Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Question
2.2. Inclusion and Exclusion Criteria
2.3. The PICO Approach
2.4. Search Strategy
2.5. Cohen’s Kappa Coefficient
3. Results and Discussion
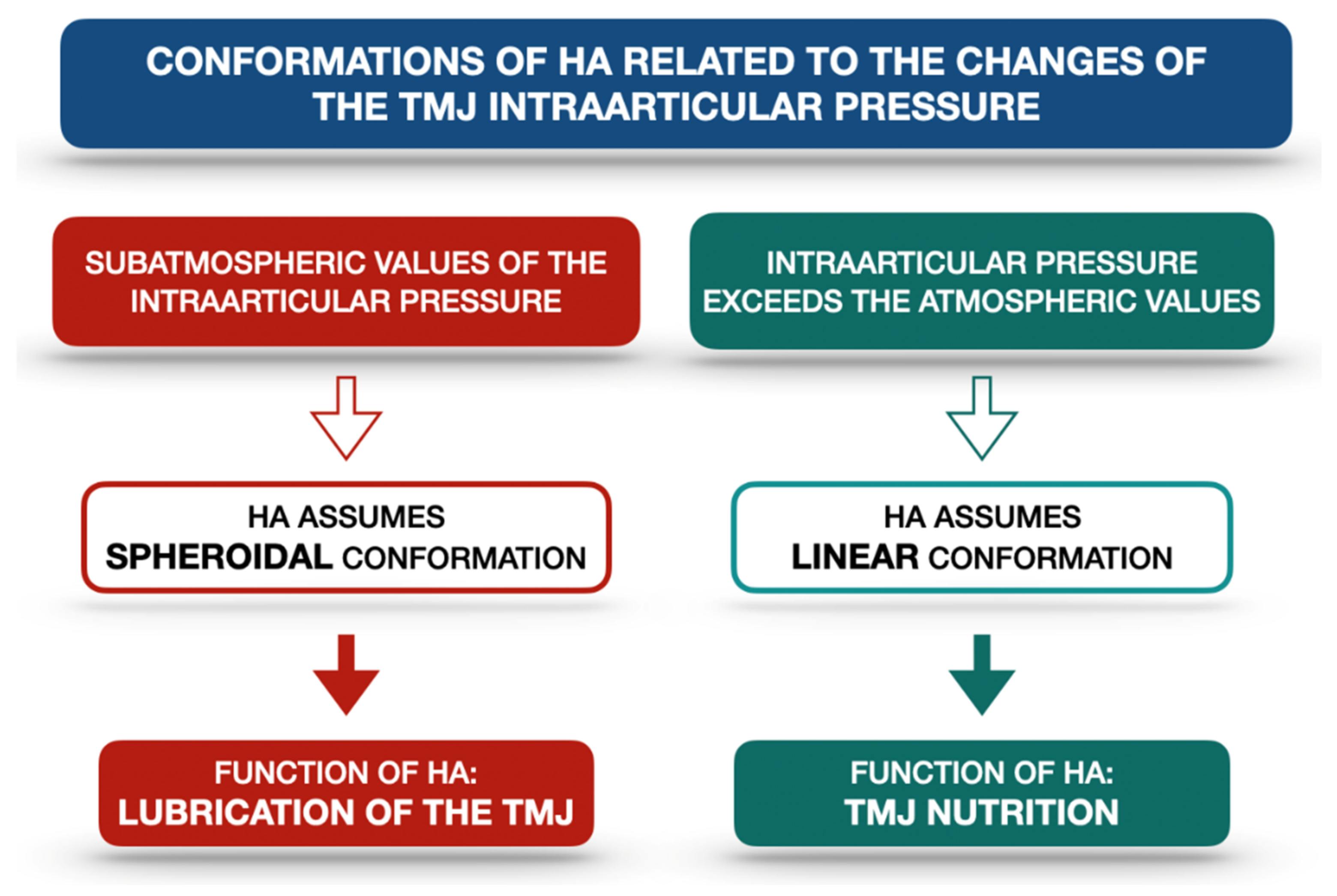
3.1. Hyaluronic Acid (HA)
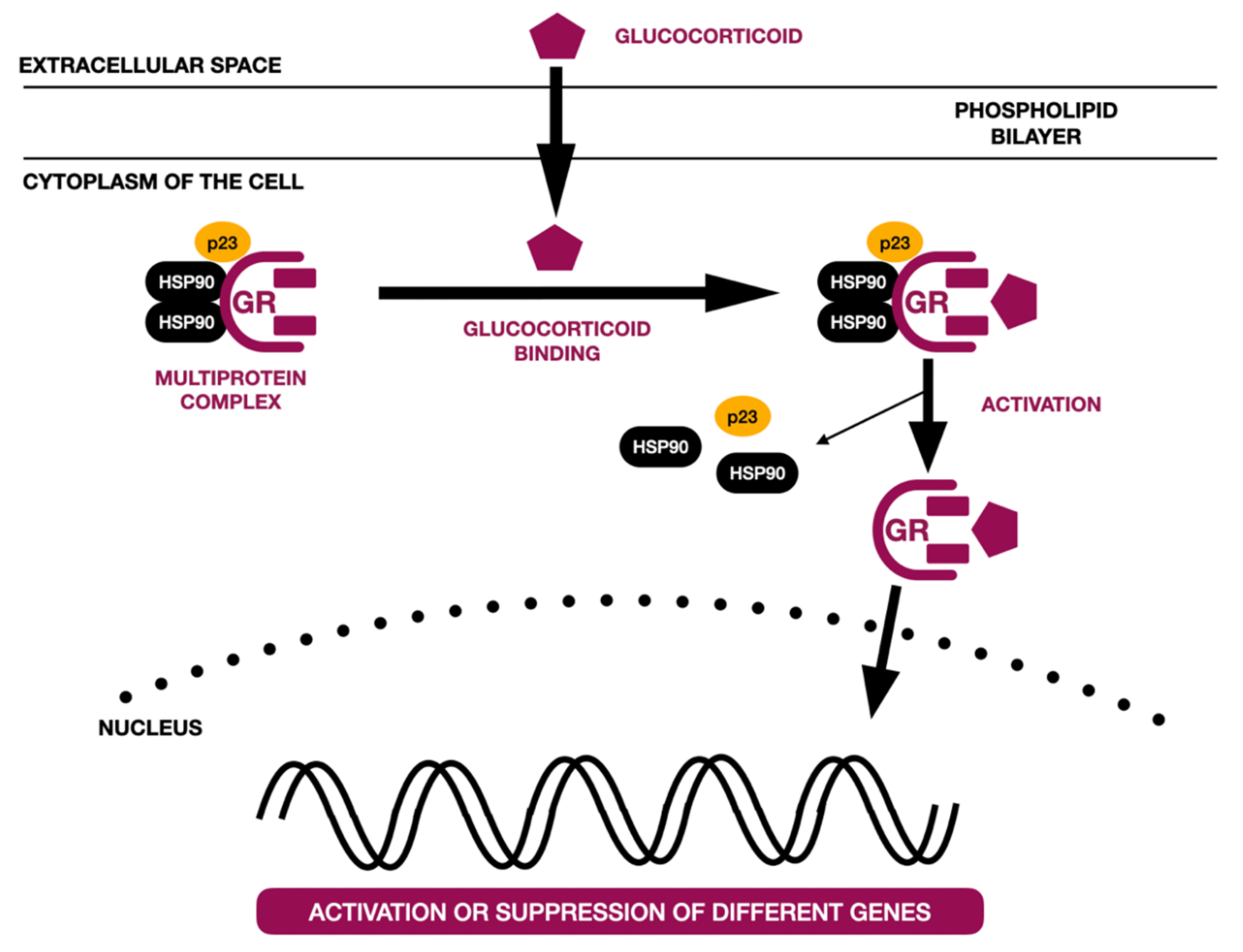
3.2. Corticosteroids (CS)
3.3. Platelet-Rich Plasma (PRP)
3.4. HA, CS and PRP in the Treatment of TMJ OA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G.; Abramowicz, S. Arthritic Conditions Affecting the Temporomandibular Joint. In Contemporary Oral Medicine; Farah, C., Balasubramaniam, R., McCullough, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Orally Administered NSAIDs-General Characteristics and Usage in the Treatment of Temporomandibular Joint Osteoarthritis-A Narrative Review. Pharmaceuticals 2021, 14, 219. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Interdisciplinary Approach to the Temporomandibular Joint Osteoarthritis-Review of the Literature. Medicina 2020, 56, 225. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Al-Moraissi, E.A.; Wolford, L.M.; Ellis E 3rd Neff, A. The hierarchy of different treatments for arthrogenous temporomandibular disorders: A network meta-analysis of randomized clinical trials. J. Craniomaxillofac. Surg. 2020, 48, 9–23. [Google Scholar] [CrossRef]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Seror, J.; Merkherm, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular cartilage proteoglycans as boundary lubricants: Structure and frictional interaction of surface-attached hyaluronan and hyaluronan—Aggrecan complexes. Biomacromolecules 2011, 12, 3432–3443. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Masiero, S.; Marioni, G. Conservative treatment of temporomandibular joint osteoarthrosis: Intra-articular injection of sodium hyaluronate. J. Oral Rehabil. 2005, 32, 729–734. [Google Scholar] [CrossRef]
- Cascone, P.; Fonzi Dagger, L.; Aboh, I.V. Hyaluronic acid’s biomechanical stabilization function in the temporomandibular joint. J. Craniofac. Surg. 2002, 13, 751–754. [Google Scholar] [CrossRef]
- Manfredini, D.; Piccotti, F.; Guarda-Nardini, L. Hyaluronic acid in the treatment of TMJ disorders: A systematic review of the literature. Cranio 2010, 28, 166–176. [Google Scholar] [CrossRef]
- Ferreira, N.; Masterson, D.; Lopes de Lima, R.; de Souza Moura, B.; Oliveira, A.T.; Kelly da Silva Fidalgo, T.; Carvalho, A.C.P.; DosSantos, M.F.; Grossmann, E. Efficacy of viscosupplementation with hyaluronic acid in temporomandibular disorders: A systematic review. J. Craniomaxillofac. Surg. 2018, 46, 1943–1952. [Google Scholar] [CrossRef]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef]
- Shinohara, T.; Izawa, T.; Mino-Oka, A.; Mori, H.; Iwasa, A.; Inubushi, T.; Yamaguchi, Y.; Tanaka, E. Hyaluronan metabolism in overloaded temporomandibular joint. J. Oral Rehabil. 2016, 43, 921–928. [Google Scholar] [CrossRef]
- Siiskonen, H.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K. Hyaluronan synthase 1: A mysterious enzyme with unexpected functions. Front. Immunol. 2015, 6, 43. [Google Scholar] [CrossRef]
- Faust, H.J.; Sommerfeld, S.D.; Rathod, S.; Rittenbach, A.; Ray Banerjee, S.; Tsui, B.M.W.; Pomper, M.; Amzel, M.L.; Singh, A.; Elisseeff, J.H. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 2018, 183, 93–101. [Google Scholar] [CrossRef]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef]
- Zhu, L.; Seror, J.; Day, A.J.; Kampf, N.; Klein, J. Ultra-low friction between boundary layers of hyaluronan-phosphatidylcholine complexes. Acta Biomater. 2017, 59, 283–292. [Google Scholar] [CrossRef]
- Das, S.; Banquy, X.; Zappone, B.; Greene, G.W.; Jay, G.D.; Israelachvili, J.N. Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules 2013, 14, 1669–1677. [Google Scholar] [CrossRef]
- Iturriaga, V.; Vásquez, B.; Bornhardt, T.; Del Sol, M. Effects of low and high molecular weight hyaluronic acid on the osteoarthritic temporomandibular joint in rabbit. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Nastasi, G.; Micali, A.; Prestipino, V.; Vaccaro, M.; D’Ascola, A.; Calatroni, A.; Campo, S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta 2011, 1812, 1170–1181. [Google Scholar] [CrossRef]
- Herzog, M.; Li, L.; Galla, H.J.; Winter, R. Effect of hyaluronic acid on phospholipid model membranes. Colloids Surf. B Biointerfaces 2019, 173, 327–334. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Greenwald, R.A.; Moy, W.W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980, 23, 455–463. [Google Scholar] [CrossRef]
- Takahashi, T.; Tominaga, K.; Takano, H.; Ariyoshi, W.; Habu, M.; Fukuda, J.; Maeda, H. A decrease in the molecular weight of hyaluronic acid in synovial fluid from patients with temporomandibular disorders. J. Oral Pathol. Med. 2004, 33, 224–229. [Google Scholar] [CrossRef]
- Guo, X.; Watari, I.; Ikeda, Y.; Yang, W.; Ono, T. Effect of functional lateral shift of the mandible on hyaluronic acid metabolism related to lubrication of temporomandibular joint in growing rats. Eur. J. Orthod. 2020, 42, 658–663. [Google Scholar] [CrossRef]
- Meszaros, M.; Kis, A.; Kunos, L.; Tarnoki, A.D.; Tarnoki, D.L.; Lazar, Z.; Bikov, A. The role of hyaluronic acid and hyaluronidase-1 in obstructive sleep apnoea. Sci. Rep. 2020, 10, 19484. [Google Scholar] [CrossRef] [PubMed]
- Tolba, Y.M.; Omar, S.S.; Nagui, D.A.; Nawwar, M.A. Effect of high molecular weight hyaluronic acid in treatment of osteoarthritic temporomandibular joints of rats. Arch. Oral Biol. 2020, 110, 104618. [Google Scholar] [CrossRef] [PubMed]
- Duygu, G.; Güler, N.; Cam, B.; Kürkçü, M. The effects of high molecular weight hyaluronic acid (Hylan G-F 20) on experimentally induced temporomandibular joint osteoartrosis: Part II. Int. J. Oral Maxillofac. Surg. 2011, 40, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Lemos, G.A.; Rissi, R.; Pimentel, E.R.; Palomari, E.T. Effects of high molecular weight hyaluronic acid on induced arthritis of the temporomandibular joint in rats. Acta Histochem. 2015, 117, 566–575. [Google Scholar] [CrossRef]
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef]
- Freire, V.; Bureau, N.J. Injectable Corticosteroids: Take Precautions and Use Caution. Semin. Musculoskelet. Radiol. 2016, 20, 401–408. [Google Scholar] [CrossRef]
- Yaftali, N.A.; Weber, K. Corticosteroids and Hyaluronic Acid Injections. Clin. Sports Med. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- MacMahon, P.J.; Eustace, S.J.; Kavanagh, E.C. Injectable corticosteroid and local anesthetic preparations: A review for radiologists. Radiology 2009, 252, 647–661. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef]
- Barnes, P.J. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. (Lond.) 1998, 94, 557–572. [Google Scholar] [CrossRef]
- Ingawale, D.K.; Mandlik, S.K. New insights into the novel anti-inflammatory mode of action of glucocorticoids. Immunopharmacol. Immunotoxicol. 2020, 42, 59–73. [Google Scholar] [CrossRef]
- Quatrini, L.; Ugolini, S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell. Mol. Immunol. 2021, 18, 269–278. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Tuckermann, J.; Libert, C. New insights into the anti-inflammatory mechanisms of glucocorticoids: An emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology 2013, 154, 993–1007. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Schaafsma, D.; Amrani, Y.; Koziol-White, C.; Ostrom, R.; Tliba, O. Non-genomic Effects of Glucocorticoids: An Updated View. Trends Pharmacol. Sci. 2019, 40, 38–49. [Google Scholar] [CrossRef]
- Buttgereit, F. Glucocorticoids: Surprising new findings on their mechanisms of actions. Ann. Rheum. Dis. 2021, 80, 137–139. [Google Scholar] [CrossRef]
- Kondo, T.; Kitazawa, R.; Yamaguchi, A.; Kitazawa, S. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J. Cell. Biochem. 2008, 103, 335–345. [Google Scholar] [CrossRef]
- Monseau, A.J.; Nizran, P.S. Common injections in musculoskeletal medicine. Prim. Care 2013, 40, 987–1000. [Google Scholar] [CrossRef]
- Céleste, C.; Ionescu, M.; Robin Poole, A.; Laverty, S. Repeated intraarticular injections of triamcinolone acetonide alter cartilage matrix metabolism measured by biomarkers in synovial fluid. J. Orthop. Res. 2005, 23, 602–610. [Google Scholar] [CrossRef]
- Murray, R.C.; DeBowes, R.M.; Gaughan, E.M.; Zhu, C.F.; Athanasiou, K.A. The effects of intra-articular methylprednisolone and exercise on the mechanical properties of articular cartilage in the horse. Osteoarthr. Cartil. 1998, 6, 106–114. [Google Scholar] [CrossRef][Green Version]
- Dragoo, J.L.; Danial, C.M.; Braun, H.J.; Pouliot, M.A.; Kim, H.J. The chondrotoxicity of single-dose corticosteroids. Knee Surg Sports Traumatol. Arthrosc. 2012, 20, 1809–1814. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Eppley, B.L. Platelet rich plasma: Biology and new technology. J. Craniofac. Surg. 2005, 16, 1043–1054. [Google Scholar] [CrossRef]
- Chung, P.Y.; Lin, M.T.; Chang, H.P. Effectiveness of platelet-rich plasma injection in patients with temporomandibular joint osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 106–116. [Google Scholar] [CrossRef]
- Bousnaki, M.; Bakopoulou, A.; Koidis, P. Platelet-rich plasma for the therapeutic management of temporomandibular joint disorders: A systematic review. Int. J. Oral Maxillofac. Surg. 2018, 47, 188–198. [Google Scholar] [CrossRef]
- Al-Hamed, F.S.; Hijazi, A.; Gao, Q.; Badran, Z.; Tamimi, F. Platelet Concentrate Treatments for Temporomandibular Disorders: A Systematic Review and Meta-analysis. JDR Clin. Trans. Res. 2021, 6, 174–183. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr. Rev. Musculoskelet. Med. 2018, 11, 624–634. [Google Scholar] [CrossRef]
- Harrison, T.E.; Bowler, J.; Levins, T.N.; Reeves, K.D.; Cheng, A.L. Platelet-Rich Plasma Centrifugation Changes Leukocyte Ratios. Cureus 2021, 13, e14470. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Reed, G.L. Development of platelet secretory granules. Semin. Cell. Dev. Biol. 2002, 13, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Nazaroff, J.; Oyadomari, S.; Brown, N.; Wang, D. Reporting in clinical studies on platelet-rich plasma therapy among all medical specialties: A systematic review of Level I and II studies. PLoS ONE 2021, 16, e0250007. [Google Scholar] [CrossRef] [PubMed]
- Grambart, S.T. Sports medicine and platelet-rich plasma: Nonsurgical therapy. Clin. Podiatr. Med. Surg. 2015, 32, 99–107. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.S.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.J.; Lenz, M.E.; Sah, R.L.; Masuda, K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr. Cartil. 2006, 14, 1272–1280. [Google Scholar] [CrossRef]
- Anitua, E.; Fernández-de-Retana, S.; Alkhraisat, M.H. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef]
- Medina-Porqueres, I.; Ortega-Castillo, M.; Muriel-Garcia, A. Effectiveness of platelet-rich plasma in the management of hip osteoarthritis: A systematic review and meta-analysis. Clin. Rheumatol. 2021, 40, 53–64. [Google Scholar] [CrossRef]
- Bergstrand, S.; Ingstad, H.K.; Møystad, A.; Bjørnland, T. Long-term effectiveness of arthrocentesis with and without hyaluronic acid injection for treatment of temporomandibular joint osteoarthritis. J. Oral Sci. 2019, 61, 82–88. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Cadorin, C.; Frizziero, A.; Ferronato, G.; Manfredini, D. Comparison of 2 hyaluronic acid drugs for the treatment of temporomandibular joint osteoarthritis. J. Oral Maxillofac. Surg. 2012, 70, 2522–2530. [Google Scholar] [CrossRef]
- Tang, Y.L.; Zhu, G.Q.; Hu, L.; Zheng, M.; Zhang, J.Y.; Shi, Z.D.; Liang, X.H. Effects of intra-articular administration of sodium hyaluronate on plasminogen activator system in temporomandibular joints with osteoarthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 541–547. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Chou, J.; Krishnan, D.; Aghaloo, T.; Kahenasa, N.; Smith, J.A.; Giannakopoulos, H. Is Hyaluronic Acid or Corticosteroid Superior to Lactated Ringer Solution in the Short-Term Reduction of Temporomandibular Joint Pain After Arthrocentesis? Part 1. J. Oral Maxillofac. Surg. 2017, 75, 52–62. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Chou, J.; Krishnan, D.; Aghaloo, T.; Kahenasa, N.; Smith, J.A.; Giannakopoulos, H. Is Hyaluronic Acid or Corticosteroid Superior to Lactated Ringer Solution in the Short Term for Improving Function and Quality of Life After Arthrocentesis? Part 2. J. Oral Maxillofac. Surg. 2017, 75, 63–72. [Google Scholar] [CrossRef]
- Huddleston Slater, J.J.; Vos, L.M.; Story, L.P.; Stegenga, B. Randomized trial on the effectiveness of dexamethasone in TMJ arthrocentesis. J. Dent. Res. 2012, 91, 173–178. [Google Scholar] [CrossRef]
- Manfredini, D.; Rancitelli, D.; Ferronato, G.; Guarda-Nardini, L. Arthrocentesis with or without additional drugs in temporomandibular joint inflammatory-degenerative disease: Comparison of six treatment protocols*. J. Oral Rehabil. 2012, 39, 245–251. [Google Scholar] [CrossRef]
- Bjørnland, T.; Gjaerum, A.A.; Møystad, A. Osteoarthritis of the temporomandibular joint: An evaluation of the effects and complications of corticosteroid injection compared with injection with sodium hyaluronate. J. Oral Rehabil. 2007, 34, 583–589. [Google Scholar] [CrossRef]
- Møystad, A.; Mork-Knutsen, B.B.; Bjørnland, T. Injection of sodium hyaluronate compared to a corticosteroid in the treatment of patients with temporomandibular joint osteoarthritis: A CT evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, e53–e60. [Google Scholar] [CrossRef]
- Isacsson, G.; Schumann, M.; Nohlert, E.; Mejersjö, C.; Tegelberg, Å. Pain relief following a single-dose intra-articular injection of methylprednisolone in the temporomandibular joint arthralgia-A multicentre randomised controlled trial. J. Oral Rehabil. 2019, 46, 5–13. [Google Scholar] [CrossRef]
- Cömert Kiliç, S. Does Injection of Corticosteroid After Arthrocentesis Improve Outcomes of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2016, 74, 2151–2158. [Google Scholar] [CrossRef]
- Cömert Kiliç, S.; Güngörmüş, M. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis plus hyaluronic acid for the treatment of temporomandibular joint osteoarthritis: A randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 1538–1544. [Google Scholar] [CrossRef]
- Cömert Kiliç, S.; Güngörmüş, M.; Sümbüllü, M.A. Is Arthrocentesis Plus Platelet-Rich Plasma Superior to Arthrocentesis Alone in the Treatment of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2015, 73, 1473–1483. [Google Scholar] [CrossRef]
- Hegab, A.F.; Ali, H.E.; Elmasry, M.; Khallaf, M.G. Platelet-Rich Plasma Injection as an Effective Treatment for Temporomandibular Joint Osteoarthritis. J. Oral Maxillofac. Surg. 2015, 73, 1706–1713. [Google Scholar] [CrossRef]
- Fernández Sanromán, J.; Fernández Ferro, M.; Costas López, A.; Arenaz Bua, J.; López, A. Does injection of plasma rich in growth factors after temporomandibular joint arthroscopy improve outcomes in patients with Wilkes stage IV internal derangement? A randomized prospective clinical study. Int. J. Oral Maxillofac. Surg. 2016, 45, 828–835. [Google Scholar] [CrossRef]
- Fernández-Ferro, M.; Fernández-Sanromán, J.; Blanco-Carrión, A.; Costas-López, A.; López-Betancourt, A.; Arenaz-Bua, J.; Stavaru Marinescu, B. Comparison of intra-articular injection of plasma rich in growth factors versus hyaluronic acid following arthroscopy in the treatment of temporomandibular dysfunction: A randomised prospective study. J. Craniomaxillofac. Surg. 2017, 45, 449–454. [Google Scholar] [CrossRef] [PubMed]

| Criteria | List of Specific Criteria |
|---|---|
| Inclusion criteria |
|
| Exclusion criteria |
|
| Ester Preparations (Insoluble in Water) | Non-Ester Preparations (Soluble in Water) |
|---|---|
| Methylprednisolone acetate | Dexamethasone sodium phosphate |
| Betamethasone acetate | Betamethasone sodium phosphate |
| Triamcinolone acetonide | |
| Hydrocortisone acetate |
| References | Study Design | Participants and Intervention | Endpoint and Results |
|---|---|---|---|
| Bergstrand et al. (2019) [66] | Randomized, double-blind study | 37 patients (30 women, 7 men, aged 23–83 years):
| Endpoint: 47 months (range: 25–79 months) No significant differences regarding maximum incisor opening and pain reduction between the examined groups. Additional HA injection did not improve the final outcome. |
| Guarda-Nardini et al. (2012) [67] | Randomized, double-blind study | 35 patients (30 women, 5 men, mean age—group A: 47.7 ± 15.0 years; group B: 52.9 ± 16.1 years):
| Endpoint: 3 months No significant differences between the examined groups regarding the effectiveness of both methods of treatment. |
| Tang et al. (2010) [68] | Randomized, double-blind study | 40 patients (21 women, 19 men, aged: 25–63 years):
| Endpoint: after 5-week treatment Only patients treated with SH presented significant pain reduction. |
| Bouloux et al. (2017) [69,70] | Randomized, double-blind study | 102 patients (89 women, 13 men, mean age—group CS: 39.6 ± 18.4 years; group HA: 44.3 ± 17.2 years; group Ringer: 51.8 ± 17.2 years):
| Endpoint: 3 months No significant differences among the examined groups regarding pain levels, maximum incisal opening, jaw function and quality of life. |
| Huddleston Slater et al. (2012) [71] | Randomized, double-blind study | 28 patients (23 women, 5 men, mean age—control group: 33.9; group CS: 32.6):
| Endpoint: 24 weeks No significant differences between the examined groups regarding pain complaints, maximal interincisal opening, as well as functional impairment. |
| Manfredini et al. (2012) [72] | Randomized, single-blind study | 60 patients (51 women, 9 men, mean age 50.1 years)
| Endpoint: 6 weeks No significant differences among the examined groups regarding the pain at rest, pain at chewing, chewing efficiency and mouth opening. |
| Bjørnland et al. (2007) [73] | Randomized, double-blind study | 40 patients (34 women, 6 men, mean age—group HA: 53.4 ± 12.9 years; group CS: 50.0 ± 13.3 years)
| Endpoint: 6 months Patients treated with HA presented:
|
| Møystad et al. (2008) [74] | Randomized, double-blind study | 36 patients (31 women, 5 men, mean age—group HA: 51.5 ± 12.9 years; group CS: 48.3 ± 13.5 years)
| Endpoint: 6 months No significant differences between the examined groups regarding the presence of the radiographic signs of osteoarthritis or regarding the progression or regression of osseous changes in the TMJs. |
| Isacsson et al. (2019) [75] | Randomized, double-blind study | 54 patients (44 women, 10 men, mean age—group A: 48 ± 18.6 years; group B: 56 ± 14.7 years):
| Endpoint: 4 weeks No significant differences between the examined groups regarding TMJ arthralgia pain reduction. Methylprednisolone led to increased pain following the intervention compared to saline. |
| Cömert Kiliç et al. (2016) [76] | Randomized clinical trial | 24 patients (21 women, 3 men, mean age—control group: 35.08 ± 14.84 years; group CS: 32.58 ± 9.58 years):
| Endpoint: 12 months No significant differences between the examined groups regarding pain complaints or range of motion. |
| Cömert Kiliç et al. (2016) [77] | Randomized clinical trial | 31 patients (26 women, 5 men, mean age: 30.48 ± 13.04 years):
| Endpoint: 12 months No significant differences between the examined groups regarding masticatory efficiency, pain complaints, joint sounds, painless mouth opening, maximum mouth opening, lateral and protrusive movement. |
| Cömert Kiliç et al. (2015) [78] | Randomized clinical trial | 30 patients (27 women, 3 men, mean age—control group: 35.08 ± 14.84 years; group PRP: 32.22 ± 14.33 years):
| Endpoint: 12 months No significant differences between the examined groups regarding pain complaints, joint sounds, painless mouth opening, maximum mouth opening, lateral and protrusive movement. Only masticatory efficiency was significantly higher in PRP group. |
| Hegab et al. (2015) [79] | Randomized single-blind study | 50 patients (29 women, 21 men, aged 31–49 years):
| Endpoint: 12 months PRP performed better than LMW HA during long-term follow-up (12 months) in terms of pain reduction and increased interincisal distance (up to 6 months better results were obtained with LMW HA). |
| Fernández Sanromán et al. (2016) [80] | Randomized single-blind study | 92 patients (87 women, 5 men, aged 17–67 years):
| Endpoint: 2 years No significant differences between the examined groups regarding pain complaints and maximum mouth opening. |
| Fernández-Ferro et al. (2017) [81] | Randomized single-blind study | 100 patients (94 women, 6 men, aged 18–77 years):
| Endpoint: 18 months No significant differences between the examined groups regarding maximum mouth opening. PRGF following arthroscopy was more effective than the injection of HA regarding pain control. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. https://doi.org/10.3390/ijms22147405
Derwich M, Mitus-Kenig M, Pawlowska E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. International Journal of Molecular Sciences. 2021; 22(14):7405. https://doi.org/10.3390/ijms22147405
Chicago/Turabian StyleDerwich, Marcin, Maria Mitus-Kenig, and Elzbieta Pawlowska. 2021. "Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review" International Journal of Molecular Sciences 22, no. 14: 7405. https://doi.org/10.3390/ijms22147405
APA StyleDerwich, M., Mitus-Kenig, M., & Pawlowska, E. (2021). Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. International Journal of Molecular Sciences, 22(14), 7405. https://doi.org/10.3390/ijms22147405






