Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer
Abstract
:1. Introduction
2. Results
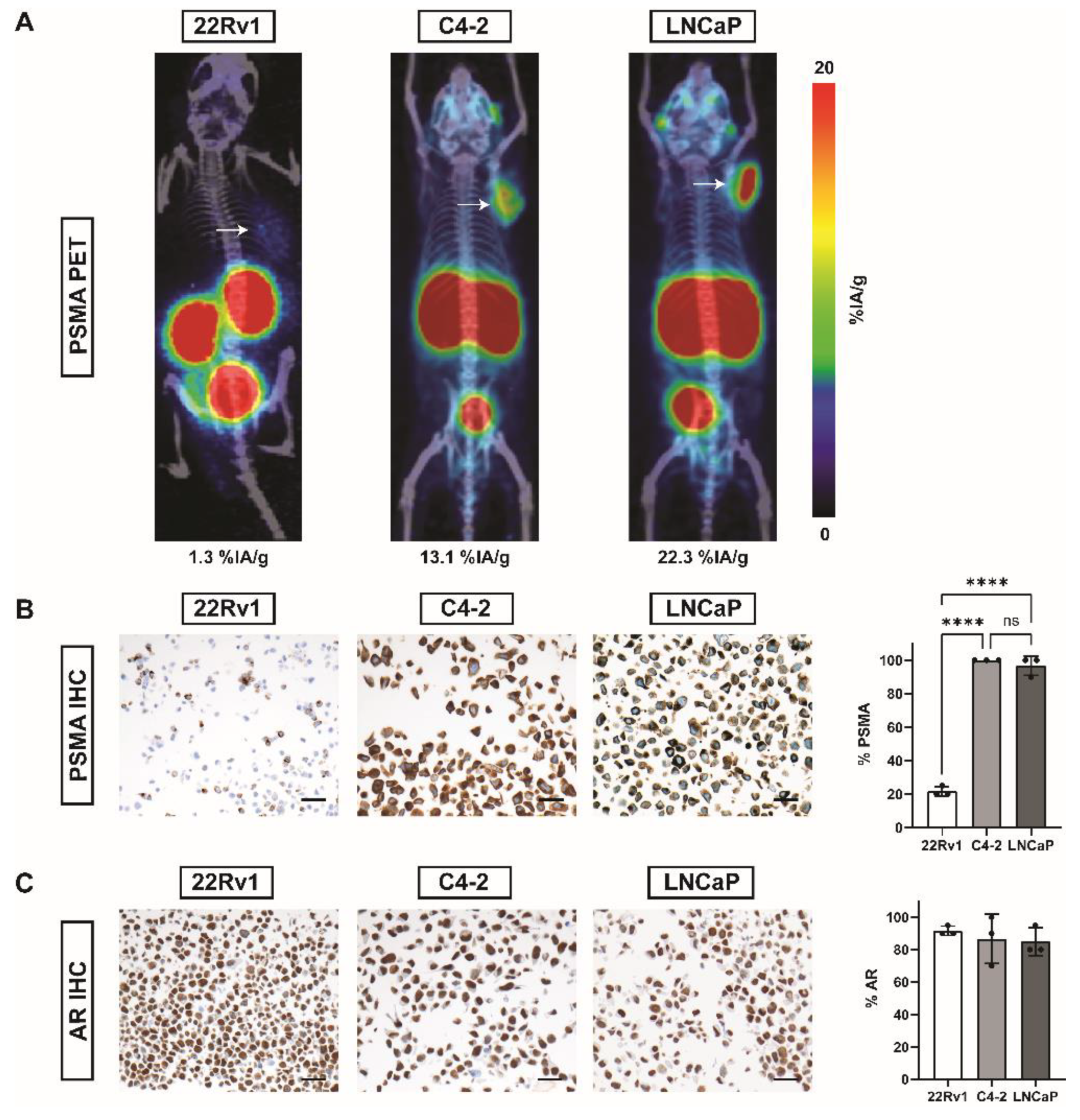
2.1. PSMA and AR Expression in Three Different PC Cell Lines
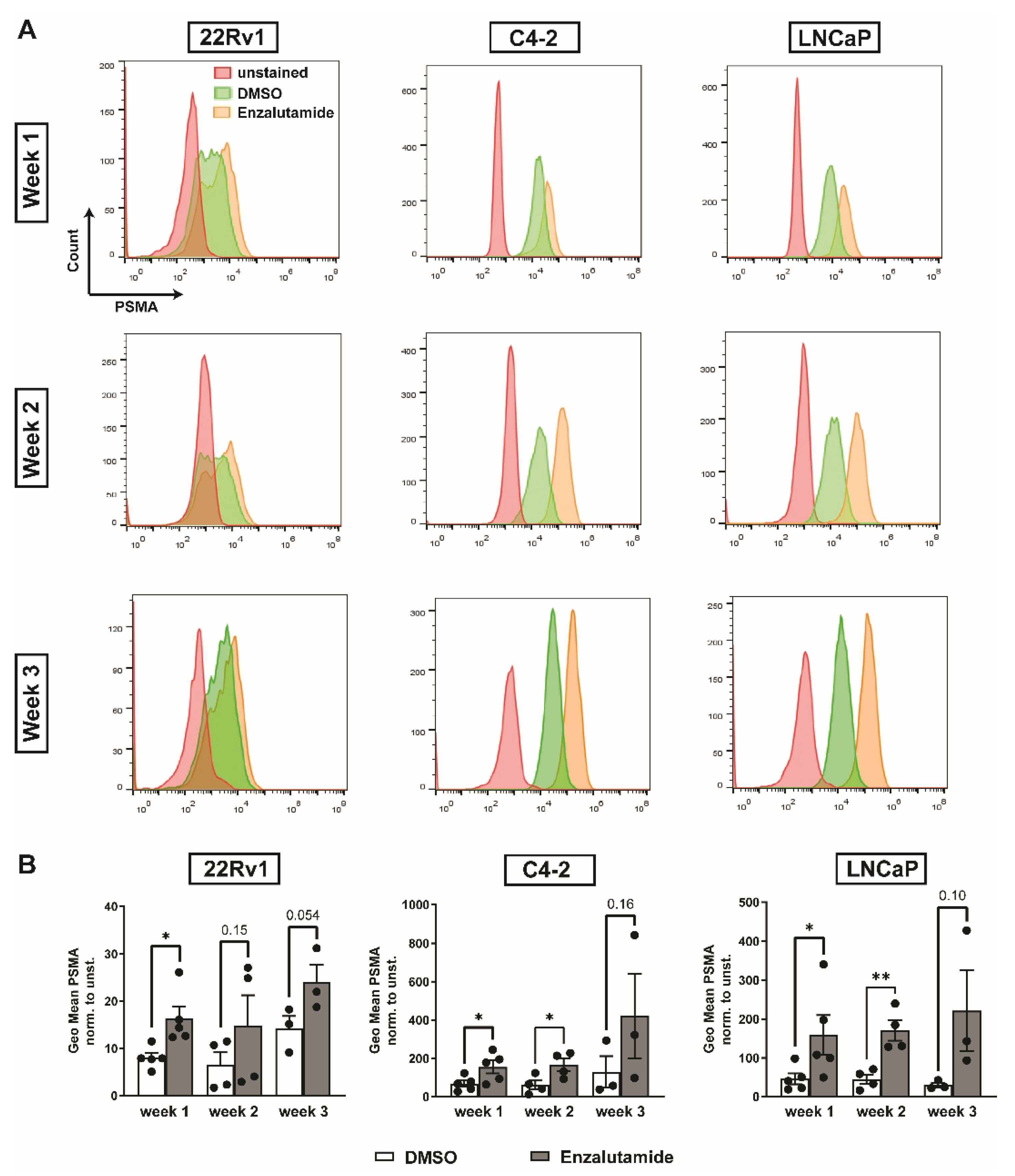
2.2. Enzalutamide Increases PSMA Expression in Three Different PC Cell Lines
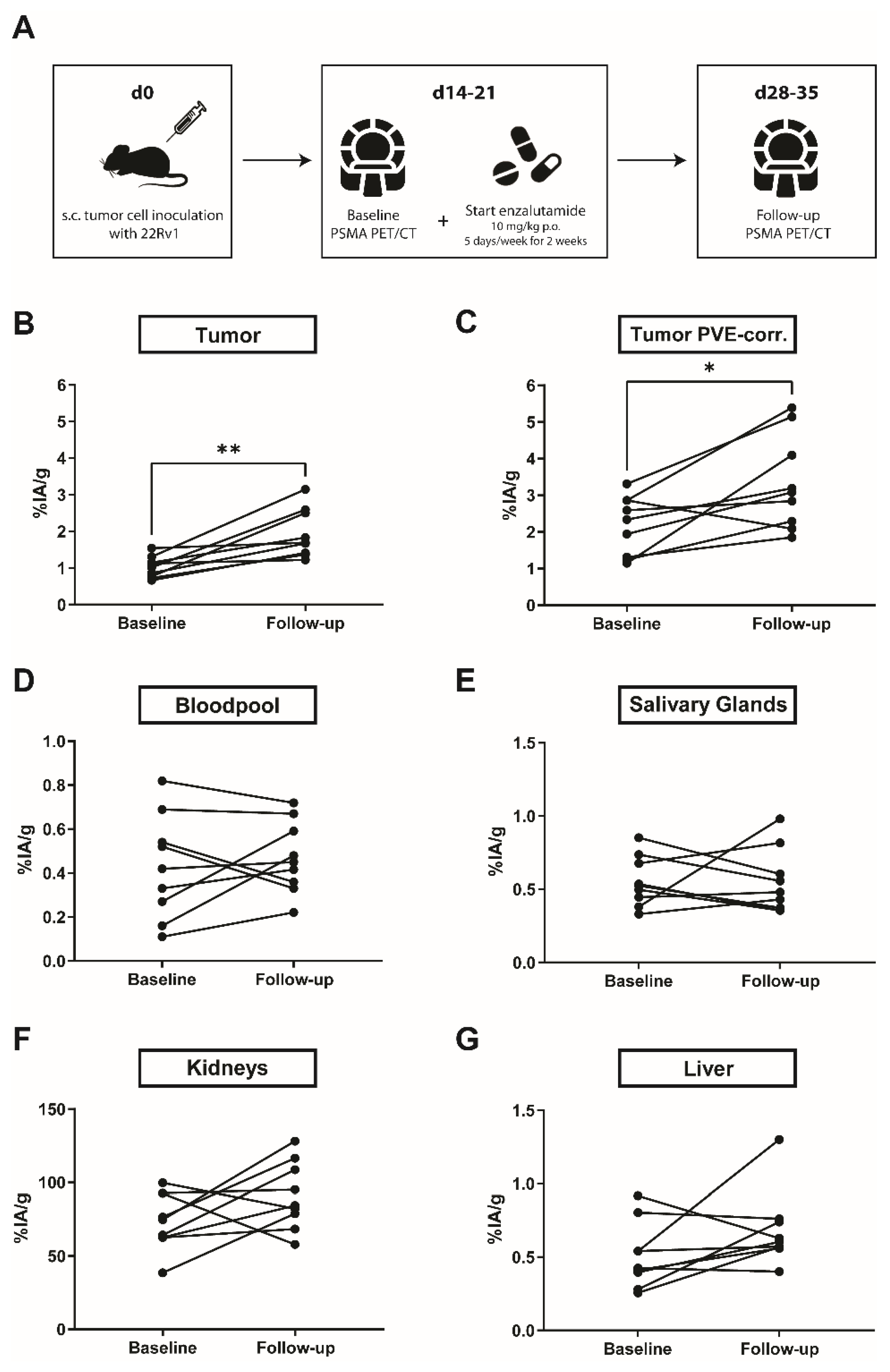
2.3. PSMA Expression in 22Rv1 Xenografts
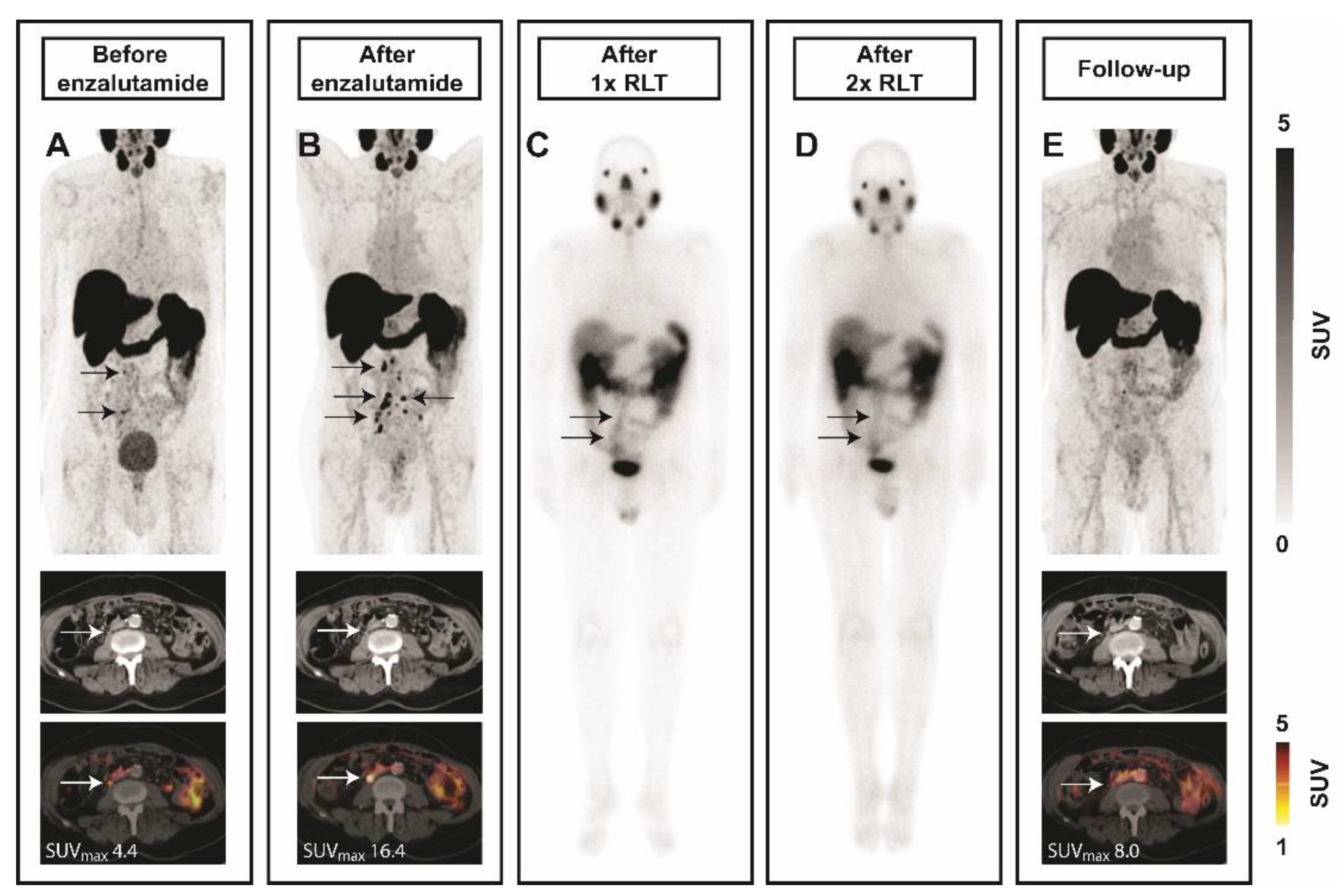
2.4. Enzalutamide Increases 68Ga-PSMA-11 Uptake in a Patient with mCRPC
3. Discussion
Limitations
4. Materials and Methods
4.1. Cell Culture
4.2. Animals and ARB
4.3. PET/CT
4.4. Flow Cytometry
4.5. IHC
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujita, K.; Nonomura, N. Role of androgen receptor in prostate cancer: A review. World J. Mens Health 2019, 3, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Iking, J.; Lückerath, K.; Hadaschik, B.; Herrmann, K.; Ferdinandus, J.; Fendler, W.P. Drug and molecular radiotherapy combinations for metastatic castration resistant prostate cancer. Nucl. Med. Biol. 2021, 96–97, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.-S.; Kimura, G.; et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: Extended analysis of the phase 3 PREVAIL study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Ping Thang, S.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German multicenter study investigating 177Lu-PSMA-617 Radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef]
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase III VISION trial and its importance for the future of theranostics. J. Nucl. Med. 2019, 60, 1504–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafita, A.; Calais, J.; Grogan, T.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcome after LuPSMA radionuclide therapy in men with metastatic castration-resistant prostate cancer: An international multicenter retrospective study. Lancet Oncol. 2021. (In press) [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 1–13. [Google Scholar] [CrossRef]
- Evans, M.J.; Smith-Jones, P.M.; Wongvipat, J.; Navarro, V.; Kim, S.; Bander, N.H.; Larson, S.M.; Sawyers, C.L. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl. Acad. Sci. USA 2011, 108, 9578–9582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murga, J.D.; Moorji, S.M.; Han, A.Q.; Magargal, W.W.; DiPippo, V.A.; Olson, W.C. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate 2015, 75, 242–254. [Google Scholar] [CrossRef] [PubMed]
- DiPippo, V.A.; Nguyen, H.M.; Brown, L.G.; Olson, W.C.; Vessella, R.L.; Corey, E. Addition of PSMA ADC to enzalutamide therapy significantly improves survival in in vivo model of castration resistant prostate cancer. Prostate 2016, 76, 325–334. [Google Scholar] [CrossRef]
- Hope, T.A.; Truillet, C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: First human experience. J. Nucl. Med. 2017, 58, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Lückerath, K.; Wei, L.; Fendler, W.P.; Evans-Axelsson, S.; Stuparu, A.D.; Slavik, R.; Mona, C.E.; Calais, J.; Rettig, M.; Reiter, R.E.; et al. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Current, K.; Meyer, C.; Magyar, C.E.; Mona, C.E.; Almajano, J.; Slavik, R.; Stuparu, A.D.; Cheng, C.; Dawson, D.W.; Radu, C.G.; et al. Investigating PSMA-targeted radioligand therapy efficacy as a function of cellular PSMA levels and intratumoral PSMA heterogeneity. Clin. Cancer Res. 2020, 26, 2946–2955. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Ravi Kumar, A.S.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid modulation of PSMA expression by androgen deprivation: Serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.L.; Grob, B.M.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen- deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Heston, W.D.W. Prostate specific membrane antigen. In Prostate Cancer; Contemporary Cancer Research; Chung, L.W.K., Isaacs, W.B., Simons, J.W., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 307–326. [Google Scholar]
- Bakht, M.K.; Oh, S.W.; Youn, H.; Cheon, G.J.; Kwak, C.; Kang, K.W. Influence of androgen deprivation therapy on the uptake of PSMA-targeted agents: Emerging opportunities and challenges. Nucl. Med. Mol. Imaging 2017, 51, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Heston, W.D.W. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; White, M.E.; Basuli, F.; Opina, A.C.L.; Wong, K.; Riba, M.; Ton, A.T.; Zhang, X.; Jansson, K.H.; Edmondson, E.; et al. Monitoring PSMA responses to ADT in prostate cancer patient-derived xenograft mouse models using [18 F] DCFPyL PET imaging. Mol. Imaging Biol. 2021. (Ahead of printing). [Google Scholar] [CrossRef]
- Yao, V.; Bacich, D.J. Prostate Specific Membrane Antigen (PSMA) Expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. Prostate 2006, 66, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Caromile, L.A.; Shapiro, L.H. PSMA redirects MAPK to PI3K-AKT signaling to promote prostate cancer progression. Mol. Cell. Oncol. 2017, 4, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Colombatti, M.; Grasso, S.; Porzia, A.; Fracasso, G.; Scupoli, M.T.; Cingarlini, S.; Poffe, O.; Naim, H.Y.; Heine, M.; Tridente, G.; et al. The prostate specific membrane antigen regulates the expression of IL-6 and CCL5 in prostate tumour cells by activating the MAPK pathways. PLoS ONE 2009, 4, e4608. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga-and 177Lu-labeled PSMA i and T: Optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniszewska, M.; Fragoso Costa, P.; Eiber, M.; Klose, J.M.; Wosniack, J.; Reis, H.; Szarvas, T.; Hadaschik, B.; Lückerath, K.; Herrmann, K.; et al. Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 7431. https://doi.org/10.3390/ijms22147431
Staniszewska M, Fragoso Costa P, Eiber M, Klose JM, Wosniack J, Reis H, Szarvas T, Hadaschik B, Lückerath K, Herrmann K, et al. Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. International Journal of Molecular Sciences. 2021; 22(14):7431. https://doi.org/10.3390/ijms22147431
Chicago/Turabian StyleStaniszewska, Magdalena, Pedro Fragoso Costa, Matthias Eiber, Jasmin M. Klose, Jasmin Wosniack, Henning Reis, Tibor Szarvas, Boris Hadaschik, Katharina Lückerath, Ken Herrmann, and et al. 2021. "Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer" International Journal of Molecular Sciences 22, no. 14: 7431. https://doi.org/10.3390/ijms22147431
APA StyleStaniszewska, M., Fragoso Costa, P., Eiber, M., Klose, J. M., Wosniack, J., Reis, H., Szarvas, T., Hadaschik, B., Lückerath, K., Herrmann, K., Fendler, W. P., & Iking, J. (2021). Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. International Journal of Molecular Sciences, 22(14), 7431. https://doi.org/10.3390/ijms22147431







