The Effect of Cucurbit[7]uril on the Antitumor and Immunomodulating Properties of Oxaliplatin and Carboplatin
Abstract
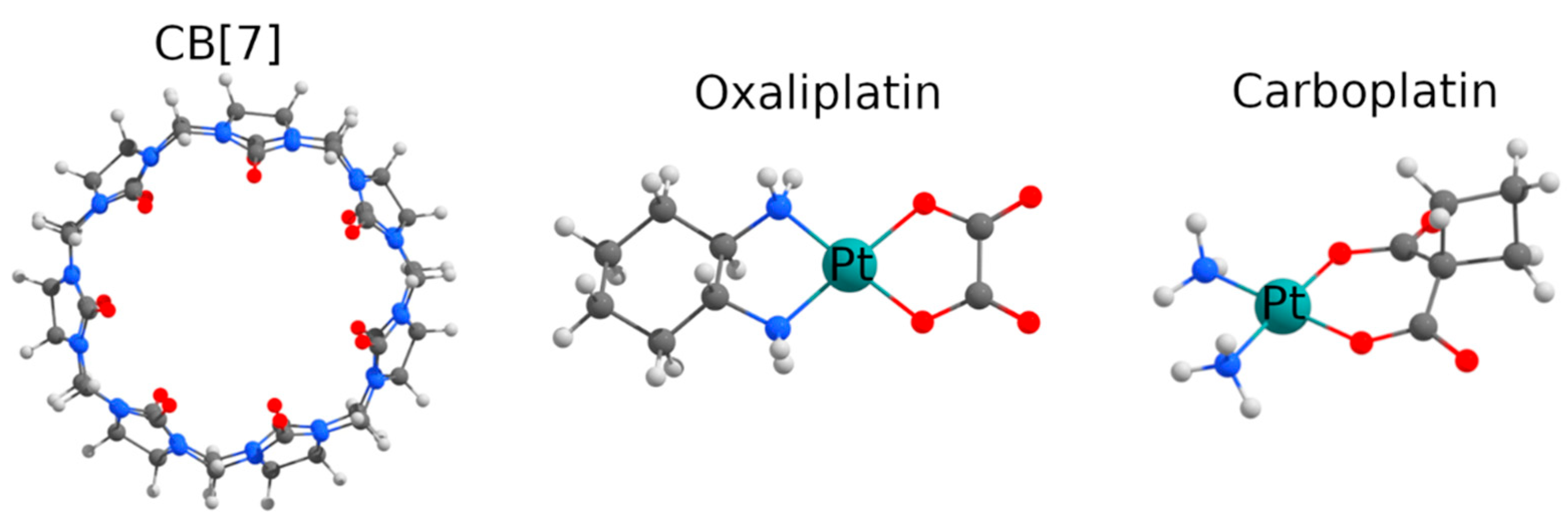
:1. Introduction
2. Results and Discussion
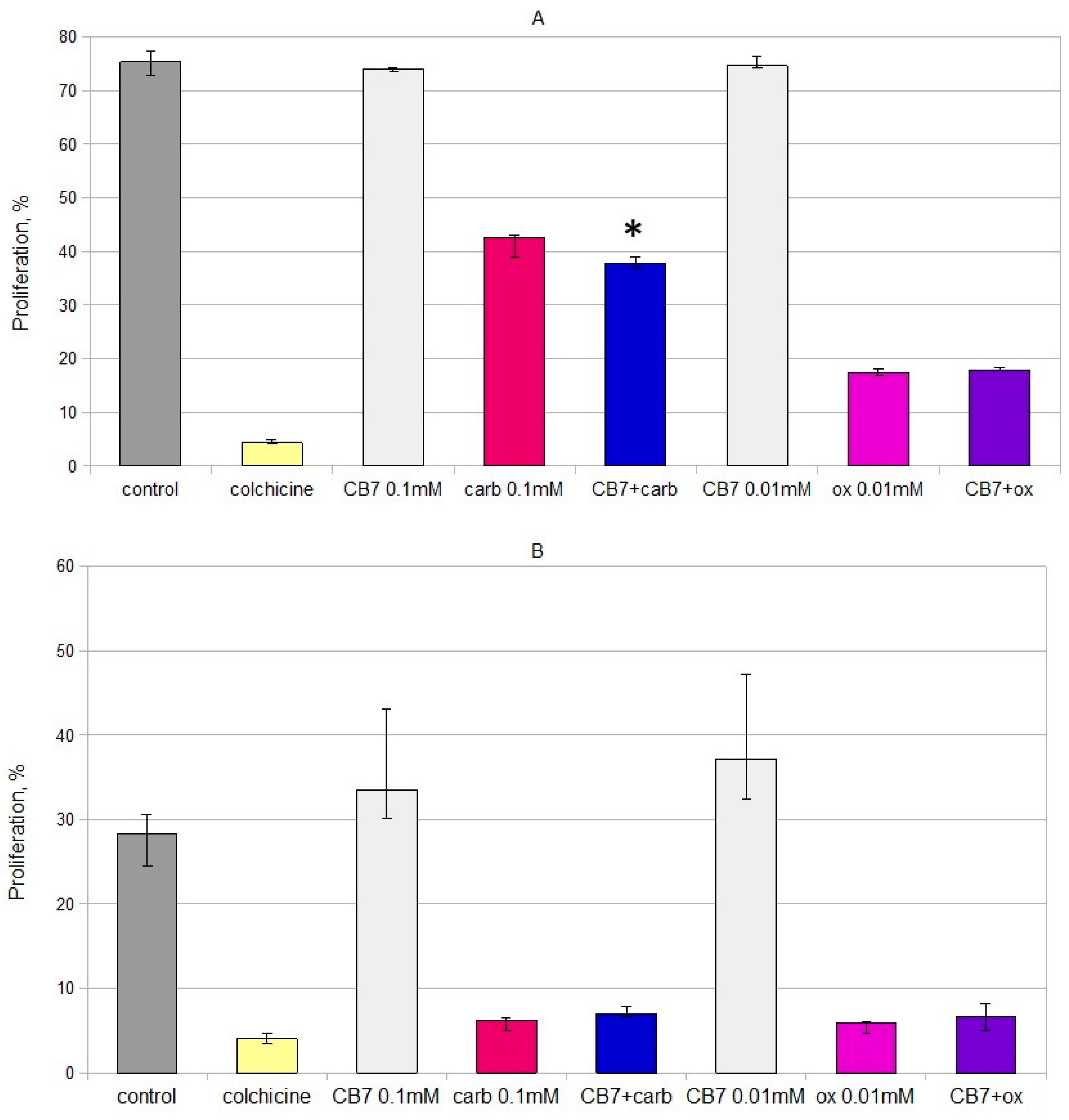
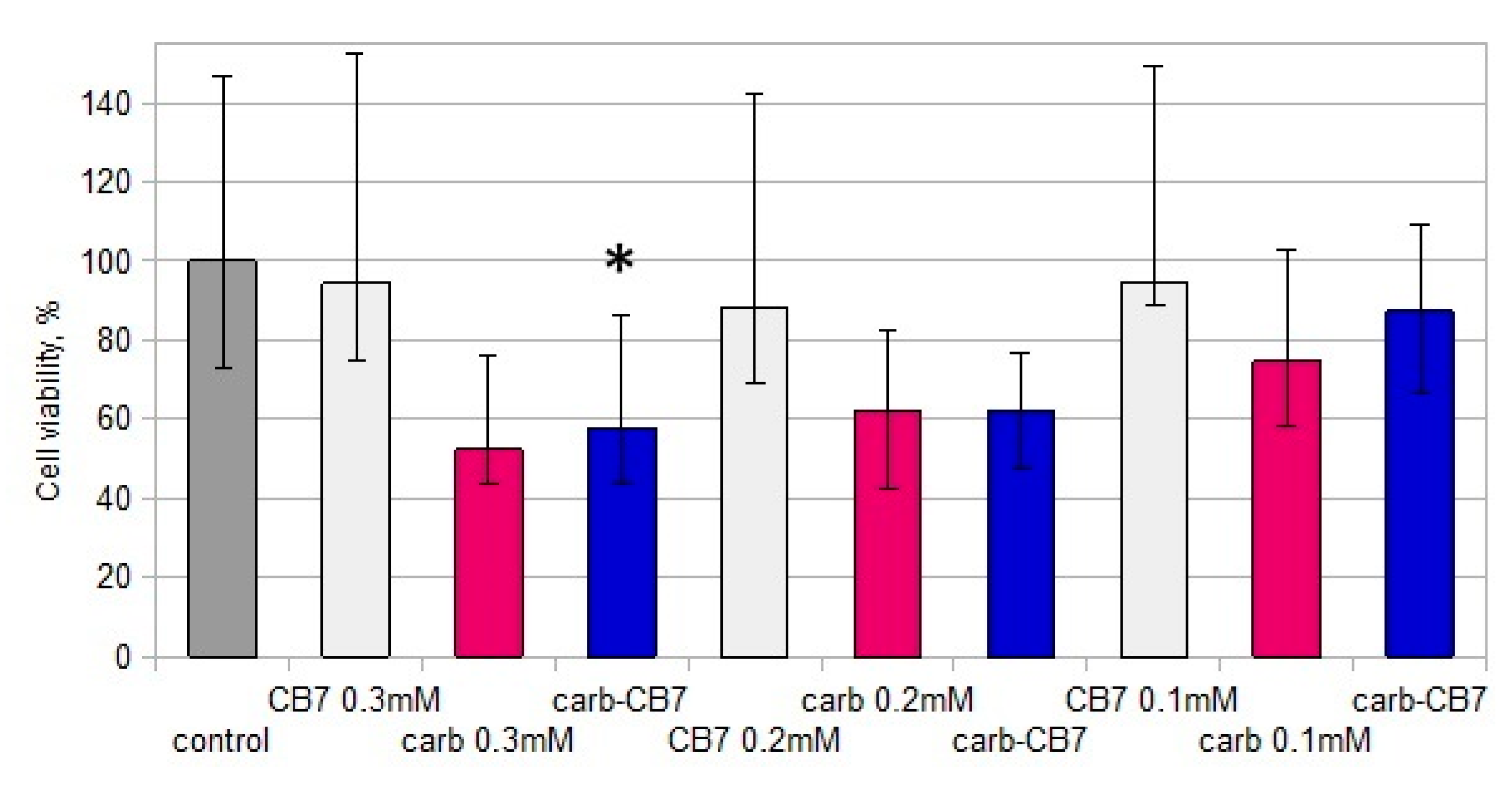
2.1. In Vitro Cytotoxicity and Immunomodulating Properties
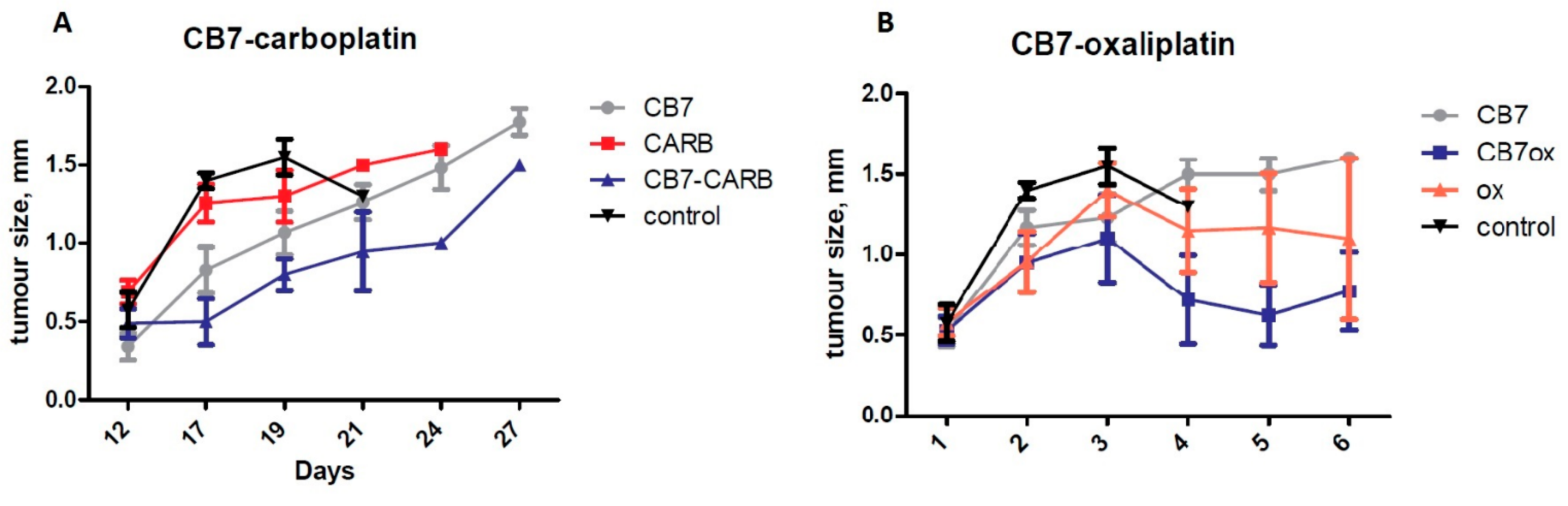
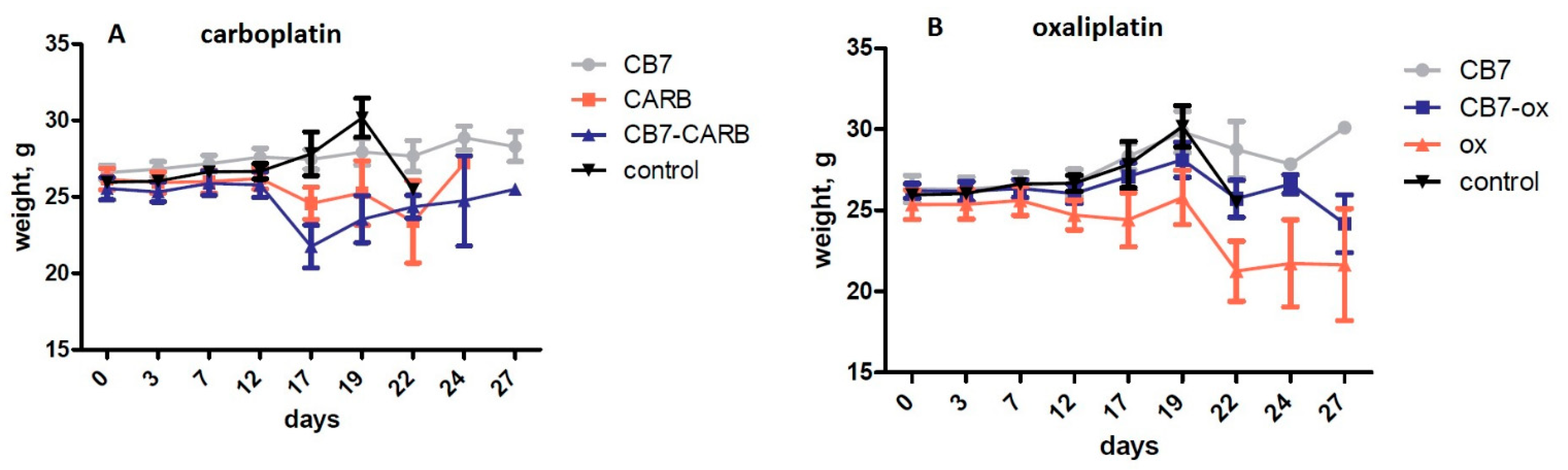
2.2. In Vivo Antitumor Activity and Side-Effect Evaluation
3. Materials and Methods
3.1. Materials
3.2. Cell Cultures
3.3. Cell Viability Assay
3.4. Cell Proliferation Assay
3.5. In Vivo Studies
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wyman, I.W.; Macartney, D.H. Host–guest complexations of local anaesthetics by cucurbit[7]uril in aqueous solution. Org. Biomol. Chem. 2010, 8, 247–252. [Google Scholar] [CrossRef]
- Wheate, N.; Vora, V.; Anthony, N.; Macdougal, F. Host–guest complexes of the antituberculosis drugs pyrazinamide and isoniazid with cucurbit[7]uril. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 359–367. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Megyesi, M.; Tárkányi, G.; Mizsei, R.; Biczók, L. Inclusion complex formation of sanguinarine alkaloid with cucurbit[7]uril: Inhibition of nucleophilic attack and photooxidation. Org. Biomol. Chem. 2011, 9, 1061–1070. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Biczók, L. Kinetics and thermodynamics of berberine inclusion in cucurbit[7]uril. J. Phys. Chem. B 2014, 118, 2499–2505. [Google Scholar] [CrossRef] [Green Version]
- Saleh, N.; Al-Handawi, M.B.; Al-Kaabi, L.; Ali, L.; Ashraf, S.S.; Thiemann, T.; Al-Hindawi, B.; Meetani, M. Intermolecular interactions between cucurbit[7]uril and pilocarpine. Int. J. Pharm. 2014, 460, 53–62. [Google Scholar] [CrossRef]
- Kovalenko, E.A.; Pashkina, E.A.; Kanazhevskaya, L.Y.; Masliy, A.N.; Kozlov, V.A. Chemical and Biological Properties of a Supramolecular Complex of Tuftsin and Cucurbit[7]uril. Int. Immunopharmacol. 2017, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Konda, S.K.; Maliki, R.; McGrath, S.; Parker, B.S.; Robinson, T.; Spurling, A.; Cheong, A.; Lock, P.; Pigram, P.J.; Phillips, D.R.; et al. Encapsulation of Mitoxantrone within Cucurbit[8]uril Decreases Toxicity and Enhances Survival in a Mouse Model of Cancer. ACS Med. Chem. Lett. 2017, 8, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.X.; Zhang, X.M.; Duan, X.C.; Liu, F.; Du, L.M. Supramolecular interaction of methotrexate with cucurbit[7]uril and analytical application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef] [Green Version]
- Kuok, K.I.; Li, S.; Wyman, I.W.; Wang, R. Cucurbit[7]uril: An emerging candidate for pharmaceutical excipients. Ann. N. Y. Acad. Sci. 2017, 1398, 108–119. [Google Scholar] [CrossRef]
- Pashkina, E.A.; Grishina, L.V.; Aktanova, A.A.; Kozlov, V.A. Antitumor activity of supramolecular complexes of cucurbituril with platinum(II) compounds. Inorg. Chim. Acta 2021, 522, 120370. [Google Scholar] [CrossRef]
- Wheate, N.; Buck, D.P.; Day, A.I.; Collins, J.G. Cucurbit[n]uril binding of platinum anticancer complexes. Dalton Trans. 2006, 3, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Wheate, N.J. Improving platinum(II)-based anticancer drug delivery using cucurbit[n]urils. J. Inorg. Biochem. 2008, 102, 2060–2066. [Google Scholar] [CrossRef]
- Oun, R.; Floriano, R.S.; Isaacs, L.; Rowan, E.G.; Wheate, N.J. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbituril-based macrocyclic drug delivery vehicles. Toxicol. Res. 2014, 3, 447. [Google Scholar] [CrossRef] [Green Version]
- Plumb, J.A.; Venugopal, B.; Oun, R.; Gomez-Roman, N.; Kawazoe, Y.; Venkataramanan, N.S.; Wheate, N.J. Cucurbit[7]uril encapsulated cisplatin overcomes cisplatin resistance via a pharmacokinetic effect. Metallomics 2012, 4, 561–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oun, R.; Plumb, J.A.; Wheate, N.J. A cisplatin slow-release hydrogel drug delivery system based on a formulation of the macrocycle cucurbit[7]uril, gelatin and polyvinyl alcohol. J. Inorg. Biochem. 2014, 134, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Roman, N.; McGregor, F.; Wheate, N.J.; Plumb, J.A. Cucurbit[7]uril encapsulated cisplatin overcomes resistance to cisplatin induced by Rab25 overexpression in an intraperitoneal ovarian cancer model. J. Ovarian Res. 2015, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.J.; Kim, S.Y.; Ko, Y.H.; Sakamoto, S.; Yamaguchi, K.; Kim, K. Novel molecular drug carrier: Encapsulation of oxaliplatin in cucurbit[7]uril and its effects on stability and reactivity of the drug. Org. Biomol. Chem. 2005, 3, 2122–2125. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huang, Z.; Zhao, H.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]uril. ACS Appl. Mater. Interfaces 2017, 9, 8602–8608. [Google Scholar] [CrossRef] [Green Version]
- Venkataramanan, N.S.; Ambigapathy, S.; Mizuseki, H.; Kawazoe, Y. Theoretical Prediction of the Complexation Behaviors of Antitumor Platinum Drugs with Cucurbiturils. J. Phys. Chem. B 2012, 116, 14029–14039. [Google Scholar] [CrossRef]
- Mirzaeva, I.V.; Moroz, N.K.; Andrienko, I.V.; Kovalenko, E.A. Interaction between carboplatin and cucurbit[7]uril studied by means of multinuclear NMR spectroscopy and DFT calculations. J. Mol. Struct. 2018, 1163, 68–76. [Google Scholar] [CrossRef]
- Mirzaeva, I.; Pashkina, E.; Andrienko, I.; Kovalenko, E.; Knauer, N.; Ermakov, A.; Aktanova, A.; Kozlov, V. NMR and DFT study of the mechanism for increased antitumor activity of carboplatin in mixture with cucurbit[7]uril. In Proceedings of the Systems Biology and Biomedicine (SBioMed-2018) Symposium, Novosibirsk, Russia, 21–22 August 2018; p. 93. [Google Scholar]
- Mirzaeva, I.V.; Andrienko, I.V.; Kovalenko, E.A.; Pashkina, E.A.; Aktanova, A.A. 1H NMR study of the effect of cucurbit[7]uril on the aquation of carboplatin in biologically relevant media. Appl. Magn. Reson. 2019, 50, 1267–1276. [Google Scholar] [CrossRef]
- de Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, H.; Kokura, S.; Ishikawa, T.; Tsuchiya, R.; Okajima, M.; Matsuyama, T.; Adachi, S.; Katada, K.; Kamada, K.; Uchiyama, K.; et al. Effects of anticancer agents on cell viability, proliferative activity and cytokine production of peripheral blood mononuclear cells. J. Clin. Biochem. Nutr. 2013, 52, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feather, C.E.; Lees, J.G.; Makker, P.G.; Goldstein, D.; Kwok, J.B.; Moalem-Taylor, G.; Polly, P. Oxaliplatin induces muscle loss and muscle—specific molecular changes in Mice. Muscle Nerve 2018, 57, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Renn, C.L.; Carozzi, V.A.; Rhee, P.; Gallop, D.; Dorsey, S.G.; Cavaletti, G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol. Pain. 2011, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chen, Y.; Wu, H.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials 2018, 178, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling Factors in the Synthesis of Cucurbituril and Its Homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef]
- Grant, M.P.; Wheate, N.; Aldrich-Wright, J.R. Diffusion Coefficient of Cucurbit[n]urils (n = 6 or 7) at Various Concentrations, Temperatures, and pH. J. Chem. Eng. Data 2009, 54, 323–326. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Tang, B.; Kang, Y.; Xu, J.; Zhang, X. Host-Guest Interactions between Oxaliplatin and Cucurbit[7]uril/Cucurbit[7]uril Derivatives under Pseudo-Physiological Conditions. Langmuir 2020, 36, 1235–1240. [Google Scholar] [CrossRef]
- Böyum, A. Separation of leukocytes from blood and bone marrow. Introduction. Scand. J. Clin. Lab. Invest. Suppl. 1968, 21, 77–89. [Google Scholar]



| CD3+CD4+ | CD3+CD4− | CD19+ | ||||
|---|---|---|---|---|---|---|
| Frequency (%) | Dividing Cells (%) | Frequency (%) | Dividing Cells (%) | Frequency (%) | Dividing Cells (%) | |
| control | 49.8 (47.3–59.0) | 1.8 (0.9–2.4) | 25.3 (19.1–31.7) | 3.1 (0.9–3.9) | 7.0 (3.5–7.6) | 2.4 (1.2–3.4) |
| CB[7] 0.3 M | 53.7 (45.3–62.7) | 2.3 (1.0–2.4) | 26.6 (20.0–32.0) | 3.3 (1.1–4.1) | 4.7 (2.7–6.3) | 2.7 (0.9–4.0) |
| carboplatin 0.3 M | 55.3 (45.3–58.7) | 2.2 (0.9–4.3) | 26.4 (20.8–31.4) | 4.5 (1.2–9.6) | 4.8 (1.9–10.6) | 2.7 (1.0–3.4) |
| carboplatin:CB[7] 1:1 0.3M | 60.3(52.8–66.6) *# | 2.5 (1.4–3.7) | 27.6 (22.1–33.1) | 4.3 (1.6–4.7) | 5.9 (1.7–7.8) | 3.1 (0.6–3.5) |
| CB[7] 0.1 M | 52.7 (42.9–55.5) | 2.4 (1.2–3.0) | 27.4 (25.7–31.2) | 4.0 (1.1–7.0) | 5.6 (2.6–6.5) | 2.8 (1.9–3.5) |
| oxaliplatin (0.1 M) | 71.6 (63.5–76.6) * | 2.5 (1.1–3.9) | 21.4 (21.1–22.9) * | 4.6 (1.9–8.8) | 1.2 (0.9–4.1) * | 1.4 (0.6–2.1) * |
| CB[7]–oxaliplatin 0.1 M | 73.4 (69.1–77.2) * | 2.4 (1.1–4.1) | 21.6 (17,9–23.2) * | 5.1 (1.6–6.6) | 1.8 (1.2–2.8) * | 2.0 (1.0–2.5) |
| CD3+CD4+ | CD3+CD4− | CD19+ | ||||
|---|---|---|---|---|---|---|
| Frequency (%) | Dividing Cells (%) | Frequency (%) | Dividing Cells (%) | Frequency (%) | Dividing Cells (%) | |
| control | 51.3 (41.2–59.2) | 28.7 (19.0–37.3) | 29.9 (23.8–45.4) | 30.6 (27.2–32.6) | 5.4 (2.0–6.2) | 5.3 (4.1–14.9) |
| CB[7] 0.3 M | 51.5 (41.1–59.9) | 27.4 (12.6–29.8) | 30.2 (23.2–50.5) | 28.9 (22.8–30.1) | 4.2 (1.7–5.9) | 5.3 (3.2–9.6) |
| carboplatin 0.3 M | 48.7 (36.8–50.8) | 2.7 (2.4–5.1) * | 24.3 (20.2–27.9) | 4.2 (3.6–7.8) * | 4.9 (1.9–10.1) | 2.8 (1.1–4.0) * |
| carboplatin:CB[7] 1:1 0.3M | 50.4 (40.5–65.8) # | 2.5 (1.5–4.8) * | 22.1 (19.4–28.6) * | 4.8 (3.1–6.7) * | 3.1 (1.5–9.1) | 1.7 (1.1–2.5) * |
| CB[7] 0.1 M | 46.3 (41.9–55.5) | 21.1 (17.1–30.1) | 32.0 (29.6–45.0) | 30.0 (27.4–30.6) | 4.0 (2.3–6.9) | 4.9 (4.0–6.4) |
| oxaliplatin 0.1 M | 63.8 (60.9–69.7) * | 2.8 (1.4–3.9) * | 19.8 (16.4–21.4) * | 5.5 (2.4–9.4) * | 1.5 (1.1–3.7) * | 2.8 (1.4–3.7) |
| CB[7]–oxaliplatin 0.1 M | 65.0 (62.4–71.6) * | 2.8 (1.6–4.6) * | 19.9 (17.8–27.5) * | 5.7 (2.4–7.2) * | 1.9 (0.9–2.2) * | 3.6 (2.7–4.3) † |
| Carboplatin | Oxaliplatin | |
|---|---|---|
| Increased cytotoxicity for tumor cell lines | + | ++ |
| Deceased cytotoxicity for non-tumor cells | 0 | 0 |
| Decreased immunosuppression in vitro | 0 | 0 |
| Increased antitumor activity in vivo | + | 0 |
| Decreased side effects in vivo | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashkina, E.; Aktanova, A.; Mirzaeva, I.; Kovalenko, E.; Andrienko, I.; Knauer, N.; Pronkina, N.; Kozlov, V. The Effect of Cucurbit[7]uril on the Antitumor and Immunomodulating Properties of Oxaliplatin and Carboplatin. Int. J. Mol. Sci. 2021, 22, 7337. https://doi.org/10.3390/ijms22147337
Pashkina E, Aktanova A, Mirzaeva I, Kovalenko E, Andrienko I, Knauer N, Pronkina N, Kozlov V. The Effect of Cucurbit[7]uril on the Antitumor and Immunomodulating Properties of Oxaliplatin and Carboplatin. International Journal of Molecular Sciences. 2021; 22(14):7337. https://doi.org/10.3390/ijms22147337
Chicago/Turabian StylePashkina, Ekaterina, Alina Aktanova, Irina Mirzaeva, Ekaterina Kovalenko, Irina Andrienko, Nadezhda Knauer, Natalya Pronkina, and Vladimir Kozlov. 2021. "The Effect of Cucurbit[7]uril on the Antitumor and Immunomodulating Properties of Oxaliplatin and Carboplatin" International Journal of Molecular Sciences 22, no. 14: 7337. https://doi.org/10.3390/ijms22147337
APA StylePashkina, E., Aktanova, A., Mirzaeva, I., Kovalenko, E., Andrienko, I., Knauer, N., Pronkina, N., & Kozlov, V. (2021). The Effect of Cucurbit[7]uril on the Antitumor and Immunomodulating Properties of Oxaliplatin and Carboplatin. International Journal of Molecular Sciences, 22(14), 7337. https://doi.org/10.3390/ijms22147337






