Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners
Abstract
:1. Introduction
2. Results
2.1. Effects of the Stimulation of PPARγ or the Inhibition of ROCKs on TGF-β2-Treated 2D HconF Monolayers
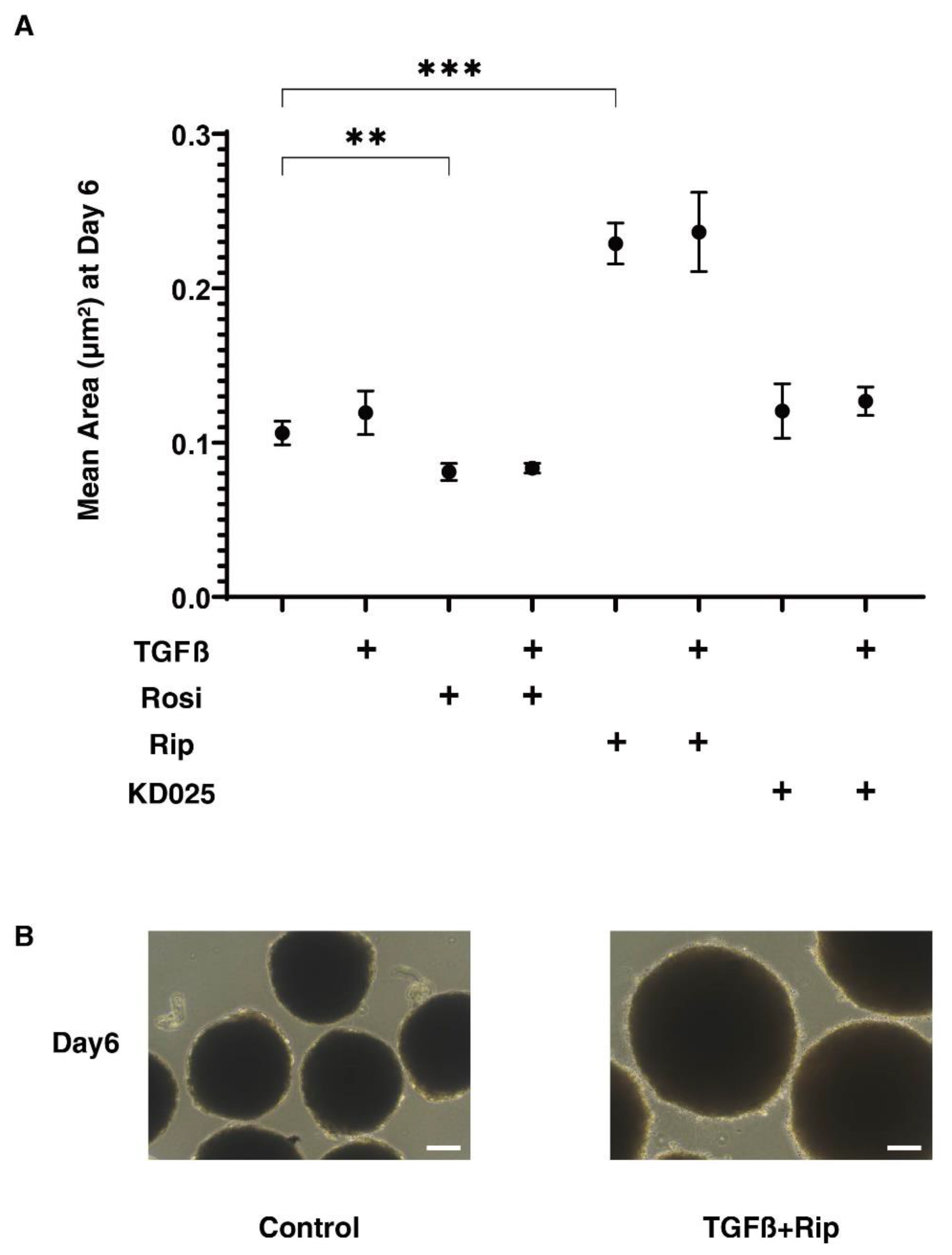
2.2. Effects of the Stimulation of PPARγ or the Inhibition of ROCKs on the Physical Properties of the TGF-β2-Treated 3D HconF Sphenoids
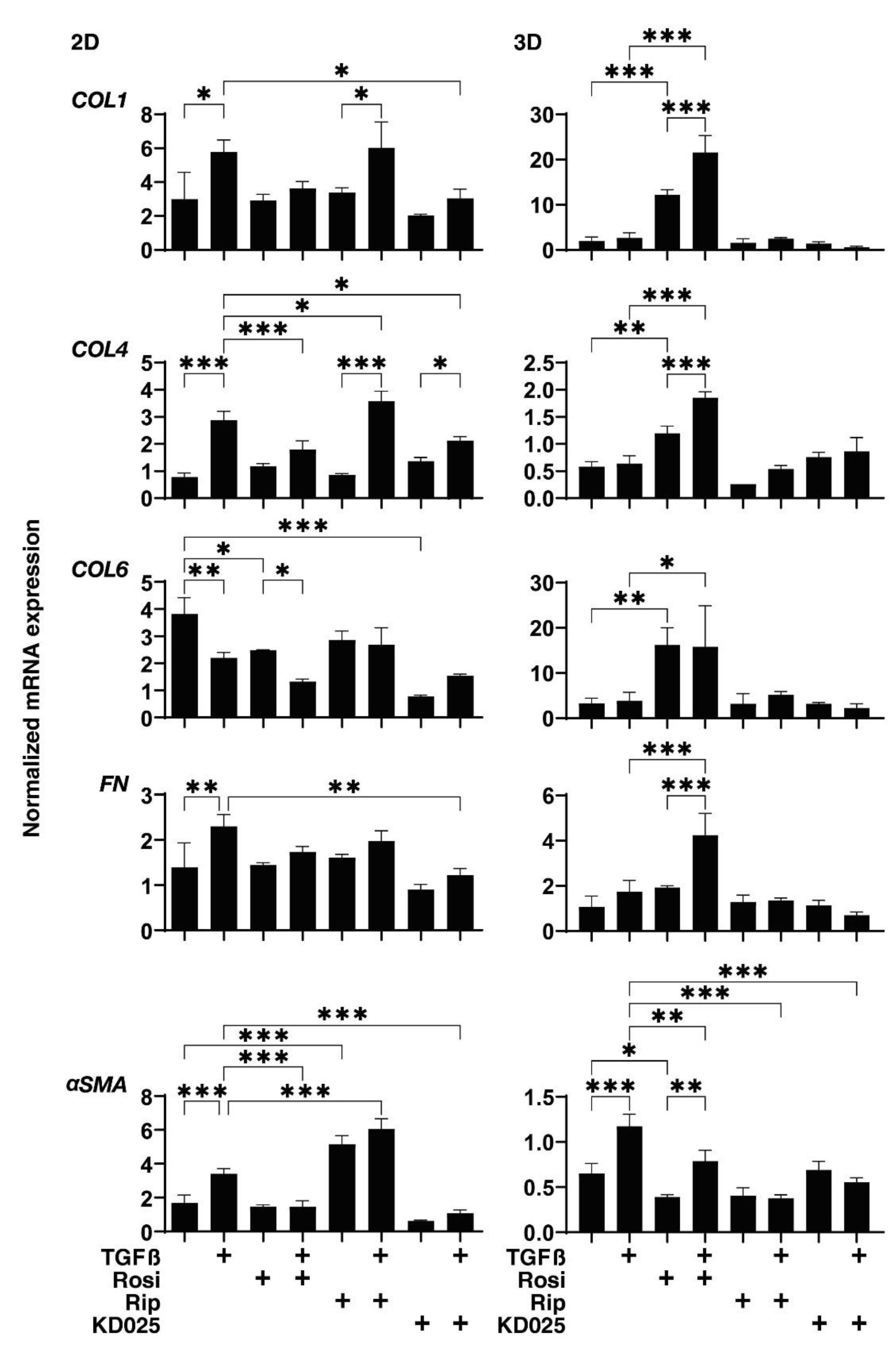
2.3. Effects of the Stimulation of PPARγ or the Inhibition of ROCKs on the mRNA Expression of TGF-β2-Treated 2D and 3D HconF Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Transendothelial Electron Resistance (TEER) Measurements of 2D HTM Culture
4.3. Quantitative PCR
4.4. Solidity Measurements of 3D Organoids
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolaños-Jiménez, R.; Navas, A.; López-Lizárraga, E.P.; de Ribot, F.M.; Peña, A.; Graue-Hernández, E.O.; Garfias, Y. Ocular Surface as Barrier of Innate Immunity. Open Ophthalmol. J. 2015, 9, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahi, M.; Shafiee, A.; Bathaiee, F.S.; Sharifzadeh, M.; Nikfar, S. Drug-induced toxic reactions in the eye: An overview. J. Infus. Nurs. Off. Publ. Infus. Nurses Soc. 2004, 27, 386–398. [Google Scholar] [CrossRef]
- Khaw, P.T.; Migdal, C.S. Current techniques in wound healing modulation in glaucoma surgery. Curr. Opin. Ophthalmol. 1996, 7, 24–33. [Google Scholar] [CrossRef]
- Chiou, A.G.; Florakis, G.J.; Kazim, M. Management of conjunctival cicatrizing diseases and severe ocular surface dysfunction. Surv. Ophthalmol. 1998, 43, 19–46. [Google Scholar] [CrossRef]
- Chui, J.; Di Girolamo, N.; Wakefield, D.; Coroneo, M.T. The pathogenesis of pterygium: Current concepts and their therapeutic implications. Ocul. Surf. 2008, 6, 24–43. [Google Scholar] [CrossRef]
- Dale, S.B.; Saban, D.R. Linking immune responses with fibrosis in allergic eye disease. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Munir, S.Z.; Aylward, J. A Review of Ocular Graft-Versus-Host Disease. Optom. Vis. Sci. 2017, 94, 545–555. [Google Scholar] [CrossRef]
- Broadway, D.C.; Chang, L.P. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J. Glaucoma 2001, 10, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Schlunck, G.; Meyer-ter-Vehn, T.; Klink, T.; Grehn, F. Conjunctival fibrosis following filtering glaucoma surgery. Exp. Eye Res. 2016, 142, 76–82. [Google Scholar] [CrossRef]
- White, E.S.; Lazar, M.H.; Thannickal, V.J. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J. Pathol. 2003, 201, 343–354. [Google Scholar] [CrossRef]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv. Wound Care 2013, 2, 195–214. [Google Scholar] [CrossRef] [Green Version]
- Phan, S.H. The myofibroblast in pulmonary fibrosis. Chest 2002, 122, 286s–289s. [Google Scholar] [CrossRef]
- Vaughan, M.B.; Howard, E.W.; Tomasek, J.J. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp. Cell Res. 2000, 257, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Y.; Phan, S.H. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am. J. Respir. Cell Mol. Biol. 1999, 21, 658–665. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: A computational approach. Front. Pharm. 2015, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Bishop-Bailey, D.; Wray, J. Peroxisome proliferator-activated receptors: A critical review on endogenous pathways for ligand generation. Prostaglandins Other Lipid Mediat. 2003, 71, 1–22. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef]
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From orphan receptors to drug discovery. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef] [Green Version]
- Day, C. Thiazolidinediones: A new class of antidiabetic drugs. Diabet. Med. 1999, 16, 179–192. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Chen, L.; Luo, H.; Chen, J.; Cheng, F.; Gui, C.; Zhang, R.; Shen, J.; Chen, K.; Jiang, H.; et al. Binding analyses between Human PPARgamma-LBD and ligands. Eur. J. Biochem. 2004, 271, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Ouyang, P.B.; Tian, J.; Guo, X.J.; Duan, X.C. Rosiglitazone inhibits TGF-β 1 induced activation of human Tenon fibroblasts via p38 signal pathway. PLoS ONE 2014, 9, e105796. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Manser, E.; Tan, L.; Lim, L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem. 1995, 270, 29051–29054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef]
- Yin, J.; Yu, F.S. Rho kinases regulate corneal epithelial wound healing. Am. J. Physiol. Cell Physiol. 2008, 295, C378–C387. [Google Scholar] [CrossRef]
- Yin, J.; Lu, J.; Yu, F.S. Role of small GTPase Rho in regulating corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 2008, 49, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sun, A.; Li, L.; Zhao, G.; Jia, J.; Wang, K.; Ge, J.; Zou, Y. Up-regulation of BMP-2 antagonizes TGF-β1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/Smad6 complex. J. Cell Mol. Med. 2012, 16, 2301–2310. [Google Scholar] [CrossRef]
- Inai, K.; Burnside, J.L.; Hoffman, S.; Toole, B.P.; Sugi, Y. BMP-2 induces versican and hyaluronan that contribute to post-EMT AV cushion cell migration. PLoS ONE 2013, 8, e77593. [Google Scholar] [CrossRef] [Green Version]
- Scimone, C.; Bramanti, P.; Ruggeri, A.; Donato, L.; Alafaci, C.; Crisafulli, C.; Mucciardi, M.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. CCM3/SERPINI1 bidirectional promoter variants in patients with cerebral cavernous malformations: A molecular and functional study. BMC Med. Genet. 2016, 17, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, C.; Bramanti, P.; Scimone, C.; Donato, L.; Alafaci, C.; D’Angelo, R.; Sidoti, A. Relevance of CCM gene polymorphisms for clinical management of sporadic cerebral cavernous malformations. J. Neurol. Sci. 2017, 380, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Bramanti, P.; Ruggeri, A.; Katsarou, Z.; Donato, L.; Sidoti, A.; D’Angelo, R. Detection of Novel Mutation in Ccm3 Causes Familial Cerebral Cavernous Malformations. J. Mol. Neurosci. MN 2015, 57, 400–403. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Invest. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Weaver, V.M.; Petersen, O.W.; Larabell, C.A.; Dedhar, S.; Briand, P.; Lupu, R.; Bissell, M.J. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 1998, 95, 14821–14826. [Google Scholar] [CrossRef]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-B.; Zhong, Y.-S.; Cheng, Y.; Shen, X. Rho/ROCK pathway and neural regeneration: A potential therapeutic target for central nervous system and optic nerve damage. Int. J. Ophthalmol. 2011, 4, 652–657. [Google Scholar]
- Stiles, J.M.; Kurisetty, V.; Mitchell, D.C.; Bryan, B.A. Rho Kinase Proteins Regulate Global miRNA Expression in Endothelial Cells. Cancer Genom. Proteom. 2013, 10, 251–263. [Google Scholar]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Waki, M.; Yoshida, Y.; Oka, T.; Azuma, M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr. Eye Res. 2001, 22, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Hamuro, J.; Kinoshita, S. The New Therapeutic Concept of Using a Rho Kinase Inhibitor for the Treatment of Corneal Endothelial Dysfunction. Cornea 2011, 30, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Trovato Battagliola, E.; D’Angelo, R.; Sidoti, A.; Donato, L. Expression of Pro-Angiogenic Markers Is Enhanced by Blue Light in Human RPE Cells. Antioxidants 2020, 9, 1154. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Pitruzzella, A.; Scalia, F.; D’Angelo, R.; Sidoti, A. Possible A2E Mutagenic Effects on RPE Mitochondrial DNA from Innovative RNA-Seq Bioinformatics Pipeline. Antioxidants 2020, 9, 1158. [Google Scholar] [CrossRef]
- Nakajima, E.; Nakajima, T.; Minagawa, Y.; Shearer, T.R.; Azuma, M. Contribution of ROCK in Contraction of Trabecular Meshwork: Proposed Mechanism for Regulating Aqueous Outflow in Monkey and Human Eyes. J. Pharm. Sci. 2005, 94, 701–708. [Google Scholar] [CrossRef]
- Tamura, M.; Nakao, H.; Yoshizaki, H.; Shiratsuchi, M.; Shigyo, H.; Yamada, H.; Ozawa, T.; Totsuka, J.; Hidaka, H. Development of specific Rho-kinase inhibitors and their clinical application. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2005, 1754, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P. Ripasudil: First Global Approval. Drugs 2014, 74, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Kassumeh, S.; von Studnitz, A.; Priglinger, S.G.; Fuchshofer, R.; Luft, N.; Moloney, G.; Dirisamer, M.; Ohlmann, A. Ex vivo excimer laser ablation of cornea guttata and ROCK inhibitor-aided endothelial recolonization of ablated central cornea. Acta Ophthalmol. 2020, 98, e773–e780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, Y.; Ikeda, Y.; Mori, K.; Yoshii, K.; Ueno, M.; Yoshikawa, H.; Sotozono, C.; Kinoshita, S. Morphological change and recovery of corneal endothelial cells after rho-associated protein kinase inhibitor eye-drop (ripasudil 0.4%) instillation. Brit. J. Ophthalmol. 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, A.; Inoue, T.; Fujimoto, T.; Inoue-Mochita, M.; Kawai, M.; Tanihara, H. The effects of ripasudil (K-115), a Rho kinase inhibitor, on activation of human conjunctival fibroblasts. Exp. Eye Res. 2016, 149, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.G.; Ko, J.A.; Iwata, W.; Okumichi, H.; Kiuchi, Y. An in vitro study of scarring formation mediated by human Tenon fibroblasts: Effect of Y-27632, a Rho kinase inhibitor. Cell Biochem. Funct. 2019, 37, 113–124. [Google Scholar] [CrossRef]
- Yamanaka, O.; Saika, S.; Ohnishi, Y.; Kim-Mitsuyama, S.; Kamaraju, A.K.; Ikeda, K. Inhibition of p38MAP kinase suppresses fibrogenic reaction in conjunctiva in mice. Mol. Vis. 2007, 13, 1730–1739. [Google Scholar] [PubMed]
- Kiyono, T. Molecular mechanisms of cellular senescence and immortalization of human cells. Expert Opin. Ther. targets 2007, 11, 1623–1637. [Google Scholar] [CrossRef]
- Kawai, T.; Masaki, T.; Doi, S.; Arakawa, T.; Yokoyama, Y.; Doi, T.; Kohno, N.; Yorioka, N. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Investig. 2009, 89, 47–58. [Google Scholar] [CrossRef]
- Dai, B.; Liu, Y.; Mei, C.; Fu, L.; Xiong, X.; Zhang, Y.; Shen, X.; Hua, Z. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin. Sci. (London England: 1979) 2010, 119, 323–333. [Google Scholar] [CrossRef]
- Fan, F.; Li, Y.; Duan, X.; Zhao, T.; Pan, D.; Chen, H. Rosiglitazone attenuates activation of human Tenon’s fibroblasts induced by transforming growth factor-β1. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1213–1220. [Google Scholar] [CrossRef]
- Nakamoto, M.; Ohya, Y.; Shinzato, T.; Mano, R.; Yamazato, M.; Sakima, A.; Takishita, S. Pioglitazone, a thiazolidinedione derivative, attenuates left ventricular hypertrophy and fibrosis in salt-sensitive hypertension. Hypertens Res. 2008, 31, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, S.; Chu, E.S.; Go, M.Y.; Lau, R.H.; Zhao, J.; Wu, C.W.; Tong, L.; Zhao, J.; Poon, T.C.; et al. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int. J. Biochem. Cell Biol. 2010, 42, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, K.; Cao, M.; Qu, J.; Zhou, D.; Pan, Z.; Duan, X.; Zhou, Y. Rosiglitazone Treatment Prevents Postoperative Fibrosis in a Rabbit Model of Glaucoma Filtration Surgery. Invest. Ophthalmol. Vis. Sci. 2019, 60, 2743–2752. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Liu, K.; Pan, Z.; Cao, M.; Zhou, D.; Liu, H.; Huang, Y.; Duan, X. Effects of rosiglitazone/PHBV drug delivery system on postoperative fibrosis in rabbit glaucoma filtration surgery model. Drug Deliv. 2019, 26, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Cerbone, A.; Toaldo, C.; Minelli, R.; Ciamporcero, E.; Pizzimenti, S.; Pettazzoni, P.; Roma, G.; Dianzani, M.U.; Ullio, C.; Ferretti, C.; et al. Rosiglitazone and AS601245 decrease cell adhesion and migration through modulation of specific gene expression in human colon cancer cells. PLoS ONE 2012, 7, e40149. [Google Scholar] [CrossRef]
- Fitzgerald, A.M.; Benz, C.; Clark, A.F.; Wordinger, R.J. The effects of transforming growth factor-β2 on the expression of follistatin and activin A in normal and glaucomatous human trabecular meshwork cells and tissues. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7358–7369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Z.M.; Elner, S.G.; Elner, V.M. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp. Eye Res. 2007, 84, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Sarret, Y.; Woodley, D.T.; Grigsby, K.; Wynn, K.; O’Keefe, E.J. Human keratinocyte locomotion: The effect of selected cytokines. J. Investig. Dermatol. 1992, 98, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef] [PubMed]


| Sequence | Exon Location | RefSeqNumber | ||
|---|---|---|---|---|
| human RPLP0 | Probe | 5′-/56-FAM/CCCTGTCTT/ZEN/CCCTGGGCATCAC/3IABkFQ/-3′ | 2–3 | NM_001002 |
| Primer2 | 5′-TCGTCTTTAAACCCTGCGTG-3′ | |||
| Primer1 | 5′-TGTCTGCTCCCACAATGAAAC-3′ | |||
| human COL1A1 | Probe | 5′-/56-FAM/TCGAGGGCC/ZEN/AAGACGAAGACATC/3IABkFQ/-3′ | 1–2 | NM_000088 |
| Primer2 | 5′-GACATGTTCAGCTTTGTGGAC-3′ | |||
| Primer1 | 5′-TTCTGTACGCAGGTGATTGG-3′ | |||
| human COL4A1 | Probe | 5′-/56-FAM/TCATACAGA/ZEN/CTTGGCAGCGGCT/3IABkFQ/-3′ | 51–52 | NM_001845 |
| Primer2 | 5′-AGAGAGGAGCGAGATGTTCA-3′ | |||
| Primer1 | 5′-TGAGTCAGGCTTCATTATGTTCT-3′ | |||
| human COL6A1 | Primer2 | 5′-CCTCGTGGACAAAGTCAAGT-3′ | 2–3 | NM_001848 |
| Primer1 | 5′-GTGAGGCCTTGGATGATCTC-3′ | |||
| human FN1 | Primer2 | 5′-CGTCCTAAAGACTCCATGATCTG-3′ | 3–4 | NM_212482 |
| Primer1 | 5′-ACCAATCTTGTAGGACTGACC-3′ | |||
| human αSMA | Probe | 5′-/56-FAM/AGACCCTGT/ZEN/TCCAGCCATCCTTC/3IABkFQ/-3′ | 8–9 | NM_001613 |
| Primer2 | 5′-AGAGTTACGAGTTGCCTGATG-3′ | |||
| Primer1 | 5′-CTGTTGTAGGTGGTTTCATGGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. https://doi.org/10.3390/ijms22147335
Oouchi Y, Watanabe M, Ida Y, Ohguro H, Hikage F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. International Journal of Molecular Sciences. 2021; 22(14):7335. https://doi.org/10.3390/ijms22147335
Chicago/Turabian StyleOouchi, Yuika, Megumi Watanabe, Yosuke Ida, Hiroshi Ohguro, and Fumihito Hikage. 2021. "Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners" International Journal of Molecular Sciences 22, no. 14: 7335. https://doi.org/10.3390/ijms22147335
APA StyleOouchi, Y., Watanabe, M., Ida, Y., Ohguro, H., & Hikage, F. (2021). Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. International Journal of Molecular Sciences, 22(14), 7335. https://doi.org/10.3390/ijms22147335







