Recent Progress in the Molecular Imaging of Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
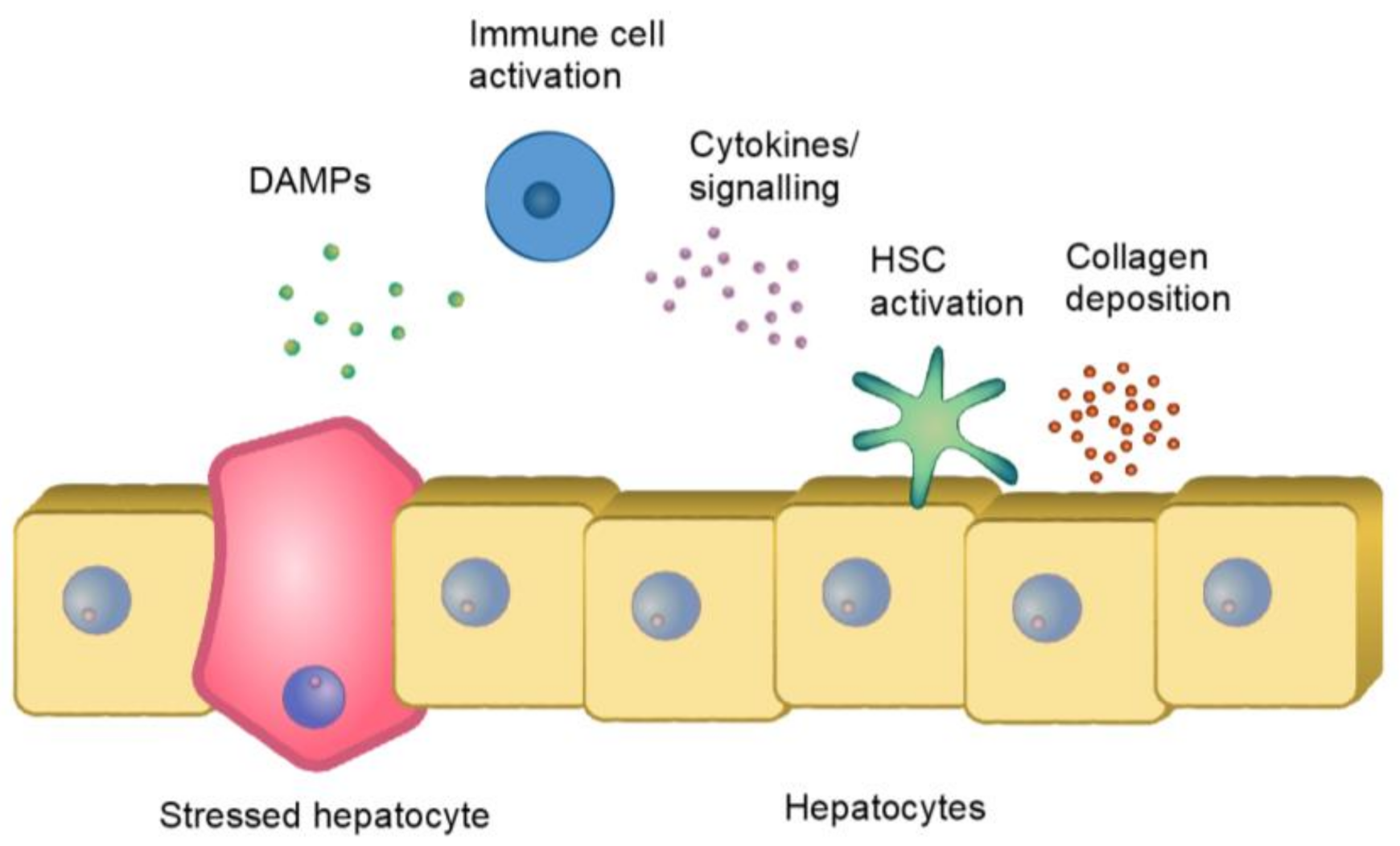
2. The Process of Pathological Fibrosis
2.1. Hepatic Steatosis
2.2. Immune Reaction and Fibrosis
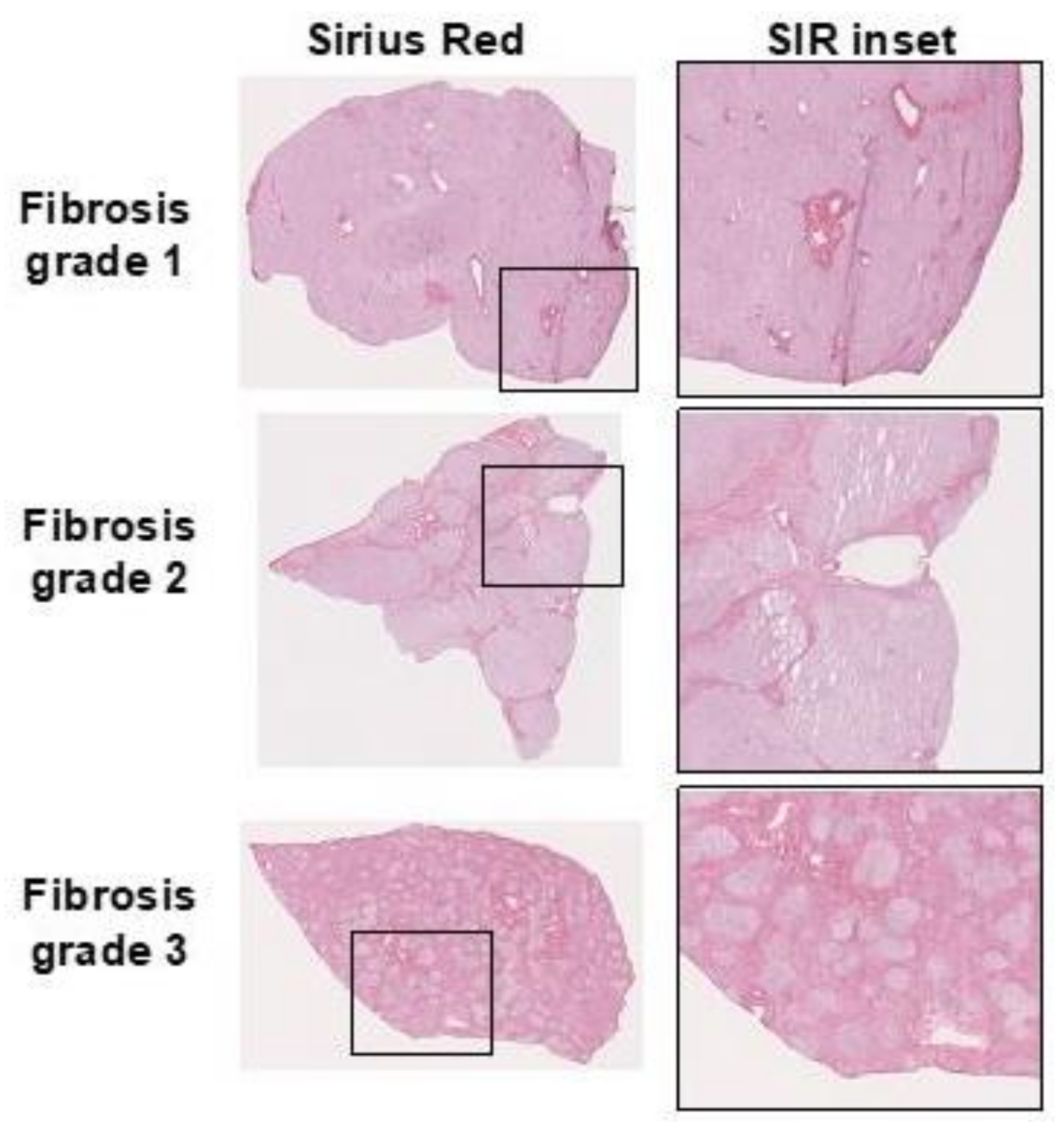
3. Clinical Assessment of Liver Fibrosis
4. Molecular Imaging of Fibrosis—State-of-the-Art
4.1. PET as a Technique to Visualize Fibrotic Processes
4.2. Imaging the Immune Context in Liver
4.3. Targeting of Fibroblasts or Stellate Cells
4.4. Targeting of Collagen Deposits
4.5. Targeting of Hepatocytes in the Liver
5. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finck, B.N. Targeting Metabolism, Insulin Resistance, and Diabetes to Treat Nonalcoholic Steatohepatitis. Diabetes 2018, 67, 2485–2493. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- WHO. Global Report On Diabetes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Wong, G.L.-H. Noninvasive biomarkers in NAFLD and NASH—Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Wong, V.W.; Chitturi, S.; Wong, G.L.; Yu, J.; Chan, H.L.; Farrell, G.C. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol. 2016, 1, 56–67. [Google Scholar] [CrossRef]
- Eriksson, O.; Laughlin, M.; Brom, M.; Nuutila, P. In vivo imaging of beta cells with radiotracers: State of the art, prospects and recommendations for development and use. Diabetologia 2016, 59, 1340–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Matos, G.; Metab, D.; Matos, A.F.G.; Júnior, W.S.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-gomez, M.; Zelber-sagi, S.; Wong, V.W.; Dufour, J.; Schattenberg, J.M.; et al. Expert Opinion A new de fi nition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; Connor, T.O.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, F.; Curci, C.; Stasi, A.; De Palma, G.; Divella, C.; Gramignoli, R.; Castellano, G.; Gallone, A. Review Article Role of Toll-Like Receptors in Actuating Stem / Progenitor Cell Repair Mechanisms: Different Functions in Different Cells. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Wang, S.; Friedman, S.L. Hepatology Snapshot: Hepatic fibrosis: A convergent response to liver injury that is reversible. J. Hepatol. 2020, 73, 210–211. [Google Scholar] [CrossRef]
- Perspectives, T. Liver Fibrosis: Mechanistic Concepts and. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Liver regeneration and fibrosis after inflammation. Inflamm. Regen. 2016, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Huang, H.; Zhang, Z.; Wang, F.S. The role of neutrophils in the development of liver diseases. Cell. Mol. Immunol. 2014, 11, 224–231. [Google Scholar] [CrossRef]
- Liu, K.; Wang, F.S.; Xu, R. Neutrophils in liver diseases: Pathogenesis and therapeutic targets. Cell. Mol. Immunol. 2021, 18, 38–44. [Google Scholar] [CrossRef]
- Lafoz, E.; Ruart, M.; Anton, A.; Oncins, A.; Hernández-Gea, V. The Endothelium as a Driver of Liver Fibrosis and Regeneration. Cells 2020, 9, 929. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Hua, X.; Xie, C.; Liu, J.; Huang, Y.; Zhou, L.; Zhang, M.; Li, X.; Gao, Z. Levels of hepatic Th17 cells and regulatory T cells upregulated by hepatic stellate cells in advanced HBV-related liver fibrosis. J. Transl. Med. 2017, 15, 75. [Google Scholar] [CrossRef]
- Mchedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.J.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Carpenter, D.H.; Rinella, M.; Harrison, S.A.; Loomba, R.; Younossi, Z.; Neuschwander-Tetri, B.A.; Sanyal, A.J. NAFLD: Reporting Histologic Findings in Clinical Practice. Concise Rev. Hepatol. 2020, 73, 2021. [Google Scholar] [CrossRef]
- Kjaer, M.B.; George, J.; Kazankov, K.; Grønbaek, H.; Breinholt Kjaer, M. Current perspectives on the pathophysiology of metabolic associated fatty liver disease: Are macrophages a viable target for therapy? Expert Rev. Gastroenterol. Hepatol. 2021, 15, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gunsten, S.P.; Sultan, D.H.; Luehmann, H.P.; Zhao, Y.; Blackwell, T.S.; Bollermann-Nowlis, Z.; Pan, J.H.; Byers, D.E.; Atkinson, J.J.; et al. PET-based imaging of chemokine receptor 2 in experimental and Disease-related lung inflammation. Radiology 2017, 283, 758–768. [Google Scholar] [CrossRef]
- Search of: CCR2 PET—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=CCR2+PET&draw=1&rank=4#rowId3 (accessed on 14 May 2021).
- Kao, W.Y.; Lin, Y.F.; Chang, I.W.; Chen, C.L.; Tang, J.H.; Chang, C.C.; Chang, Y.J.; Wang, W. Interleukin-2 receptor alpha as a biomarker for nonalcoholic fatty liver disease diagnosis. J. Chin. Med. Assoc. 2021, 84, 261–266. [Google Scholar] [CrossRef]
- IL2 Imaging in Metastatic Melanoma—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02922283 (accessed on 14 May 2021).
- van der Veen, E.L.; Suurs, F.V.; Cleeren, F.; Bormans, G.; Elsinga, P.H.; Hospers, G.A.P.; Lub-de Hooge, M.N.; de Vries, E.G.E.; de Vries, E.F.J.; Antunes, I.F. Development and Evaluation of Interleukin-2-Derived Radiotracers for PET Imaging of T Cells in Mice. J. Nucl. Med. 2020, 61, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rosenkrans, Z.T.; Wang, J.; Cai, W. PET imaging of macrophages in cardiovascular diseases. Am. J. Transl. Res. 2020, 12, 1491–1514. [Google Scholar]
- Pulli, B.; Wojtkiewicz, G.; Iwamoto, Y.; Ali, M.; Zeller, M.W.; Bure, L.; Wang, C.; Choi, Y.; Masia, R.; Guimaraes, A.R.; et al. Molecular MR imaging of myeloperoxidase distinguishes steatosis from steatohepatitis in nonalcoholic fatty liver disease. Radiology 2017, 284, 390–400. [Google Scholar] [CrossRef]
- Wang, C.; Keliher, E.; Zeller, M.W.G.; Wojtkiewicz, G.R.; Aguirre, A.D.; Buckbinder, L.; Kim, H.Y.; Chen, J.; Maresca, K.; Ahmed, M.S.; et al. An activatable PET imaging radioprobe is a dynamic reporter of myeloperoxidase activity in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 11966–11971. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Seki, E. TNFα in Liver Fibrosis. Curr. Pathobiol. Rep. 2015, 3, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Stephens, S. Certolizumab pegol. MAbs 2010, 2, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Beckford-Vera, D.R.; Gonzalez-Junca, A.; Janneck, J.S.; Huynh, T.L.; Blecha, J.E.; Seo, Y.; Li, X.; VanBrocklin, H.F.; Franc, B.L. PET/CT Imaging of Human TNFα Using [89Zr]Certolizumab Pegol in a Transgenic Preclinical Model of Rheumatoid Arthritis. Mol. Imaging Biol. 2020, 22, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Haas, J.T.; Vonghia, L.; Mogilenko, D.A.; Verrijken, A.; Molendi-Coste, O.; Fleury, S.; Deprince, A.; Nikitin, A.; Woitrain, E.; Ducrocq-Geoffroy, L.; et al. Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nat. Metab. 2019, 1, 604–614. [Google Scholar] [CrossRef]
- Gibson, H.M.; McKnight, B.N.; Malysa, A.; Dyson, G.; Wiesend, W.N.; McCarthy, C.E.; Reyes, J.; Wei, W.Z.; Viola-Villegas, N.T. IFNg PET imaging as a predictive tool for monitoring response to tumor immunotherapy. Cancer Res. 2018, 78, 5706–5717. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M.; Adolph, T.E. A role for IL-1 inhibitors in the treatment of non-alcoholic fatty liver disease (NAFLD)? Expert Opin. Invest. Drugs 2020, 29, 103–106. [Google Scholar] [CrossRef]
- Mirea, A.M.; Tack, C.J.; Chavakis, T.; Joosten, L.A.B.; Toonen, E.J.M. IL-1 Family Cytokine Pathways Underlying NAFLD: Towards New Treatment Strategies. Trends Mol. Med. 2018, 24, 458–471. [Google Scholar] [CrossRef]
- Dmochowska, N.; Tieu, W.; Keller, M.D.; Wardill, H.R.; Mavrangelos, C.; Campaniello, M.A.; Takhar, P.; Hughes, P.A. Immuno-PET of innate immune markers CD11b and IL-1b detects inflammation in murine colitis. J. Nucl. Med. 2019, 60, 858–863. [Google Scholar] [CrossRef]
- Taylor, J.M.W.; Allen, A.M.; Graham, A. Targeting mitochondrial 18 kDa translocator protein (TSPO) regulates macrophage cholesterol efflux and lipid phenotype. Clin. Sci. 2014, 127, 603–613. [Google Scholar] [CrossRef]
- Hatori, A.; Yui, J.; Xie, L.; Kumata, K.; Yamasaki, T.; Fujinaga, M.; Wakizaka, H.; Ogawa, M.; Nengaki, N.; Kawamura, K.; et al. Utility of translocator protein (18 kDa) as a molecular imaging biomarker to monitor the progression of liver fibrosis. Sci. Rep. 2015, 5, 17327. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, L.; Guo, H.; Chen, H.; Shan, H. Targeting activated hepatic stellate cells (aHSCs) for liver fibrosis imaging. EJNMMI Res. 2015, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Varasteh, Z.; Honarvar, H.; Hosseinimehr, S.J.; Eriksson, O.; Jonasson, P.; Frejd, F.Y.; Abrahmsen, L.; Orlova, A. Imaging of platelet-derived growth factor receptor β expression in glioblastoma xenografts using affibody molecule 111In-DOTA-Z09591. J. Nucl. Med. 2014, 55, 294–300. [Google Scholar] [CrossRef]
- Patsenker, E.; Stickel, F. Role of integrins in fibrosing liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301. [Google Scholar] [CrossRef]
- Ulmasov, B.; Noritake, H.; Carmichael, P.; Oshima, K.; Griggs, D.W.; Neuschwander-Tetri, B.A. An Inhibitor of Arginine-Glycine-Aspartate-Binding Integrins Reverses Fibrosis in a Mouse Model of Nonalcoholic Steatohepatitis. Hepatol. Commun. 2019, 3, 246–261. [Google Scholar] [CrossRef]
- Shao, T.; Chen, Z.; Belov, V.; Wang, X.; Rwema, S.H.; Kumar, V.; Fu, H.; Deng, X.; Rong, J.; Yu, Q.; et al. [18F]-Alfatide PET imaging of integrin αvβ3 for the non-invasive quantification of liver fibrosis. J. Hepatol. 2020, 73, 161–169. [Google Scholar] [CrossRef]
- Iordanskaia, T.; Koeck, E.; Rossi, C.; Mohan, P.; Schwarz, K.; Anders, R.; Nadler, E.P. Integrin β-8, but not β-5 or -6, protein expression is increased in livers of children with biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 679–683. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Quigley, N.G.; Steiger, K.; Richter, F.; Weichert, W.; Hoberück, S.; Kotzerke, J.; Notni, J. Tracking a TGF-β activator in vivo: Sensitive PET imaging of αvβ8-integrin with the Ga-68-labeled cyclic RGD octapeptide trimer Ga-68-Triveoctin. EJNMMI Res. 2020, 10, 133. [Google Scholar] [CrossRef]
- Lay, A.J.; Zhang, H.E.; McCaughan, G.W.; Douglas, M. Gorrell Fibroblast activation protein in liver fibrosis. Front. Biosci. 2019, 1, 1–17. [Google Scholar]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- 68Ga-FAPI PET/CT in Liver Fibrosis Patients—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04533828?term=68Ga-FAPI&draw=2&rank=8 (accessed on 15 May 2021).
- Dibba, P.; Li, A.; Cholankeril, G.; Iqbal, U.; Gadiparthi, C.; Khan, M.; Kim, D.; Ahmed, A. Mechanistic Potential and Therapeutic Implications of Cannabinoids in Nonalcoholic Fatty Liver Disease. Medicines 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Dierckx Rudi, A.J.O.; Otte, A.; de Vries Erik, F.J.; van Waarde, A.; Lammertsma, A.A. PET and SPECT of Neurobiological Systems, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Shao, T.; Yu, Q.; Liang, S. Molecular imaging of murine hepatic encephalopathy with cannabinoid receptor type I targeted [18F]MK-9470 by positron emission tomography. J. Nucl. Med. 2020, 61, 194. [Google Scholar]
- Calamita, G.; Perret, J.; Delporte, C. Aquaglyceroporins: Drug targets for metabolic diseases? Front. Physiol. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Du, M.; Dong, C.; Cao, L.; Wei, R.; Liu, C.; Zhai, W.; Wang, B.; Xin, J. In vivo preclinical PET/CT imaging of carbon-11-labeled aminoglycerol probe for the diagnosis of liver fibrosis. Ann. Nucl. Med. 2019, 33, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Montesi, S.B.; Désogère, P.; Fuchs, B.C.; Caravan, P. Molecular imaging of fibrosis: Recent advances and future directions. J. Clin. Investig. 2019, 129, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Wang, H.; Yang, Y.; Wei, L.; Polasek, M.; Schühle, D.T.; Lauwers, G.Y.; Parkar, A.; Sinskey, A.J.; Tanabe, K.K.; et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. J. Hepatol. 2013, 59, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Das, B.; Dumas, S.; Epstein, F.H.; Helm, P.A.; Jacques, V.; Koerner, S.; Kolodziej, A.; Shen, L.; Sun, W.C.; et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew. Chem. Int. Ed. 2007, 46, 8171–8173. [Google Scholar] [CrossRef]
- Désogère, P.; Tapias, L.F.; Hariri, L.P.; Rotile, N.J.; Rietz, T.A.; Probst, C.K.; Blasi, F.; Day, H.; Mino-Kenudson, M.; Weinreb, P.; et al. Type I collagen-Targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Montesi, S.B.; Izquierdo-Garcia, D.; Désogère, P.; Abston, E.; Liang, L.L.; Digumarthy, S.; Seethamraju, R.; Lanuti, M.; Caravan, P.; Catana, C. Type I collagen-targeted positron emission tomography imaging in idiopathic pulmonary fibrosis: First-in-human studies. Am. J. Respir. Crit. Care Med. 2019, 200, 258–261. [Google Scholar] [CrossRef]
- Muzard, J.; Sarda-Mantel, L.; Loyau, S.; Meulemans, A.; Louedec, L.; Bantsimba-Malanda, C.; Hervatin, F.; Marchal-Somme, J.; Michel, J.B.; Le Guludec, D.; et al. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Rosenström, U.; Estrada, S.; Ljungvall, I.; Häggström, J.; Eriksson, O.; Antoni, G. Synthesis and preclinical evaluation of 68Ga-labeled collagelin analogs for imaging and quantification of fibrosis. Nucl. Med. Biol. 2014, 41, 728–736. [Google Scholar] [CrossRef]
- Salarian, M.; Turaga, R.C.; Xue, S.; Nezafati, M.; Hekmatyar, K.; Qiao, J.; Zhang, Y.; Tan, S.; Ibhagui, O.Y.; Hai, Y.; et al. Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, X.; Liu, K.; Feng, S.; Tang, B.; Li, Q.; Huang, D.; Yang, S. Molecular imaging of fibrosis using a novel collagen-binding peptide labelled with 99mTc on SPECT/CT. Amino Acids 2017, 49, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.; Pierce, B.F.; Piluso, S.; Wischke, C.; Lendlein, A.; Neffe, A.T. Design of Decorin-Based Peptides That Bind to Collagen i and their Potential as Adhesion Moieties in Biomaterials. Angew. Chem. Int. Ed. 2015, 54, 10980–10984. [Google Scholar] [CrossRef] [PubMed]
- Rosestedt, M.; Velikyan, I.; Rosenström, U.; Estrada, S.; Åberg, O.; Weis, J.; Westerlund, C.; Ingvast, S.; Korsgren, O.; Nordeman, P.; et al. Radiolabelling and positron emission tomography imaging of a high-affinity peptide binder to collagen type 1. Nucl. Med. Biol. 2021, 93, 54–62. [Google Scholar] [CrossRef]
- Karageorgis, A.; Lenhard, S.C.; Yerby, B.; Forsgren, M.F.; Liachenko, S.; Johansson, E.; Pilling, M.A.; Peterson, R.A.; Yang, X.; Williams, D.P.; et al. A multi-center preclinical study of gadoxetate DCE-MRI in rats as a biomarker of drug induced inhibition of liver transporter function. PLoS ONE 2018, 13, e0197213. [Google Scholar] [CrossRef] [PubMed]
- Leporq, B.; Daire, J.L.; Pastor, C.M.; Deltenre, P.; Sempoux, C.; Schmidt, S.; Van Beers, B.E. Quantification of hepatic perfusion and hepatocyte function with dynamic gadoxetic acid-enhanced MRI in patients with chronic liver disease. Clin. Sci. 2018, 132, 813–824. [Google Scholar] [CrossRef]
- Mishra, A.; Castañeda, T.R.; Bader, E.; Elshorst, B.; Cummings, S.; Scherer, P.; Bangari, D.S.; Loewe, C.; Schreuder, H.; Pöverlein, C.; et al. Triantennary GalNAc Molecular Imaging Probes for Monitoring Hepatocyte Function in a Rat Model of Nonalcoholic Steatohepatitis. Adv. Sci. 2020, 7, 2002997. [Google Scholar] [CrossRef]
- Eriksson, O.; Velikyan, I.; Haack, T.; Bossart, M.; Evers, A.; Laitinen, I.; Larsen, P.J.; Plettenburg, O.; Takano, A.; Halldin, C.; et al. Assessment of glucagon receptor occupancy by Positron Emission Tomography in non-human primates. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Velikyan, I.; Haack, T.; Bossart, M.; Laitinen, I.; Larsen, P.J.; Berglund, J.-E.; Antoni, G.; Johansson, L.; Pierrou, S.; et al. Imaging of the glucagon receptor in subjects with type 2 diabetes. J. Nucl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegrzyniak, O.; Rosestedt, M.; Eriksson, O. Recent Progress in the Molecular Imaging of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 7348. https://doi.org/10.3390/ijms22147348
Wegrzyniak O, Rosestedt M, Eriksson O. Recent Progress in the Molecular Imaging of Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2021; 22(14):7348. https://doi.org/10.3390/ijms22147348
Chicago/Turabian StyleWegrzyniak, Olivia, Maria Rosestedt, and Olof Eriksson. 2021. "Recent Progress in the Molecular Imaging of Nonalcoholic Fatty Liver Disease" International Journal of Molecular Sciences 22, no. 14: 7348. https://doi.org/10.3390/ijms22147348
APA StyleWegrzyniak, O., Rosestedt, M., & Eriksson, O. (2021). Recent Progress in the Molecular Imaging of Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 22(14), 7348. https://doi.org/10.3390/ijms22147348





