org/10.3390/ijms22147433
Chicago/Turabian StylePastuszak, Jakub, Anna Szczerba, Michał Dziurka, Marta Hornyák, Przemysław Kopeć, Marek Szklarczyk, and Agnieszka Płażek. 2021. "Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage" International Journal of Molecular Sciences 22, no. 14: 7433. https://doi.org/10.3390/ijms22147433
APA StylePastuszak, J., Szczerba, A., Dziurka, M., Hornyák, M., Kopeć, P., Szklarczyk, M., & Płażek, A. (2021). Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. International Journal of Molecular Sciences, 22(14), 7433. https://doi.org/10.3390/ijms22147433








