TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Is Upregulated in Lymphocytes Stimulated with Concanavalin A
Abstract
:1. Introduction
2. Results
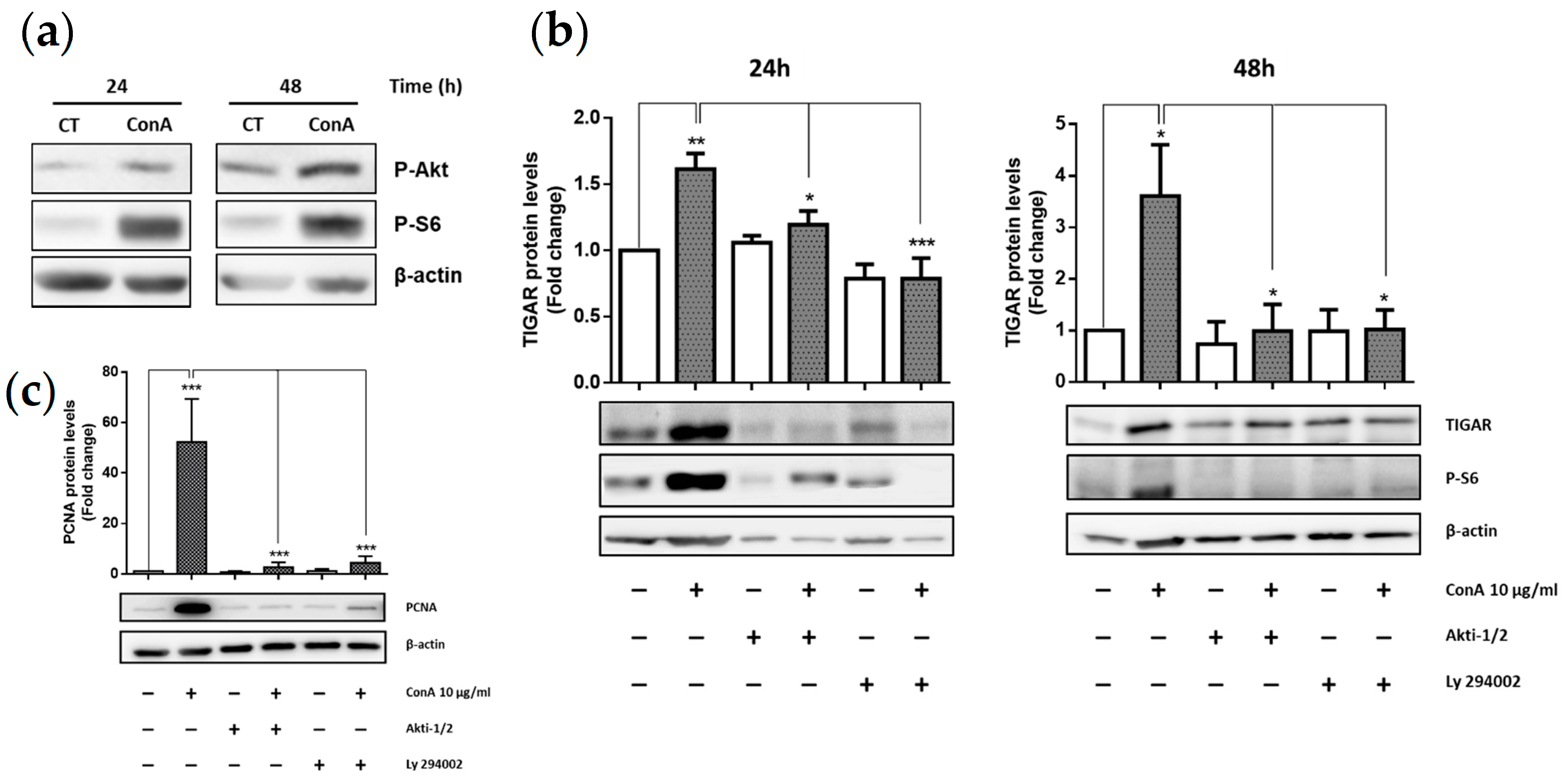
2.1. TIGAR Is Induced in ConA-Activated Human Lymphocytes
2.2. TIGAR Induction by ConA Is Mediated by PI3K/Akt Signaling Pathway
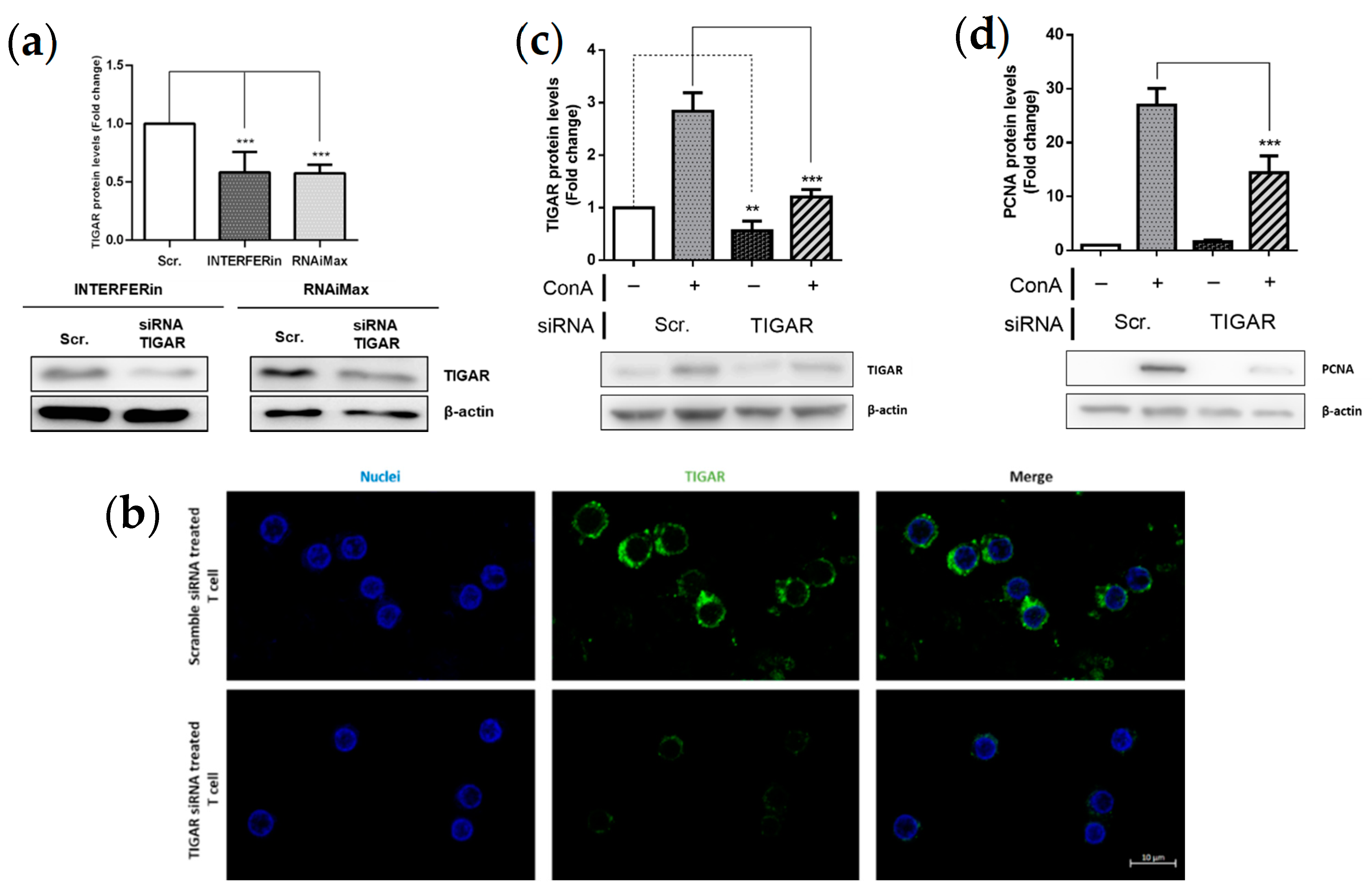
2.3. TIGAR Knockdown Decreases the PCNA Proliferation Marker in ConA-Stimulated Lymphocytes
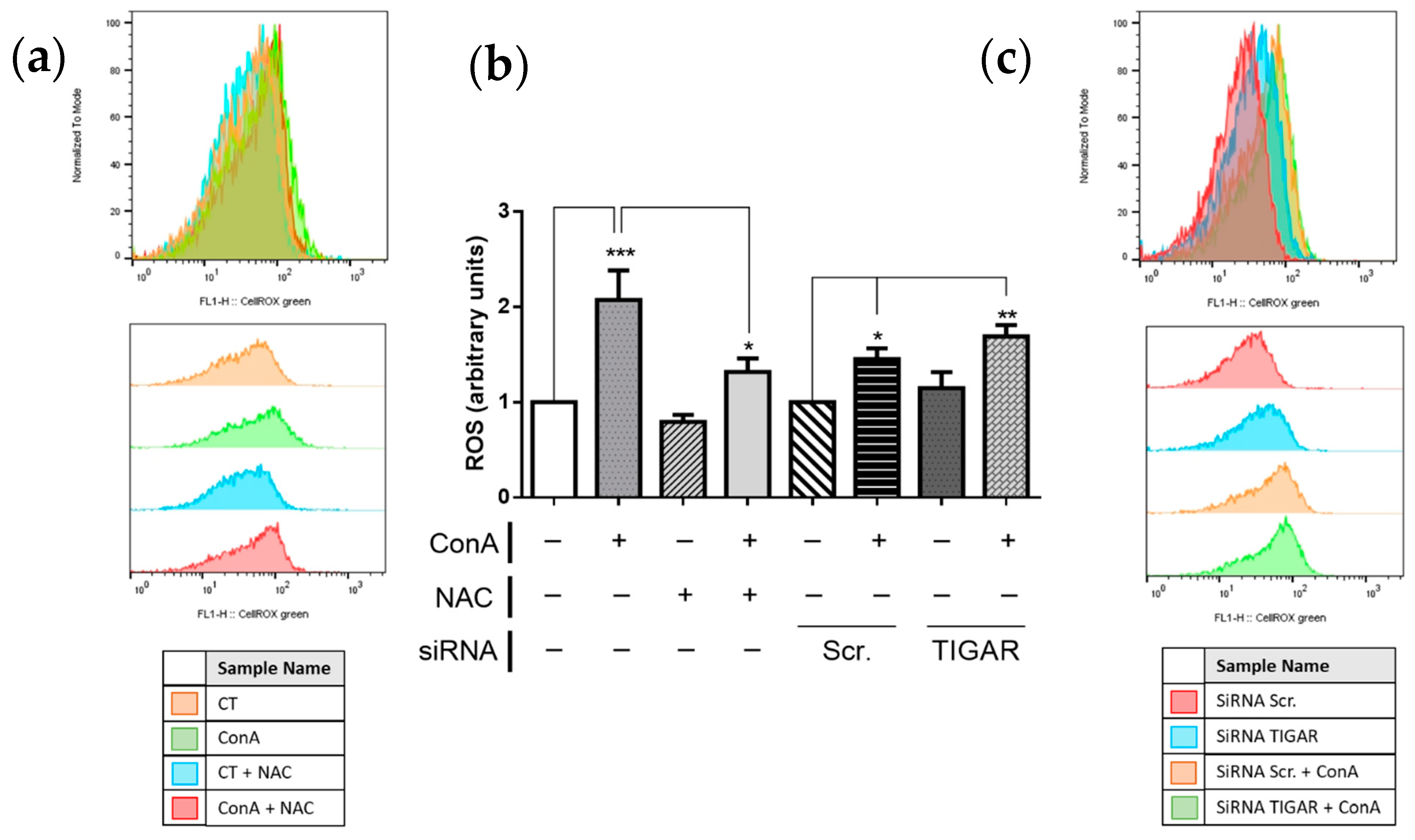
2.4. TIGAR Controls ROS Levels in ConA-Stimulated Lymphocytes
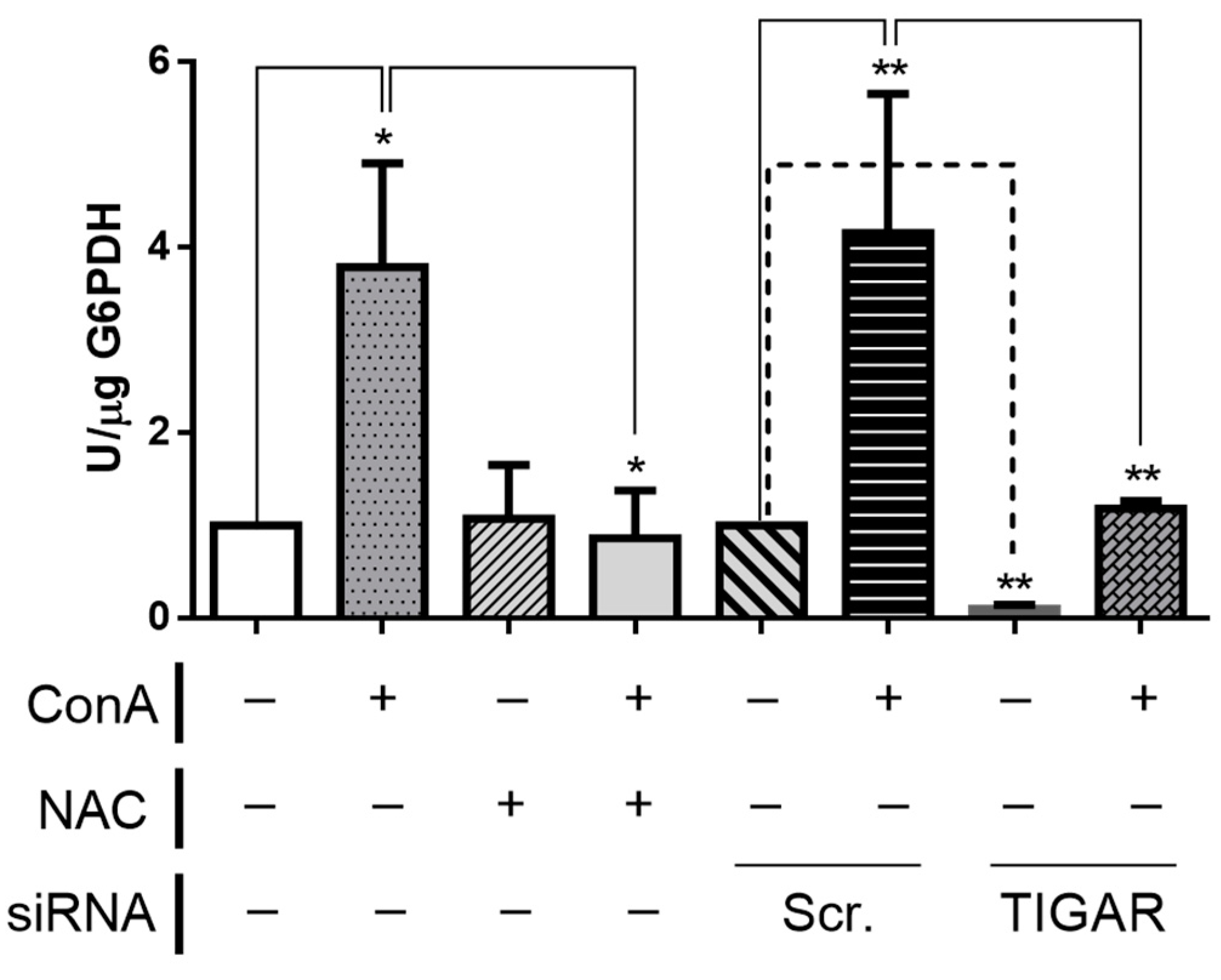
2.5. TIGAR Knockdown Results in Decreased Activity of G6PDH
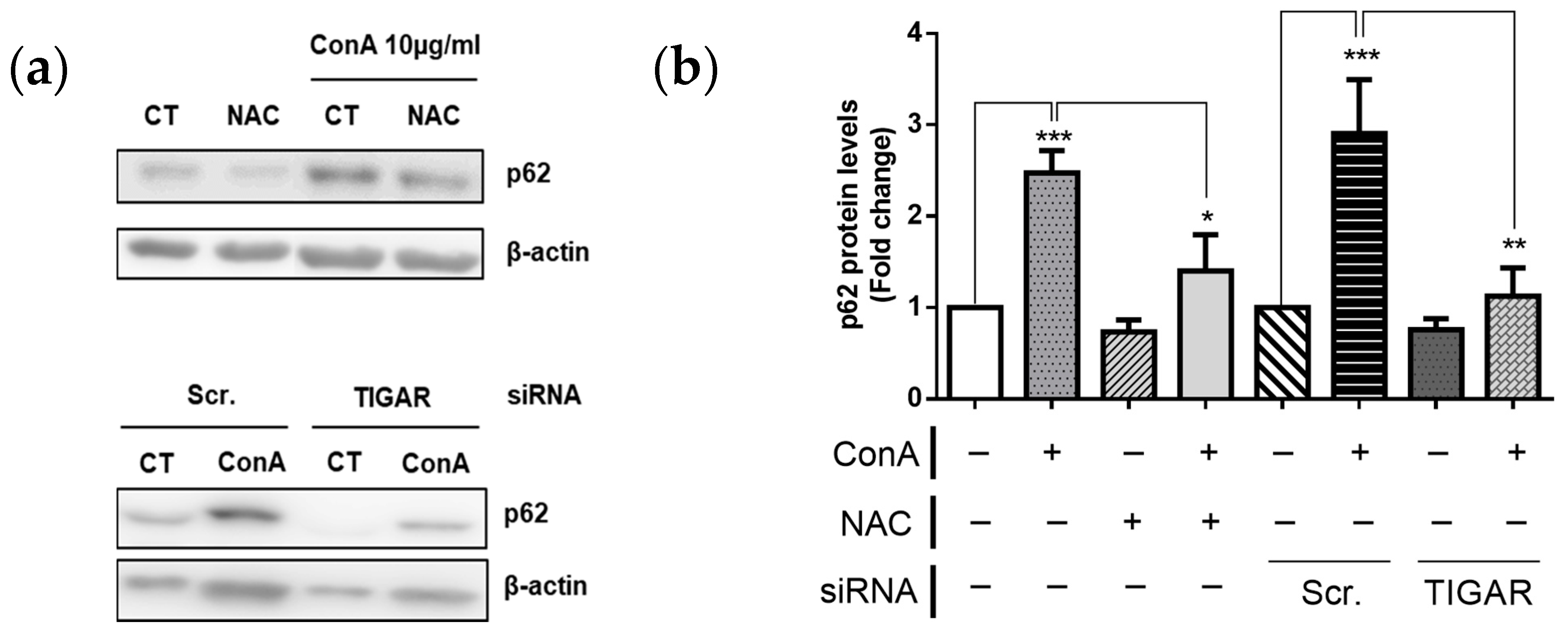
2.6. TIGAR Prevents Autophagy in ConA-Stimulated Lymphocytes
3. Discussion
4. Materials and Methods
4.1. Lymphocyte Sample Collection
4.2. Lymphocyte Purification
4.3. Lymphocyte Treatments and Sample Collection
4.4. Lymphocyte Transfection with siRNA
4.5. Inhibition of PI3K-Akt Signaling Pathway
4.6. Protein Extraction and Analysis
4.7. Metabolic Determinations
4.8. Immunohistochemistry
4.9. Redox Analysis
4.10. Statistics and Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dugnani, E.; Pasquale, V.; Bordignon, C.; Canu, A.; Piemonti, L.; Monti, P. Integrating T cell metabolism in cancer immunotherapy. Cancer Lett. 2017, 411, 12–18. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Park, B.V.; Pan, F. Metabolic regulation of T cell differentiation and function. Mol. Immunol. 2015, 68, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagman, J. Editorial overview: Lymphocyte development and activation: Lymphoid cell differentiation: Choosing paths to traverse the immune landscape. Curr. Opin. Immunol. 2016, 39, v–vii. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 2019, 20, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Riley, J.L.; Harris, M.H.; Parry, R.V.; Rathmell, J.C.; Plas, D.R.; Elstrom, R.L.; June, C.; Thompson, C.B. The CD28 Signaling Pathway Regulates Glucose Metabolism. Immunity 2002, 16, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Maciolek, J.A.; Pasternak, J.A.; Wilson, H.L. Metabolism of activated T lymphocytes. Curr. Opin. Immunol. 2014, 27, 60–74. [Google Scholar] [CrossRef]
- Bartrons, R.; Simon-Molas, H.; Rodríguez-García, A.; Castaño, E.; Navarro-Sabaté, À.; Manzano, A.; Martinez-Outschoorn, U.E. Fructose 2,6-Bisphosphate in Cancer Cell Metabolism. Front. Oncol. 2018, 8, 331. [Google Scholar] [CrossRef]
- Telang, S.; Clem, B.F.; Klarer, A.C.; Clem, A.L.; Trent, J.O.; Bucala, R.; Chesney, J. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J. Transl. Med. 2012, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Simon-Molas, H.; Arnedo-Pac, C.; Fontova, P.; Vidal-Alabró, A.; Castaño, E.; Rodríguez-García, A.; Navarro-Sabaté, À.; Lloberas, N.; Manzano, A.; Bartrons, R. PI3K–Akt signaling controls PFKFB3 expression during human T-lymphocyte activation. Mol. Cell. Biochem. 2018, 448, 187–197. [Google Scholar] [CrossRef]
- Houddane, A.; Bultot, L.; Novellasdemunt, L.; Johanns, M.; Gueuning, M.A.; Vertommen, D.; Coulie, P.G.; Bartrons, R.; Hue, L.; Rider, M.H. Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes. Cell. Signal. 2017, 34, 23–37. [Google Scholar] [CrossRef]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.C.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a p53-Inducible Regulator of Glycolysis and Apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.P.; Xie, J.M.; Li, B.; Sun, Y.H.; Gao, Q.G.; Ding, Z.H.; Wu, H.R.; Qin, Z.H. TIGAR regulates DNA damage and repair through pentosephosphate pathway and Cdk5-ATM pathway. Sci. Rep. 2015, 5, 9853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.; Vousden, K.H.; Cheung, E.C. TIGAR, TIGAR, burning bright. Cancer Metab. 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; He, Z. TIGAR promotes growth, survival and metastasis through oxidation resistance and AKT activation in glioblastoma. Oncol. Lett. 2019, 18, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Cha, Y.H.; Kim, H.S.; Kim, N.H.; Yook, J.I. The Pentose Phosphate Pathway as a Potential Target for Cancer Therapy. Biomol. Ther. 2018, 26, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Cheung, E.C.; Athineos, D.; Lee, P.; Ridgway, R.A.; Lambie, W.; Nixon, C.; Strathdee, D.; Blyth, K.; Sansom, O.J.; Vousden, K.H. TIGAR Is Required for Efficient Intestinal Regeneration and Tumorigenesis. Dev. Cell 2013, 25, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Iqbal, M.A.; Singh, R.K.; Bamezai, R.N. Resveratrol inhibits TIGAR to promote ROS induced apoptosis and autophagy. Biochimie 2015, 118, 26–35. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Jiang, L.B.; Cao, L.; Ma, Y.Q.; Chen, Q.; Liang, Y.; Yuan, F.L.; Li, X.L.; Dong, J.; Chen, N. TIGAR mediates the inhibitory role of hypoxia on ROS production and apoptosis in rat nucleus pulposus cells. Osteoarthr. Cartil. 2018, 26, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Z.; Han, X.; Chen, D.; Xu, Y.; Xu, L.; Yin, L.; Sun, H.; Qi, Y.; Fang, L.; Liu, K.; et al. Potent effects of dioscin against hepatocellular carcinoma through regulating TP53-induced glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis, autophagy, and DNA damage. Br. J. Pharmacol. 2019, 176, 919–937. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.-M.; Li, B.; Yu, H.-P.; Gao, Q.-G.; Li, W.; Wu, H.-R.; Qin, Z.-H. TIGAR Has a Dual Role in Cancer Cell Survival through Regulating Apoptosis and Autophagy. Cancer Res. 2014, 74, 5127–5138. [Google Scholar] [CrossRef] [Green Version]
- Simon-Molas, H.; Calvo-Vidal, M.N.; Castaño, E.; Rodríguez-García, A.; Navarro-Sabaté, À.; Bartrons, R.; Manzano, A. Akt mediates TIGAR induction in HeLa cells following PFKFB3 inhibition. FEBS Lett. 2016, 590, 2915–2926. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef] [Green Version]
- Bensaad, K.; Cheung, E.C.; Vousden, K.H. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009, 28, 3015–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, H.; Yamada, Y.; Iha, H.; Tsukasaki, K.; Nagai, K.; Atogami, S.; Sugahara, K.; Tsuruda, K.; Ishizaki, A.; Kamihira, S. Activation of p53 by Nutlin-3a, an antagonist of MDM2, induces apoptosis and cellular senescence in adult T-cell leukemia cells. Leukemia 2009, 23, 2090–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, T.; Malu, A.; Yapindi, L.; Bergeson, R.; Peck, K.; Romeo, M.; Harrod, C.; Pope, J.; Smitherman, L.; Gwinn, W.; et al. The TP53-Induced Glycolysis and Apoptosis Regulator mediates cooperation between HTLV-1 p30II and the retroviral oncoproteins Tax and HBZ and is highly expressed in an in vivo xenograft model of HTLV-1-induced lymphoma. Virology 2018, 520, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayal, K.; Abdulla, M.; Al-Obeed, O.; AL Kattan, W.; Zubaidi, A.; Vaali-Mohammed, M.A.; Alsheikh, A.; Ahmad, R. Identification of the TP53-induced glycolysis and apoptosis regulator in various stages of colorectal cancer patients. Oncol. Rep. 2016, 35, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; DeNicola, G.M.; Nixon, C.; Blyth, K.; Labuschagne, C.F.; Tuveson, D.A.; Vousden, K.H. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 2020, 37, 168–182. [Google Scholar] [CrossRef] [Green Version]
- Peña-Rico, M.A.; Calvo-Vidal, M.N.; Villalonga-Planells, R.; Martínez-Soler, F.; Giménez-Bonafé, P.; Navarro-Sabaté, A.; Tortosa, A.; Bartrons, R.; Manzano, A. TP53 induced glycolysis and apoptosis regulator (TIGAR) knockdown results in radiosensitization of glioma cells. Radiother. Oncol. 2011, 101, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, G.D.; Heller, S.; Wanka, C.; Rieger, J.; Steinbach, J.P. Knockdown of the TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Sensitizes Glioma Cells to Hypoxia, Irradiation and Temozolomide. Int. J. Mol. Sci. 2019, 20, 1061. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.H.; Domingo-Vidal, M.; Roche, M.; Lin, Z.; Whitaker-Menezes, D.; Seifert, E.; Capparelli, C.; Tuluc, M.; Birbe, R.C.; Tassone, P.; et al. TP53-inducible Glycolysis and Apoptosis Regulator (TIGAR) Metabolically Reprograms Carcinoma and Stromal Cells in Breast Cancer. J. Biol. Chem. 2016, 291, 26291–26303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.P.; Siegel, R.M. Mitochondrial ROS Fire up T Cell Activation. Immunity 2013, 38, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.O.J.; Liener, I.E. Some Physical and Chemical Properties of Concanavalin A, the Phytohemagglutinin of the Jack Bean. Biochemistry 1967, 6, 105–111. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon-Molas, H.; Vallvé-Martínez, X.; Caldera-Quevedo, I.; Fontova, P.; Arnedo-Pac, C.; Vidal-Alabró, A.; Castaño, E.; Navarro-Sabaté, À.; Lloberas, N.; Bartrons, R.; et al. TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Is Upregulated in Lymphocytes Stimulated with Concanavalin A. Int. J. Mol. Sci. 2021, 22, 7436. https://doi.org/10.3390/ijms22147436
Simon-Molas H, Vallvé-Martínez X, Caldera-Quevedo I, Fontova P, Arnedo-Pac C, Vidal-Alabró A, Castaño E, Navarro-Sabaté À, Lloberas N, Bartrons R, et al. TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Is Upregulated in Lymphocytes Stimulated with Concanavalin A. International Journal of Molecular Sciences. 2021; 22(14):7436. https://doi.org/10.3390/ijms22147436
Chicago/Turabian StyleSimon-Molas, Helga, Xavier Vallvé-Martínez, Irene Caldera-Quevedo, Pere Fontova, Claudia Arnedo-Pac, Anna Vidal-Alabró, Esther Castaño, Àurea Navarro-Sabaté, Núria Lloberas, Ramon Bartrons, and et al. 2021. "TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Is Upregulated in Lymphocytes Stimulated with Concanavalin A" International Journal of Molecular Sciences 22, no. 14: 7436. https://doi.org/10.3390/ijms22147436
APA StyleSimon-Molas, H., Vallvé-Martínez, X., Caldera-Quevedo, I., Fontova, P., Arnedo-Pac, C., Vidal-Alabró, A., Castaño, E., Navarro-Sabaté, À., Lloberas, N., Bartrons, R., & Manzano, A. (2021). TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Is Upregulated in Lymphocytes Stimulated with Concanavalin A. International Journal of Molecular Sciences, 22(14), 7436. https://doi.org/10.3390/ijms22147436





