Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen
Abstract
:1. Introduction
2. Results
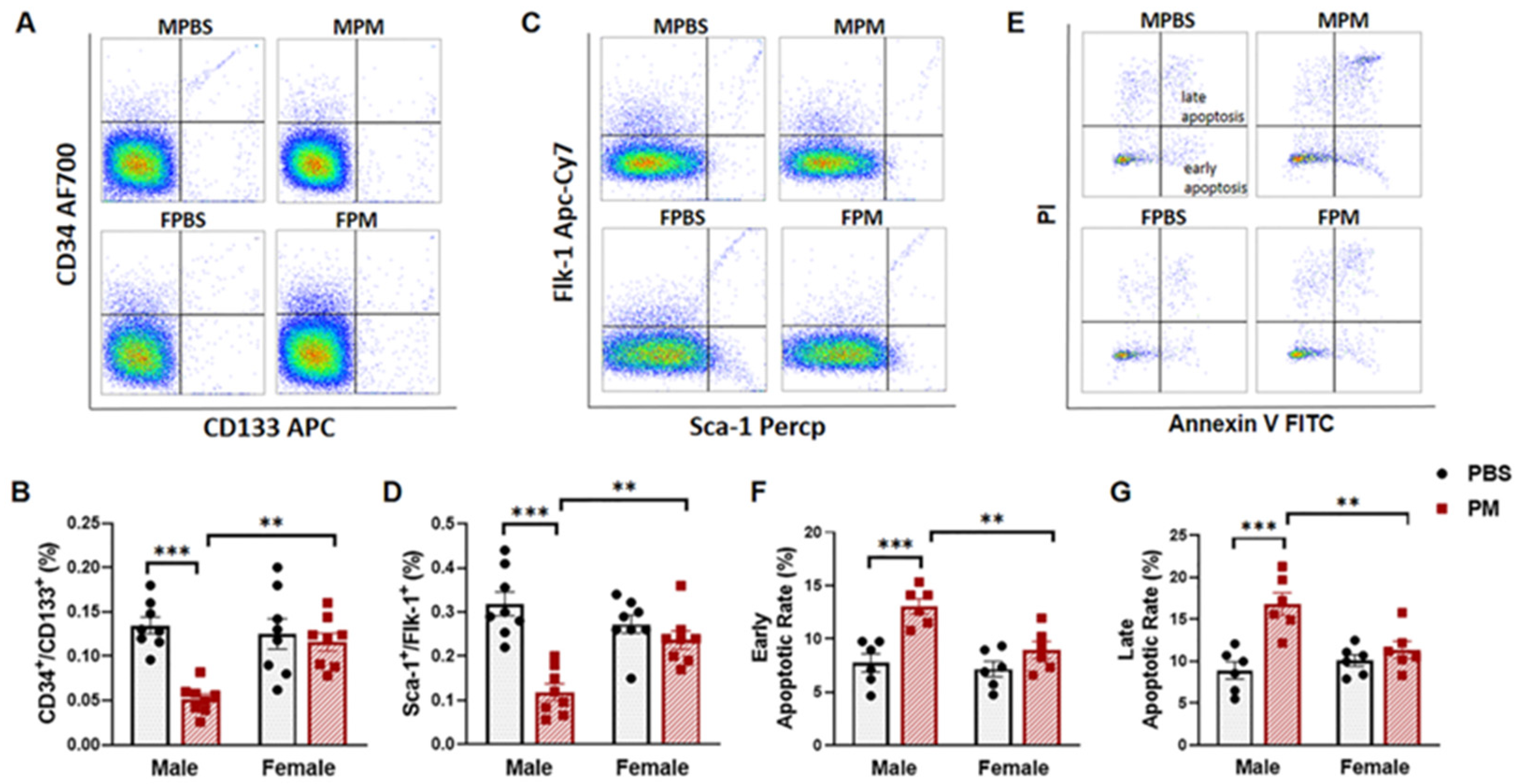
2.1. PM Exposure Decreased Circulating EPCs with an Increased Apoptosis Rate in Male Mice but Not in Female Mice
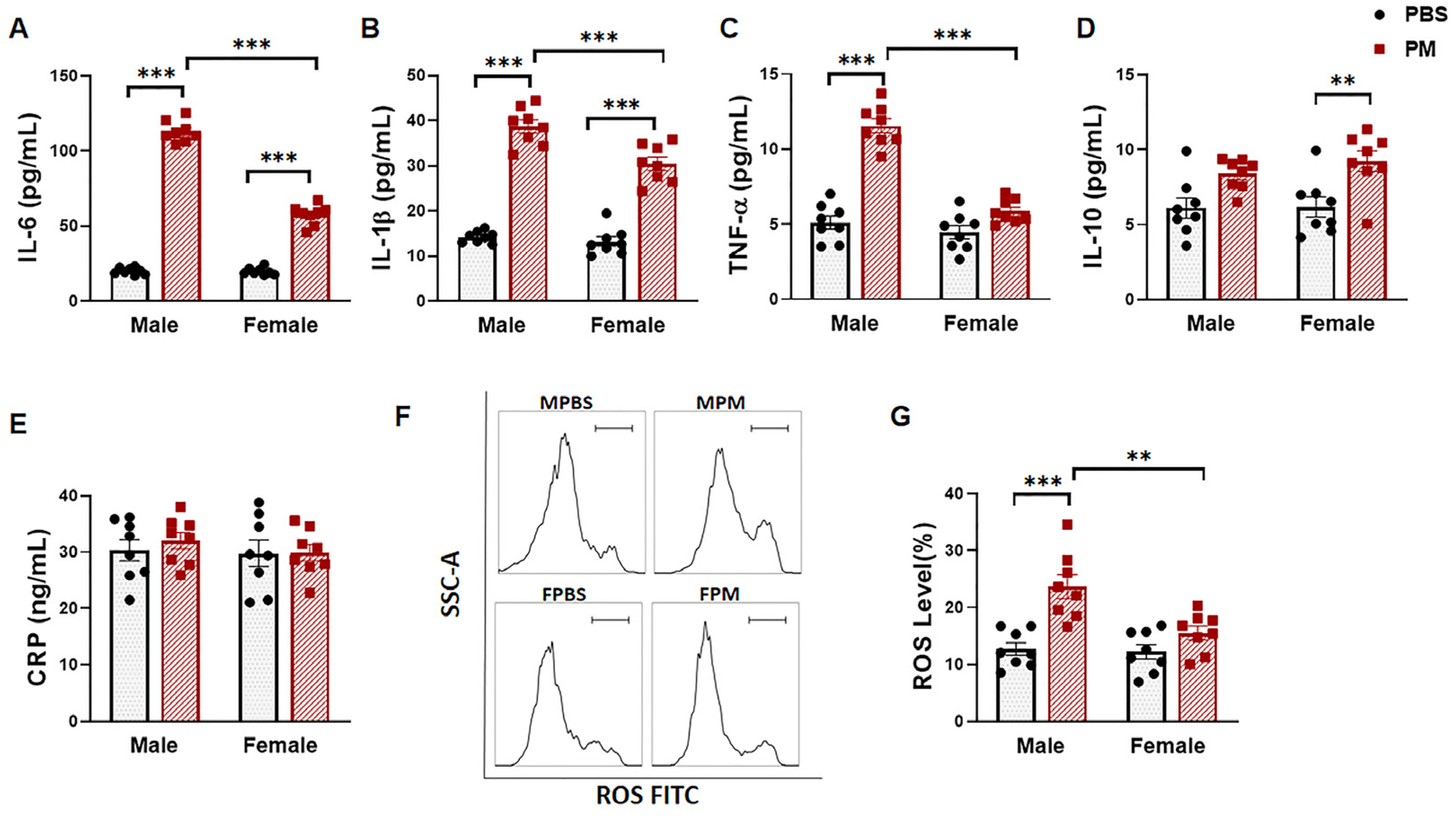
2.2. PM Exposure Significantly Increased Serum Pro-Inflammatory Cytokines and Intracellular ROS Levels
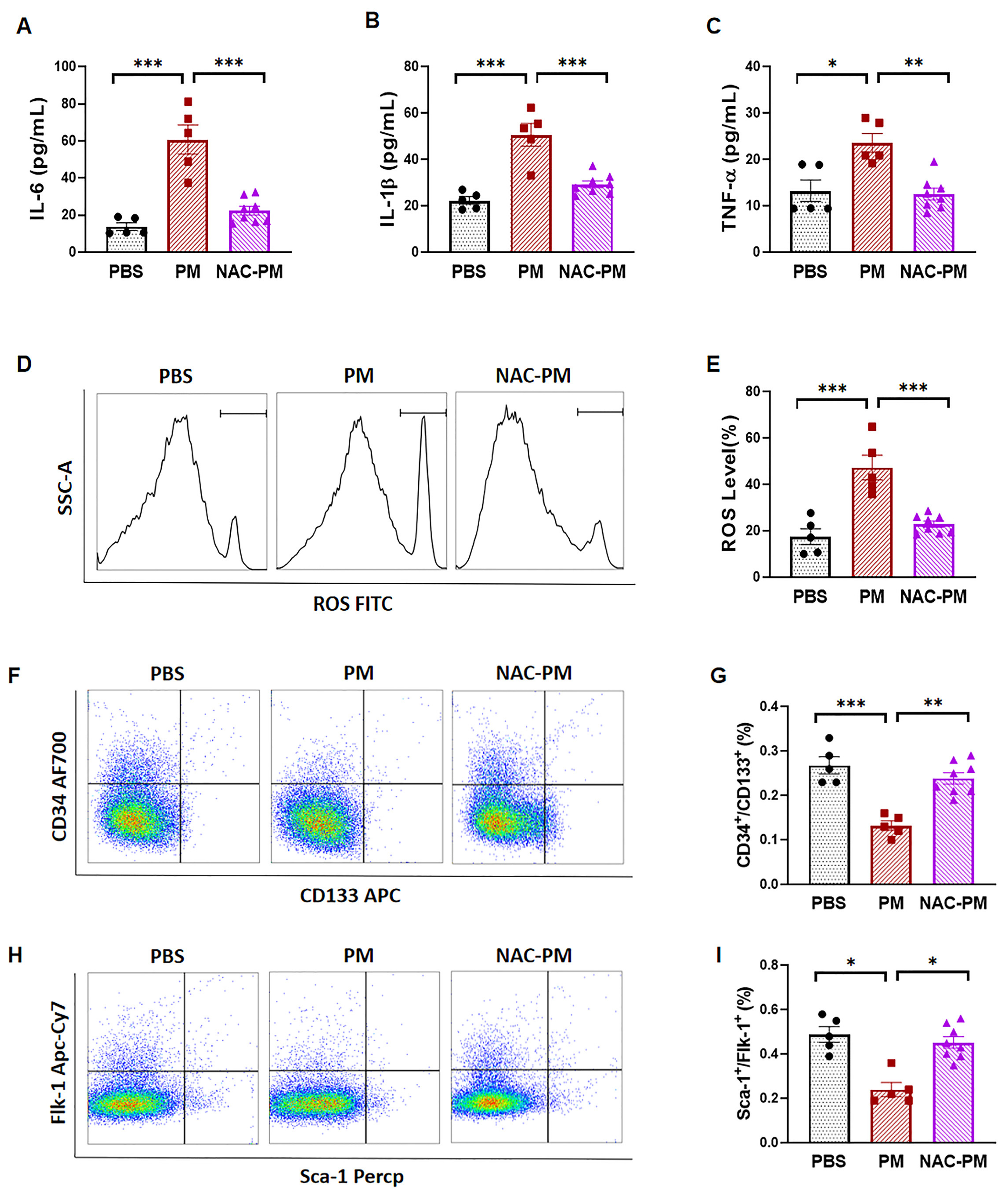
2.3. Antioxidant NAC Treatment Prevented PM Exposure-Induced Production of Inflammatory Cytokines and Reduction of Circulating EPCs in Male Mice
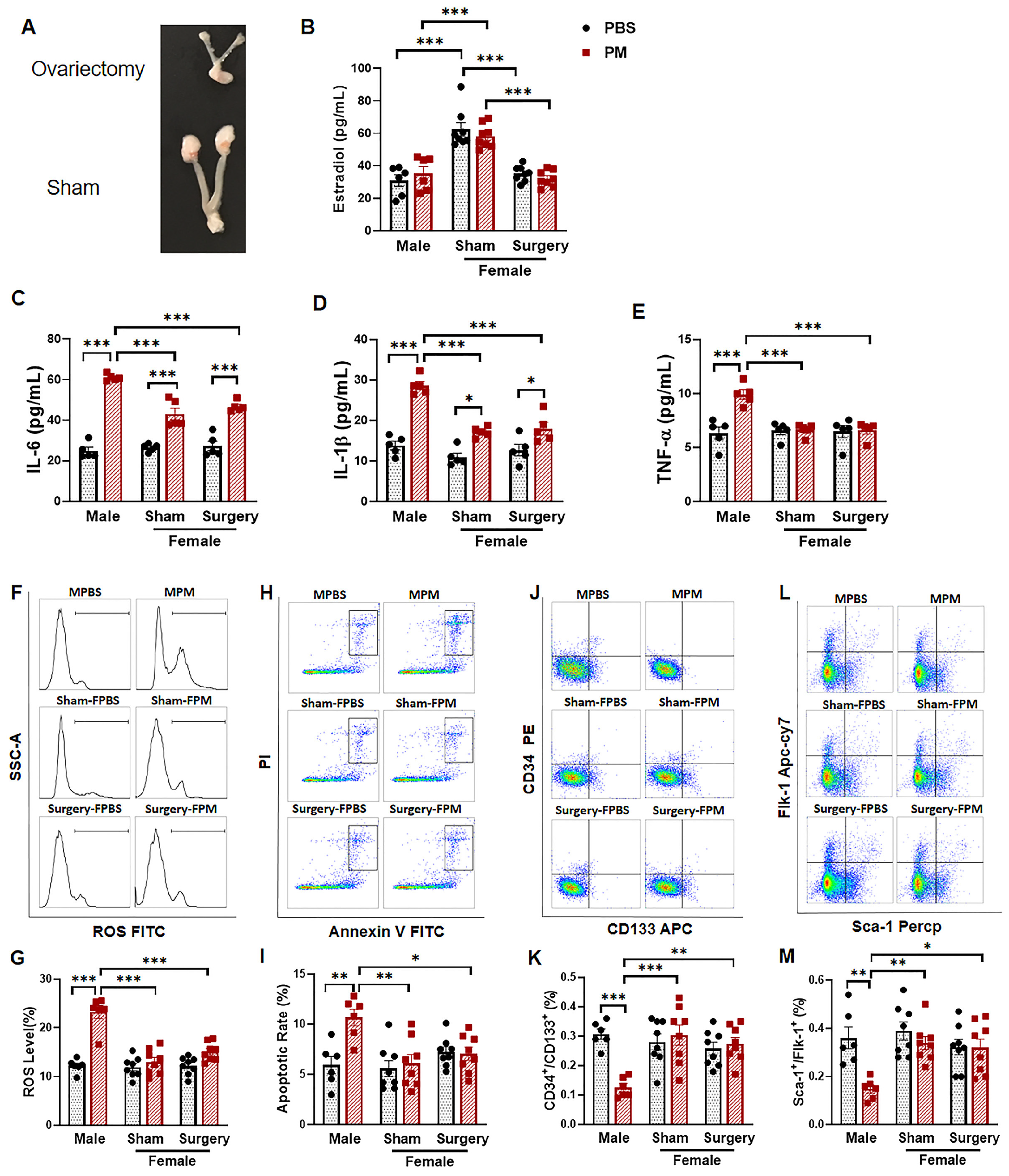
2.4. Ovariectomy Had no Effect on the Circulating EPC Population in Female Mice with PM Exposure
3. Discussion
4. Materials and Methods
4.1. PM Exposure and Animal Model
4.2. Measurement of Inflammatory Cytokines and Estradiol
4.3. Circulating Endothelial Progenitor Cell Analysis
4.4. Intracellular ROS Measurement
4.5. Cell Apoptosis Analysis
4.6. Ovariectomy
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [CrossRef] [Green Version]
- Meo, S.A.; Suraya, F. Effect of environmental air pollution on cardiovascular diseases. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4890–4897. [Google Scholar] [PubMed]
- Brunekreef, B.; Forsberg, B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [Green Version]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Gimbrone, M.J.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arter. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; O’Toole, T.E.; Conklin, D.J.; Hill, B.G.; Haberzettl, P. Endothelial progenitor cells as critical mediators of environmental air pollution-induced cardiovascular toxicity. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1440–H1455. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Weber, C. Ambivalence of progenitor cells in vascular repair and plaque stability. Curr. Opin. Lipidol. 2008, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wils, J.; Favre, J.; Bellien, J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol. Ther. 2017, 170, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xie, X.; Jia, F.; He, J.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, J.Z.; Cowan, P.J.; et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell Physiol. Biochem. 2015, 35, 353–363. [Google Scholar] [CrossRef]
- O’Toole, T.E.; Hellmann, J.; Wheat, L.; Haberzettl, P.; Lee, J.; Conklin, D.J.; Bhatnagar, A.; Pope, C.R. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ. Res. 2010, 107, 200–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattoor, A.J.; Pothineni, N.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Dabla, P.K. Oxidative stress and antioxidants in hypertension-a current review. Curr. Hypertens. Rev. 2015, 11, 132–142. [Google Scholar] [CrossRef]
- Haberzettl, P.; Conklin, D.J.; Abplanalp, W.T.; Bhatnagar, A.; O’Toole, T.E. Inhalation of Fine Particulate Matter Impairs Endothelial Progenitor Cell Function Via Pulmonary Oxidative Stress. Arter. Thromb Vasc Biol 2018, 38, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox. Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Liberda, E.N.; Cuevas, A.K.; Qu, Q.; Chen, L.C. The acute exposure effects of inhaled nickel nanoparticles on murine endothelial progenitor cells. Inhal. Toxicol. 2014, 26, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Liberda, E.N.; Qu, S.; Guo, X.; Li, X.; Zhang, J.; Meng, J.; Yan, B.; Li, N.; Zhong, M.; et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS ONE 2013, 8, e83782. [Google Scholar] [CrossRef]
- Haberzettl, P.; Lee, J.; Duggineni, D.; McCracken, J.; Bolanowski, D.; O’Toole, T.E.; Bhatnagar, A.; Conklin, D.J. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ. Health Perspect. 2012, 120, 848–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poss, J.; Lorenz, D.; Werner, C.; Pavlikova, V.; Gensch, C.; Speer, T.; Alessandrini, F.; Berezowski, V.; Kuntz, M.; Mempel, M.; et al. Diesel exhaust particles impair endothelial progenitor cells, compromise endothelial integrity, reduce neoangiogenesis, and increase atherogenesis in mice. Cardiovasc. Toxicol. 2013, 13, 290–300. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Munzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Xu, Z.; Wu, G.C.; Mao, Y.M.; Liu, L.N.; Wu, Q.; Dan, Y.L.; Tao, S.S.; Zhang, Q.; Sam, N.B.; et al. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 2019, 18, 607–614. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Nakao, N.; Kurokawa, T.; Nonami, T.; Tumurkhuu, G.; Koide, N.; Yokochi, T. Hydrogen peroxide induces the production of tumor necrosis factor-alpha in RAW 264.7 macrophage cells via activation of p38 and stress-activated protein kinase. Innate Immun. 2008, 14, 190–196. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- Voigt, A.; Rahnefeld, A.; Kloetzel, P.M.; Kruger, E. Cytokine-induced oxidative stress in cardiac inflammation and heart failure-how the ubiquitin proteasome system targets this vicious cycle. Front. Physiol. 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Fraguas, D.; Diaz-Caneja, C.M.; Ayora, M.; Hernandez-Alvarez, F.; Rodriguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P.; Cassis, L.A.; Eghbali, M.; Reue, K.; Sandberg, K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arter. Thromb. Vasc. Biol. 2017, 37, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Casimir, G.J.; Duchateau, J. Gender differences in inflammatory processes could explain poorer prognosis for males. J. Clin. Microbiol. 2011, 49, 478, author reply 478–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, D.; Cooper, L.J.; Blauwet, L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindl, L.; Buderus, S.; Dahlem, P.; Demirakca, S.; Goldner, M.; Huth, R.; Kohl, M.; Krause, M.; Kuhl, P.; Lasch, P.; et al. Gender-based differences in children with sepsis and ARDS: The ESPNIC ARDS Database Group. Intensive Care Med. 2003, 29, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Zemskova, M.; Kurdyukov, S.; James, J.; McClain, N.; Rafikov, R.; Rafikova, O. Sex-specific stress response and HMGB1 release in pulmonary endothelial cells. PLoS ONE 2020, 15, e0231267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, K.M.; Johansen, A.K.; Wright, A.F.; Wallace, E.; MacLean, M.R. Pulmonary arterial hypertension: Basis of sex differences in incidence and treatment response. Br. J. Pharmacol. 2014, 171, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Parker, W.H.; Jacoby, V.; Shoupe, D.; Rocca, W. Effect of bilateral oophorectomy on women’s long-term health. Womens Health 2009, 5, 565–576. [Google Scholar] [CrossRef]
- Esmailidehaj, M.; Kuchakzade, F.; Rezvani, M.E.; Farhadi, Z.; Esmaeili, H.; Azizian, H. 17beta-Estradiol improves insulin signalling and insulin resistance in the aged female hearts: Role of inflammatory and anti-inflammatory cytokines. Life Sci. 2020, 253, 117673. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Du, S.; Itoh, N.; Askarinam, S.; Hill, H.; Arnold, A.P.; Voskuhl, R.R. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2014, 111, 2806–2811. [Google Scholar] [CrossRef] [Green Version]
- Tamosiuniene, R.; Manouvakhova, O.; Mesange, P.; Saito, T.; Qian, J.; Sanyal, M.; Lin, Y.C.; Nguyen, L.P.; Luria, A.; Tu, A.B.; et al. Dominant Role for Regulatory T Cells in Protecting Females Against Pulmonary Hypertension. Circ. Res. 2018, 122, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Barp, J.; Araujo, A.S.; Fernandes, T.R.; Rigatto, K.V.; Llesuy, S.; Bello-Klein, A.; Singal, P. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz. J. Med. Biol. Res. 2002, 35, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater oxidative stress in healthy young men compared with premenopausal women. Arter. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ji, L.L.; Liu, T.Y.; Wang, Z.T. Evaluation of gender-related differences in various oxidative stress enzymes in mice. Chin. J. Physiol. 2011, 54, 385–390. [Google Scholar]
- Nocun, M.S.; Schantz, M.M. Determination of selected oxygenated polycyclic aromatic hydrocarbons (oxy-PAHs) in diesel and air particulate matter standard reference materials (SRMs). Anal. Bioanal. Chem. 2013, 405, 5583–5593. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2’,7’-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Zielonka, J.; Kalyanaraman, B. Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic. Biol. Med. 2018, 128, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Brauchle, E.; Thude, S.; Brucker, S.Y.; Schenke-Layland, K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci. Rep. 2014, 4, 4698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, V.R.; Mendes, E.; Casaro, M.; Antiorio, A.; Oliveira, F.A.; Ferreira, C.M. Description of Ovariectomy Protocol in Mice. Methods Mol. Biol. 2019, 1916, 303–309. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xiao, Y.; Zhu, Q.; Cui, Y.; Hao, H.; Wang, M.; Cowan, P.J.; Korthuis, R.J.; Li, G.; Sun, Q.; et al. Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen. Int. J. Mol. Sci. 2021, 22, 7200. https://doi.org/10.3390/ijms22137200
Liu X, Xiao Y, Zhu Q, Cui Y, Hao H, Wang M, Cowan PJ, Korthuis RJ, Li G, Sun Q, et al. Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen. International Journal of Molecular Sciences. 2021; 22(13):7200. https://doi.org/10.3390/ijms22137200
Chicago/Turabian StyleLiu, Xuanyou, Yichao Xiao, Qingyi Zhu, Yuqi Cui, Hong Hao, Meifang Wang, Peter J. Cowan, Ronald J. Korthuis, Guangfu Li, Qinghua Sun, and et al. 2021. "Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen" International Journal of Molecular Sciences 22, no. 13: 7200. https://doi.org/10.3390/ijms22137200
APA StyleLiu, X., Xiao, Y., Zhu, Q., Cui, Y., Hao, H., Wang, M., Cowan, P. J., Korthuis, R. J., Li, G., Sun, Q., & Liu, Z. (2021). Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen. International Journal of Molecular Sciences, 22(13), 7200. https://doi.org/10.3390/ijms22137200





