Regenerative Effects of Hypoxia Primed Flowable Placental Formulation in Muscle and Dermal Injury
Abstract
1. Introduction
2. Results
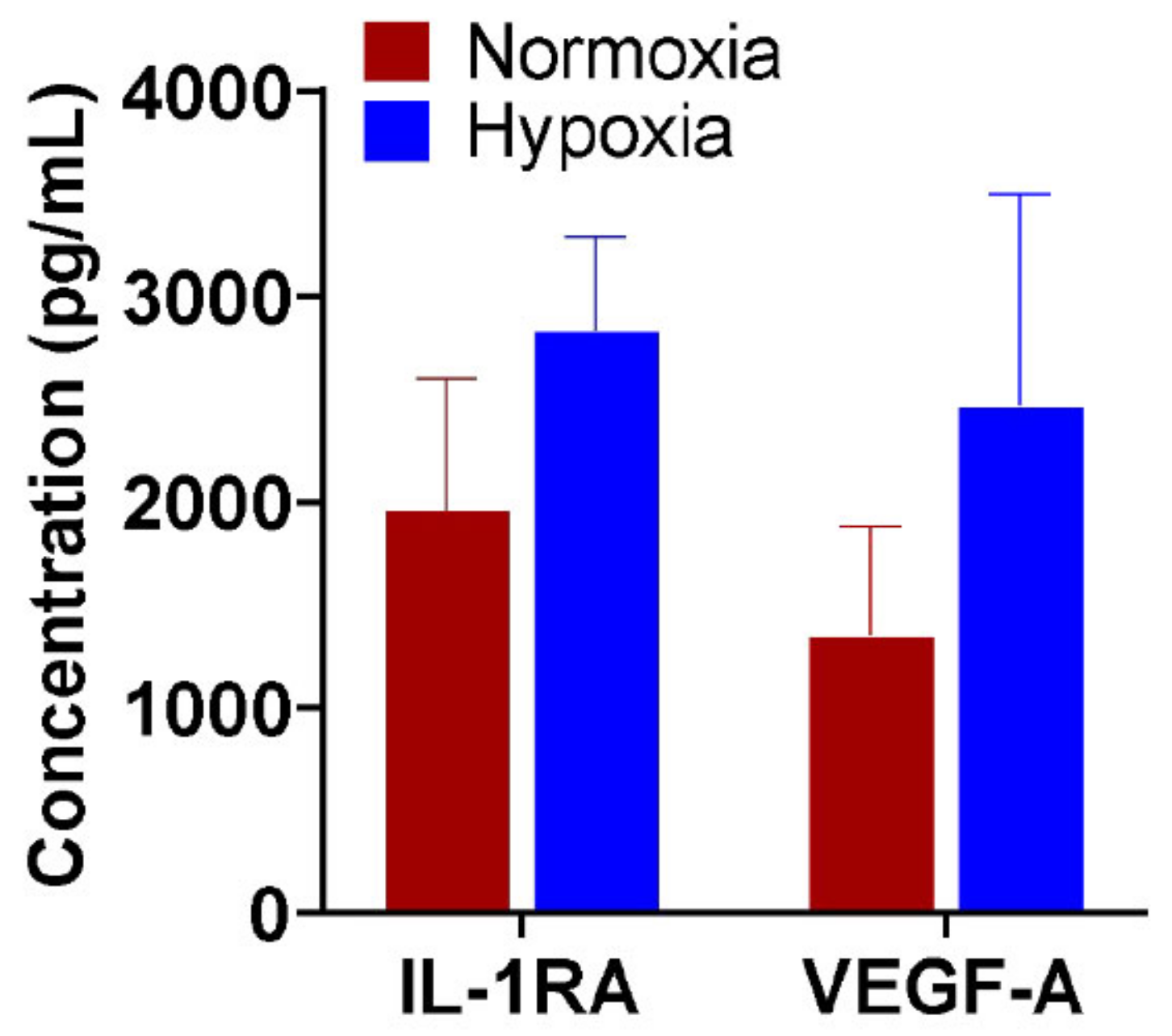
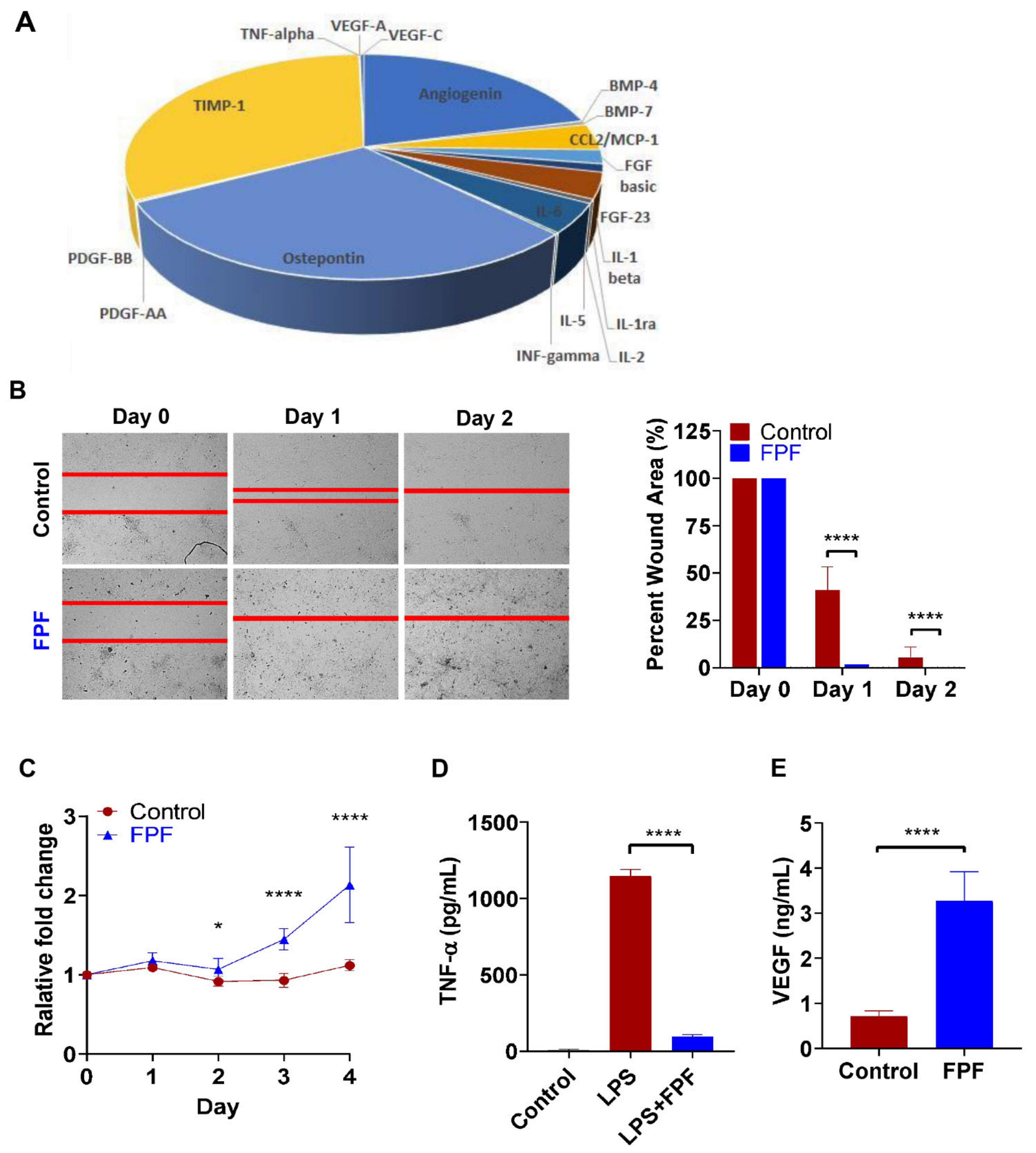
2.1. Regenerative Properties of Hypoxia Primed FPF
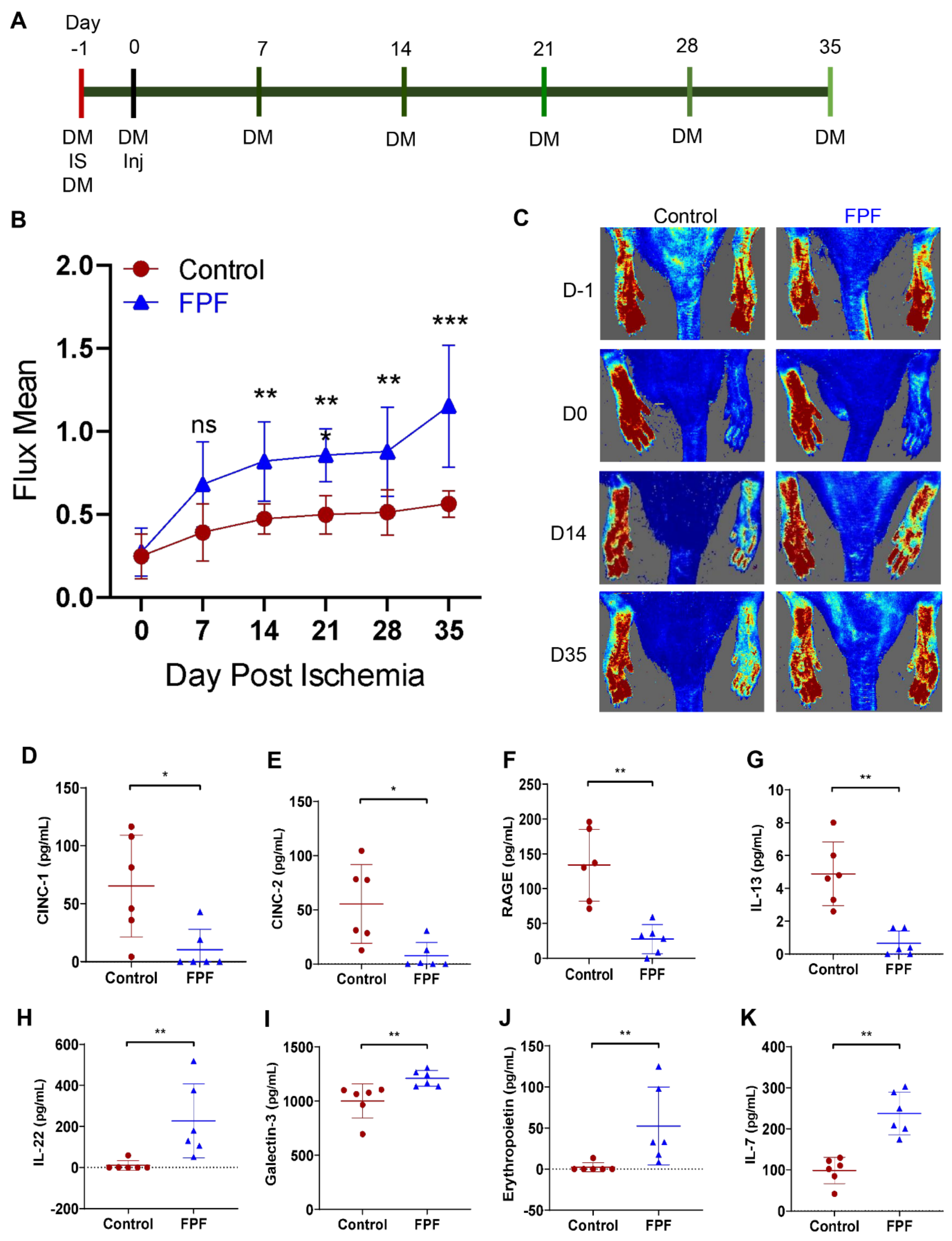
2.2. FPF Increases Hindlimb Tissue Perfusion Upon Ischemic Tissue Injury
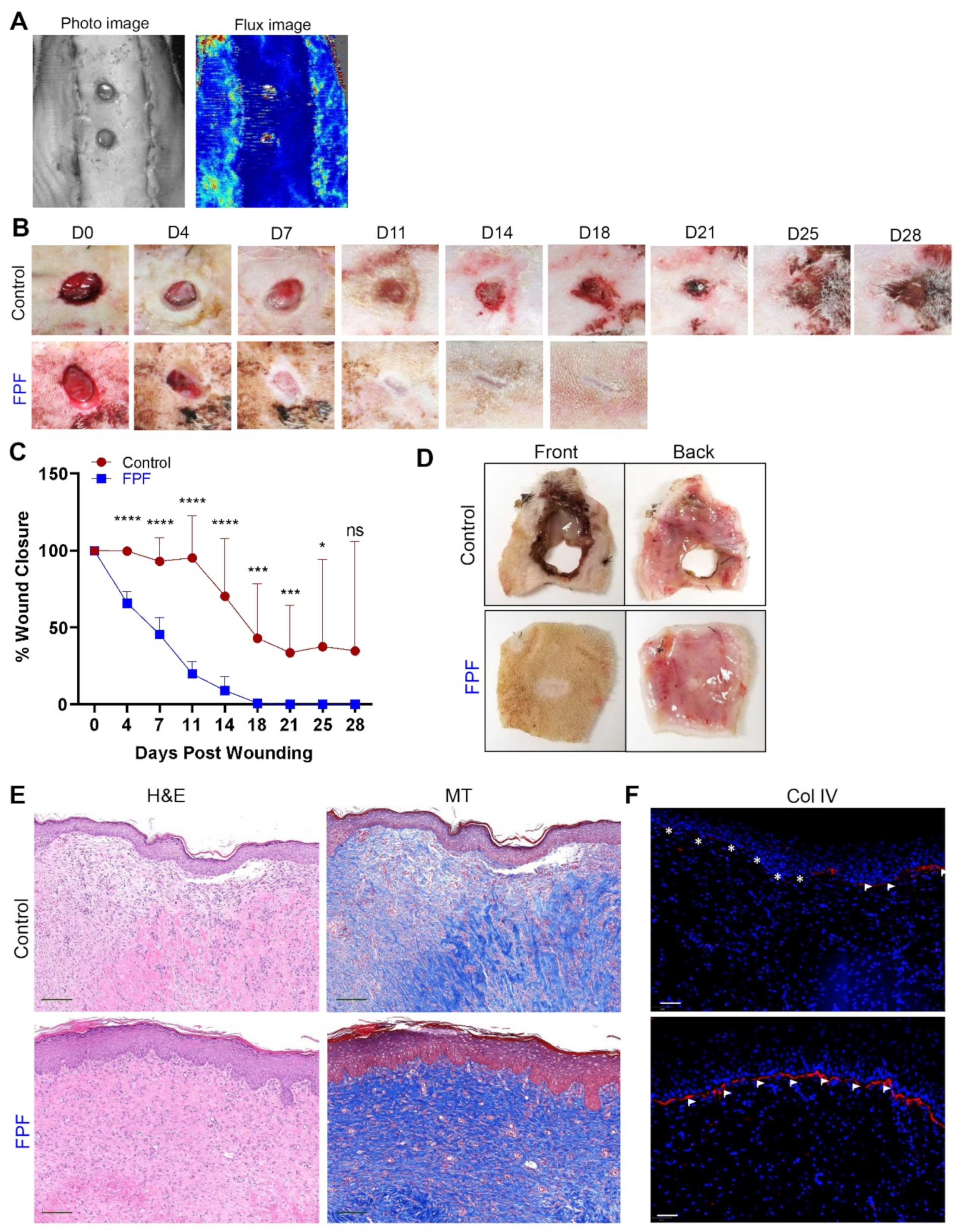
2.3. FPF Accelerates Ischemic Wound Healing and Closure with Improved Tissue Architecture
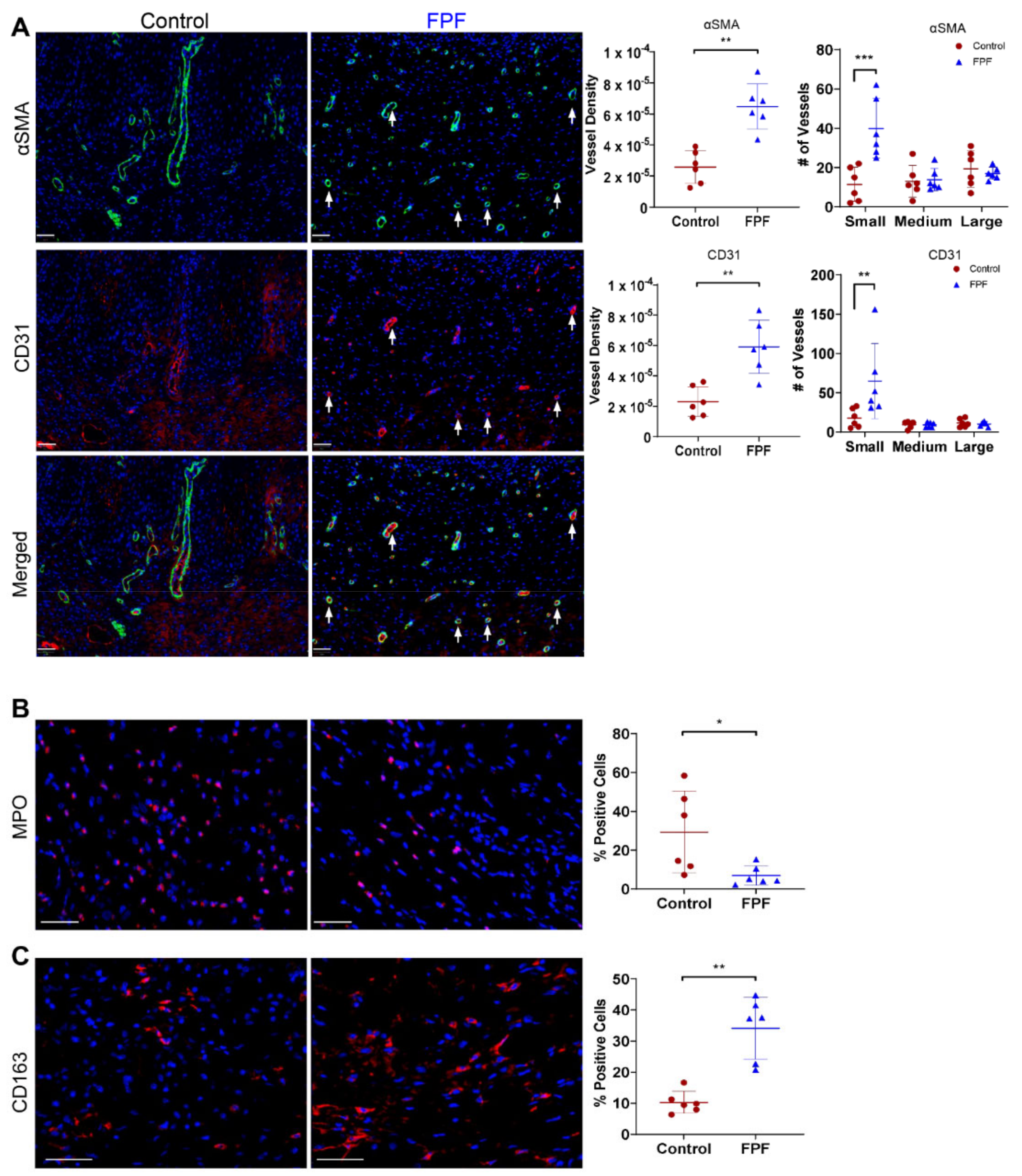
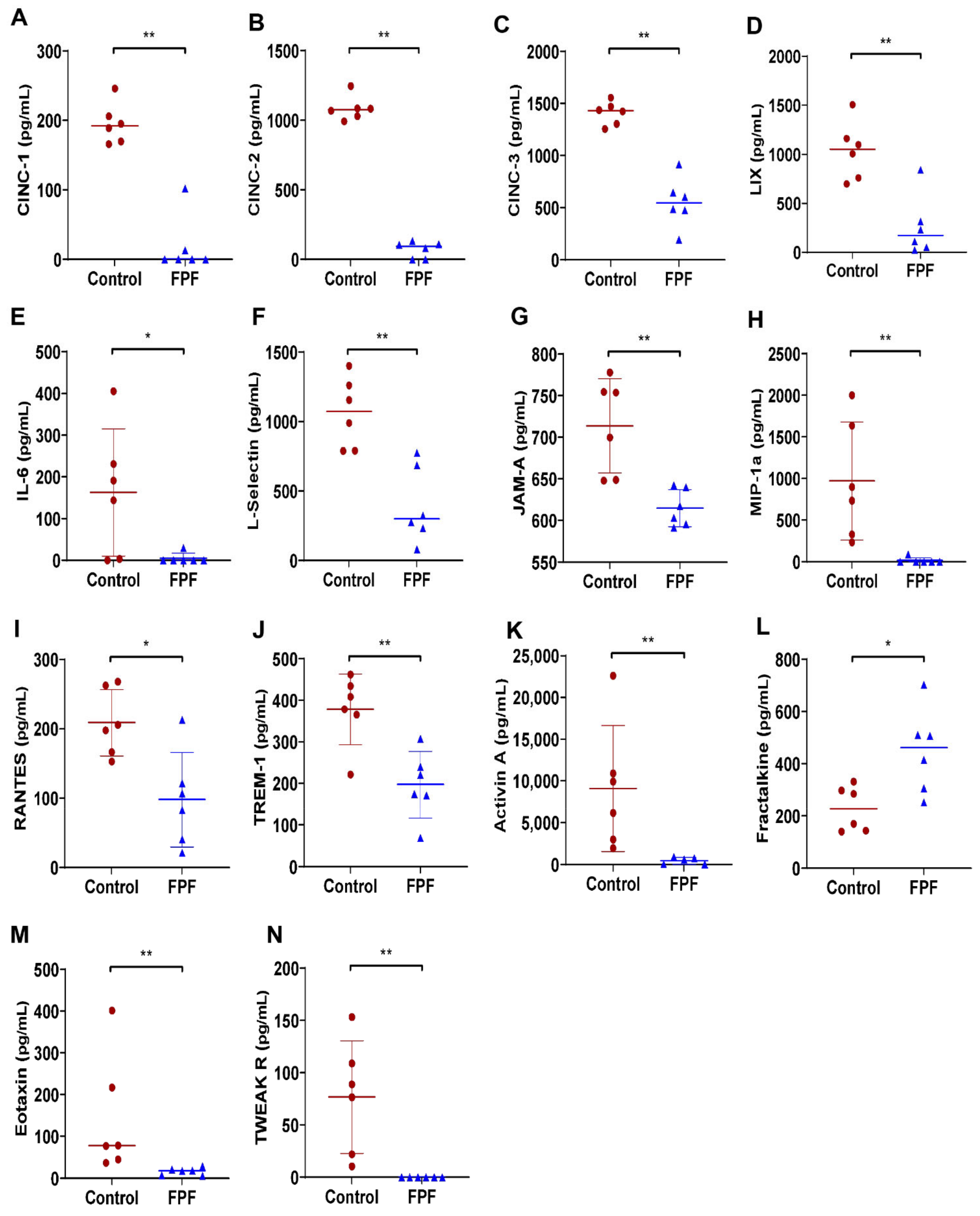
2.4. FPF Treatment Is Conducive for a Proangiogenic and an Anti-inflammatory Wound Microenvironment
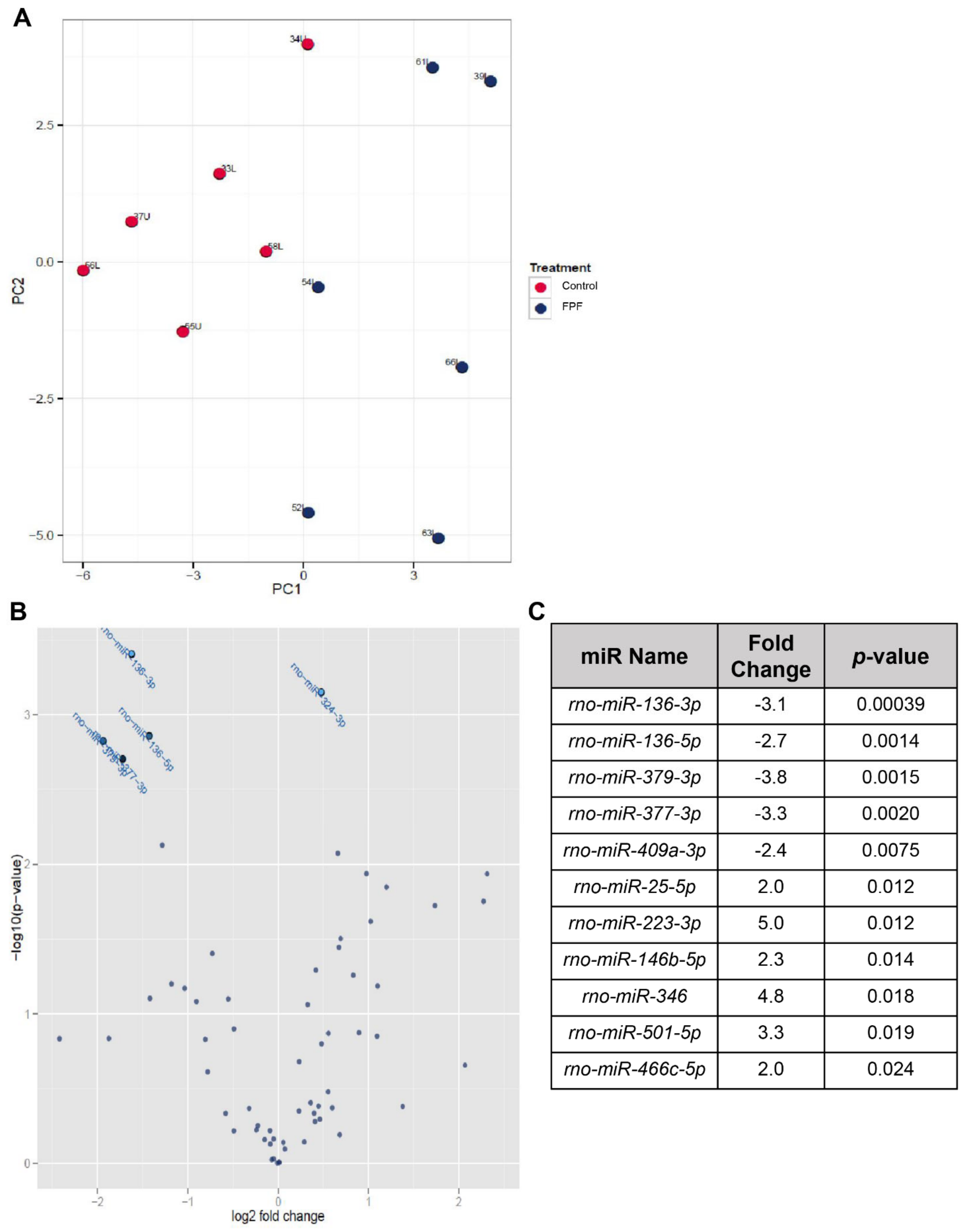
2.5. FPF Induces Transcriptional Regulation Favorable for Ischemic Wound Healing
3. Discussion
4. Materials and Methods
4.1. Tissue Procurement, Processing, and Ethics Statement
4.2. Preparation of Flowable Placental Formulation (FPF)
4.3. In Vitro Testing of Placental Membranes and FPF
4.3.1. Growth Factor Analysis of FPF
4.3.2. VEGF Reporter Bioassay
4.3.3. TNFα Inhibition Bioassay
4.3.4. Preparation of FPF Conditioned Medium for the Scratch Wound Assay and MTT Assay
4.3.5. Effect of FPF on the Migration of Human Dermal Fibroblasts (HDF) in Scratch Wound Assay
4.3.6. MTT Assay
4.4. In Vivo Testing of FPF
4.5. Histological and Immunohistochemical Assessment
4.6. Cytokine/Chemokine Multiplex Analysis of Necropsied Animal Tissue Samples
4.7. MicroRNA Array and PCR Gene Expression Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ennis, W.J.; Sui, A.; Bartholomew, A. Stem Cells and Healing: Impact on Inflammation. Adv. Wound Care 2013, 2, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chase, D.M.; Gallicchio, V.S. The effect of mesenchymal stem cells and exosomes to treat idiopathic pulmonary fibrosis. J. Stem. Cell Res. Ther. 2019, 5, 48–59. [Google Scholar] [CrossRef]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Moodley, Y.; Borlongan, C.V.; Parolini, O. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011, 32, S320–325. [Google Scholar] [CrossRef]
- Zare-Bidaki, M.; Sadrinia, S.; Erfani, S.; Afkar, E.; Ghanbarzade, N. Antimicrobial Properties of Amniotic and Chorionic Membranes: A Comparative Study of Two Human Fetal Sacs. J. Reprod. Infertil. 2017, 18, 218–224. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28868246 (accessed on 14 June 2021).
- Eskandarlou, M.; Azimi, M.; Rabiee, S.; Seif Rabiee, M.A. The Healing Effect of Amniotic Membrane in Burn Patients. World J. Plast. Surg. 2016, 5, 39–44. [Google Scholar]
- Pianta, S.; Bonassi Signoroni, P.; Muradore, I.; Francis Rodrigues, M.; Rossi, D.; Silini, A.; Parolini, O.; Pianta, S.; Bonassi Signoroni, P.; Muradore, I.; et al. Amniotic Membrane Mesenchymal Cells-Derived Factors Skew T Cell Polarization Toward Treg and Downregulate Th1 and Th17 Cells Subsets. Stem. Cell Rev. Rep. 2015, 11, 394–407. [Google Scholar] [CrossRef]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, 65S–70S. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Goren, I.; Müller, E.; Schiefelbein, D.; Christen, U.; Pfeilschifter, J.; Mühl, H.; Frank, S. Systemic Anti-TNFα Treatment Restores Diabetes-Impaired Skin Repair in ob/ob Mice by Inactivation of Macrophages. J. Invest. Dermatol. 2007, 127, 2259–2267. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Jeong, M.-J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef]
- Dhall, S.; Hoffman, T.; Sathyamoorthy, M.; Lerch, A.; Jacob, V.; Moorman, M.; Kuang, J.-Q.; Danilkovitch, A. A Viable Lyopreserved Amniotic Membrane Modulates Diabetic Wound Microenvironment and Accelerates Wound Closure. Adv. Wound Care 2019. [Google Scholar] [CrossRef]
- Jacob, V.; Johnson, N.; Lerch, A.; Jones, B.; Dhall, S.; Sathyamoorthy, M.; Danilkovitch, A.; Al, J.E.T. Structural and Functional Equivalency Between Lyopreserved and Cryopreserved Chorions with Viable Cells. Adv. Wound Care 2020, 1–14. [Google Scholar] [CrossRef]
- Dhall, S.; Coksaygan, T.; Hoffman, T.; Moorman, M.; Lerch, A.; Kuang, J.-Q.; Sathyamoorthy, M.; Danilkovitch, A. Viable cryopreserved umbilical tissue (vCUT) reduces post-operative adhesions in a rabbit abdominal adhesion model. Bioact. Mater. 2019, 4. [Google Scholar] [CrossRef]
- Warrier, S.; Haridas, N.; Bhonde, R. Inherent propensity of amnion-derived mesenchymal stem cells towards endothelial lineage: Vascularization from an avascular tissue. Placenta 2012, 33, 850–858. [Google Scholar] [CrossRef]
- Fidel, P.L.; Romero, R.; Ramirez, M.; Cutright, J.; Edwin, S.S.; Lamarche, S.; Cotton, D.B.; Mitchell, M.D. Interleukin-1 Receptor Antagonist (IL-1ra) Production by Human Amnion, Chorion, and Decidua. Am. J. Reprod. Immunol. 1994, 32, 1–7. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Farr, J.; Cole, B.J.; Flanigan, D.C.; Lattermann, C.; Mandelbaum, B.R.; Strickland, S.M.; Zaslav, K.R.; Kimmerling, K.A.; Mowry, K.C. Safety and Efficacy of an Amniotic Suspension Allograft Injection Over 12 Months in a Single-Blinded, Randomized Controlled Trial for Symptomatic Osteoarthritis of the Knee. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 1–11. [Google Scholar] [CrossRef]
- Madhusoodanan, J. Matrix mimics shape cell studies. Nature 2019, 566, 563–565. [Google Scholar] [CrossRef]
- Norgren, L.; Weiss, N.; Nikol, S.; Hinchliffe, R.J.; Lantis, J.C.; Patel, M.R.; Reinecke, H. PLX-PAD Cell Treatment of Critical Limb Ischaemia: Rationale and Design of the PACE Trial. Eur. J. Endovasc. Surg. 2019, 57, 538–545. [Google Scholar] [CrossRef]
- Ischemia, L.; Masaki, I.; Yonemitsu, Y.; Yamashita, A.; Sata, S.; Tanii, M.; Komori, K.; Nakagawa, K.; Hou, X.; Nagai, Y.; et al. Angiogenic Gene Therapy for Experimental Critical Acceleration of Limb Loss by Overexpression of Vascular Endothelial. Circ. Res. 2002, 966–973. [Google Scholar] [CrossRef]
- Avenue, G. Therapeutic Angiogenesis Induced by Human Hepatocyte Growth Factor Gene in Rat Diabetic Hind Limb. Circulation 2001. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, S.; Liu, N.; Zongjin, L. Extracellular Matrix Enhances Therapeutic Effects of Stem Cells in Regenerative Medicine. Composition and Function of the Extracellular Matrix in the Human Body. Colloids Surf. 2016, 323–340. [Google Scholar] [CrossRef]
- Zahavi-Goldstein, E.; Blumenfeld, M.; Fuchs-Telem, D.; Pinzur, L.; Rubin, S.H.Y.; Aberman, Z.; Sher, N.O.A.; Ofir, R. Placenta-Derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy 2017, 19, 1438–1446. [Google Scholar] [CrossRef]
- Gremmels, H.; Teraa, M.; de Jager, S.C.A.; Pasterkamp, G.; de Borst, G.J.; Verhaar, M.C. A Pro-Inflammatory Biomarker-Profile Predicts Amputation-Free Survival in Patients with Severe Limb Ischemia. J. Vasc. Surg. 2010, 51. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Song, J.; Herndon, D.N.; Finnerty, C.C.; Boehning, D.; Barral, J.M.; Jeschke, M.G. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 2008, 30, 503–507. [Google Scholar] [CrossRef]
- Jalkanen, J.; Maksimow, M.; Hollmén, M.; Jalkanen, S.; Hakovirta, H. Compared to Intermittant Claudication Critical Limb Ischemia Is Associated with Elevated Levels of Cytokines. PLoS ONE 2016, 11, e0162353. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009, 17. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Yu, T.; Li, Y.; Tang, Y. Interleukin-22 receptor 1 upregulation and activation in hypoxic endothelial cells improves perfusion recovery in experimental peripheral arterial disease. Biochem. Biophys. Res. Commun. 2018, 505, 60–66. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Lee, N.H.; Moon, J.Y.; Han, W.M.; Anderson, S.E.; Choi, J.J.; Shin, E.; Nakhai, S.A.; Tran, T.; Aliya, B.; et al. Critical Limb Ischemia Induces Remodeling of Skeletal Muscle Motor Unit, Myonuclear-, and Mitochondrial-Domains. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2010, 298. [Google Scholar] [CrossRef] [PubMed]
- Wesley, U.V.; Sutton, I.C.; Cunningham, K.; Jaeger, J.W.; Phan, A.Q.; Hatcher, J.F.; Dempsey, R.J. Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation. J. Cereb. Blood Flow Metab. 2021, 41, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Rancourt, A.; Dufresne, S.S.; St-Pierre, G.; Julie-Christine, L.; Nakamura, H.; Kikuchi, Y.; Masahiko, S.S.; Frenette, J.; Sato, S. Galectin-3 and N-acetylglucosamine promote myogenesis and improve skeletal muscle function in the mdx model of Duchenne muscular dystrophy. FASEB J. 2018, 32, 6445–6455. [Google Scholar] [CrossRef]
- Rotter, R.; Menshykova, M.; Winkler, T.; Matziolis, G.; Stratos, I.; Schoen, M.; Bittorf, T.; Mittlmeier, T.; Vollmar, B. Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J. Orthop. Res. 2008, 26, 1618–1626. [Google Scholar] [CrossRef]
- Trujillo, A.N.; Kesl, S.L.; Sherwood, J.; Wu, M.; Gould, L.J. Demonstration of the rat ischemic skin wound model. J. Vis. Exp. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Mustoe, T.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef]
- Velez, A.M.A.; Howard, M.S. Collagen IV in normal and in disease process. North. Am. J. Med. Sci. 2012, 1–8. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt Ka Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Liu, Y.; Petreaca, M.; Martins-Green, M. Cell and molecular mechanisms of insulin-induced angiogenesis. J. Cell Mol. Med. 2009, 13, 4492–4504. [Google Scholar] [CrossRef]
- Rovai, L.E.; Herschman, H.R.; Smith, J.B. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukoc. Biol. 1998, 64, 494–502. [Google Scholar] [CrossRef]
- Yager, D.R.; Nwomeh, B.C. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999, 7, 433–441. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10633002 (accessed on 14 June 2021). [CrossRef]
- Fielding, C.A.; McLoughlin, R.M.; McLeod, L.; Colmont, C.S.; Najdovska, M.; Grail, D.; Ernst, M.; Jones, S.A.; Topley, N.; Jenkins, B.J. IL-6 Regulates Neutrophil Trafficking during Acute Inflammation via STAT3. J. Immunol. 2008, 181, 2189–2195. [Google Scholar] [CrossRef]
- Witherel, C.E.; Yu, T.; Concannon, M.; Dampier, W.; Spiller, K.L. Immunomodulatory Effects of Human Cryopreserved Viable Amniotic Membrane in a Pro-Inflammatory Environment In Vitro. Cell Mol. Bioeng. 2017, 10, 451–462. [Google Scholar] [CrossRef]
- Carlson M a Smith, L.M.; Cordes, C.M.; Chao, J.; Eudy, J.D. Attachment-Regulated signaling networks in the fibroblast-populated 3D collagen matrix. Sci. Rep. 2013, 3, 1880. [Google Scholar] [CrossRef]
- Bu, X.; Zhao, C.; Dai, X. Involvement of COX-2/PGE(2) Pathway in the Upregulation of MMP-9 Expression in Pancreatic Cancer. Gastroenterol. Res. Pract. 2011, 2011, 214269. [Google Scholar] [CrossRef][Green Version]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69, 513–521. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11310836 (accessed on 14 June 2021).
- Woodfin, A.; Reichel, C.A.; Khandoga, A.; Corada, M.; Voisin, M.B.; Scheiermann, C.; Haskard, D.O.; Dejana, E.; Krombach, F.; Nourshargh, S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: Evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood Am. Soc. Hematol. 2007, 110, 1848–1856. [Google Scholar] [CrossRef]
- Murai, T.; Luscinskas, F.W.; Alon, R.; Ivetic, A.; Louise, H.; Green, H.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 1, 1068. [Google Scholar] [CrossRef]
- Ball, C.J.; Reiffel, A.J.; Chintalapani, S.; Kim, M.; Spector, J.A.; King, M.R. Hydrogen sulfide reduces neutrophil recruitment in hind-limb ischemia-reperfusion injury in an l-selectin and ADAM-17-dependent manner. Plast. Reconstr. Surg. 2013, 131, 487–497. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Ying, S.; Sabroe, I.; Stubbs, V.L.; Soler, D.; Williams, T.J.; Kay, A.B. Eotaxin (CCL11) and Eotaxin-2 (CCL24) Induce Recruitment of Eosinophils, Basophils, Neutrophils, and Macrophages as Well as Features of Early- and Late-Phase Allergic Reactions Following Cutaneous Injection in Human Atopic and Nonatopic Volunteers. J. Immunol. 2002, 169, 2712–2718. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Kunkel, S.L.; Strieter, R.M.; DiPietro, L.A.; Burdick, M.; Low, Q.E.; Kunkel, S.L.; Strieter, R.M. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. MIP-1 as a Critical Macrophage Chemoattractant in Murine Wound Repair words: Monocyte • macrophage • chemoattractant • wound healing • neovascularization. J. Clin. Invest. 1998, 101, 1693–1698. [Google Scholar] [CrossRef]
- Anogianaki, A.; Castellani, M.L.; Madhappan, B.; Salini, V.; Vecchiet, J.; Tetè, S.; Frydas, S.; Perrella, A.; Lutiis Made Neri, G.; Cerulli, G.; et al. RANTES (CCL5) potentiates calcium ionophore in the production of LTB4 in rat adherent macrophages from granuloma induced by KMnO4: Inhibiton by NDGA. Pharmacol. Res. 2008, 57, 49–55. [Google Scholar] [CrossRef]
- Feng, C.W.; Chen, N.F.; Sung, C.S.; Kuo, H.M.; Yang, S.N.; Chen, C.L.; Hung, H.C.; Chen, B.H.; Wen, Z.H.; Chen, W.F. Therapeutic Effect of Modulating TREM-1 via Anti-inflammation and Autophagy in Parkinson’s Disease. Front. Genet. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Filardi, E.; Puig-Kröger, A.; Blanco, F.J.; Nieto, C.; Bragado, R.; Palomero, M.I.; Bernabéu, C.; Vega, M.A.; Corbí, A.L. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011, 117, 5092–5101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xiao, S.; Xia, Y. TWEAK/Fn14 Activation Participates in Skin Inflammation. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Salentin, R.; Gemsa, D.; Sprenger, H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1α during differentiation of human monocytes to macrophages. J. Leukoc. Biol. 2001, 69, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, J.; Kwak, J.-Y.; Noh, K.; Yim, E.; Kim, H.-K.; Kim, Y.J.; Broxmeyer, H.E.; Kim, J.-A. Fractalkine induces angiogenic potential in CX3CR1-expressing monocytes. J. Leukoc. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Børsheim, E.; Carvalho, E. The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing. Genes 2014, 5, 926–956. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, T.; Hao, Y.; Zhang, F.; Tang, X.; Wang, D.; Wei, Z.; Qi, J. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem. Cell Res. Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem. Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108–119. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16415491 (accessed on 14 June 2021).
- Woo, K.Y. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab. Res. Rev. 2008, 24, 25–30. [Google Scholar] [CrossRef]
- Semaan, N.; Frenzel, L.; Alsaleh, G.; Suffert, G.; Gottenberg, J.E.; Sibilia, J.; Pfeffer, S.; Wachsmann, D. MiR-346 controls release of TNF-α protein and stability of its mRNA in rheumatoid arthritis via tristetraprolin stabilization. PLoS ONE 2011, 6, e19827. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Li, Q.; Li, J.; Wu, Y.; Liu, J. MicroRNA-25–5p counteracts oxidized LDL-induced pathological changes by targeting neuronal growth regulator 1 (NEGR1) in human brain micro-vessel endothelial cells. Biochimie 2019, 165, 141–149. [Google Scholar] [CrossRef]
- Balducci, E.; Leroyer, A.S.; Lacroix, R.; Robert, S.; Todorova, D.; Simoncini, S.; Lyonnet, L.; Chareyre, C.; Zaegel-Faucher, O.; Micallef, J.; et al. Extracellular vesicles from T cells overexpress miR-146b-5p in HIV-1 infection and repress endothelial activation. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Di, Y.F.; Li, D.C.; Shen, Y.Q.; Wang, C.L.; Zhang, D.Y.; Shang, A.Q.; Hu, T. MiR-146b protects cardiomyocytes injury in myocardial ischemia/reperfusion by targeting Smad4. Am. J. Transl. Res. 2017, 9, 656–663. [Google Scholar]
- Nata, T.; Fujiya, M.; Ueno, N.; Moriichi, K.; Konishi, H.; Tanabe, H.; Ohtake, T.; Ikuta, K.; Kohgo, Y. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-κB and improving epithelial barrier function. J. Gene Med. 2013, 15, 249–260. [Google Scholar] [CrossRef]
- Deng, G.; Gao, Y.; Cen, Z.; He, J.; Cao, B.; Zeng, G.; Zong, S. miR-136-5p Regulates the Inflammatory Response by Targeting the IKKβ/NF-κB/A20 Pathway After Spinal Cord Injury. Cell Physiol. Biochem. 2018, 50, 512–524. [Google Scholar] [CrossRef]
- Gupta, Y.; Möller, S.; Witte, M.; Belheouane, M.; Sezin, T.; Hirose, M.; Vorobyev, A.; Niesar, F.; Bischof, J.; Ludwig, R.J.; et al. Dissecting genetics of cutaneous miRNA in a mouse model of an autoimmune blistering disease. BMC Genom. 2016, 17, 112. [Google Scholar] [CrossRef][Green Version]
- Akasaka, Y.; Ishikawa, Y.; Ichiro, O.; Fujita, K.; Masuda, T.; Asuwa, N.; Inuzuka, K.; Kiguchi, H.; Ishii, T. Enhanced expression of caspase-3 in hypertrophic scars and keloid: Induction of caspase-3 and apoptosis in keloid fibroblasts in vitro. Lab. Investig. 2000, 80, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.D.; Ciszek, R.; Heiskanen, M.; Lapinlampi, N.; Kukkonen, J.; Leinonen, V.; Puhakka, N.; Pitkänen, A. Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 1563. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Feng, J.; Lu, M.; Tang, W.; Han, J.; Luo, X.G.; Zhao, Q.; Yang, L. microRNA-501-5p promotes cell proliferation and migration in gastric cancer by downregulating LPAR1. J. Cell Biochem. 2020, 121, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.P.; Leelahavanichkul, A. Over-Expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS ONE 2020, 15, e236038. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.H.; Liu, N.; Zhu, J.W.; Yao, J.; Yang, C.; Ma, P.Z.; Song, X.B. Qi-Shen-Yi-Qi Dripping Pills Promote Angiogenesis of Ischemic Cardiac Microvascular Endothelial Cells by Regulating MicroRNA-223-3p Expression. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Wen, Z.; Huang, W.; Feng, Y.; Cai, W.; Wang, Y.; Wang, X.; Liang, J.; Wani, M.; Chen, J.; Zhu, P.; et al. MicroRNA-377 Regulates Mesenchymal Stem Cell-Induced Angiogenesis in Ischemic Hearts by Targeting VEGF. PLoS ONE 2014, 9, e104666. [Google Scholar] [CrossRef]
- Brantley, J.N.; Verla, T.D. Use of Placental Membranes for the Treatment of Chronic Diabetic Foot Ulcers. Adv. Wound Care 2015, 4, 545–559. [Google Scholar] [CrossRef]
- McGinness, K.; Kurtz Phelan, D.H. Use of Viable Cryopreserved Umbilical Tissue for Soft Tissue Defects in Patients with Gas Gangrene: A Case Series. Wounds Compend. Clin. Res. Pract. 2018, 30, 90–95. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29718818 (accessed on 14 June 2021).
- Dhall, S.; Lerch, A.; Johnson, N.; Jacob, V.; Jones, B.; Park, M.S.; Sathyamoorthy, M. A flowable placental formulation prevents bleomycin-induced dermal fibrosis in aged mice. Int J. Mol. Sci. 2020, 21, 4242. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhall, S.; Park, M.S.; Li, C.; Sathyamoorthy, M. Regenerative Effects of Hypoxia Primed Flowable Placental Formulation in Muscle and Dermal Injury. Int. J. Mol. Sci. 2021, 22, 7151. https://doi.org/10.3390/ijms22137151
Dhall S, Park MS, Li C, Sathyamoorthy M. Regenerative Effects of Hypoxia Primed Flowable Placental Formulation in Muscle and Dermal Injury. International Journal of Molecular Sciences. 2021; 22(13):7151. https://doi.org/10.3390/ijms22137151
Chicago/Turabian StyleDhall, Sandeep, Min Sung Park, Chaoyang Li, and Malathi Sathyamoorthy. 2021. "Regenerative Effects of Hypoxia Primed Flowable Placental Formulation in Muscle and Dermal Injury" International Journal of Molecular Sciences 22, no. 13: 7151. https://doi.org/10.3390/ijms22137151
APA StyleDhall, S., Park, M. S., Li, C., & Sathyamoorthy, M. (2021). Regenerative Effects of Hypoxia Primed Flowable Placental Formulation in Muscle and Dermal Injury. International Journal of Molecular Sciences, 22(13), 7151. https://doi.org/10.3390/ijms22137151





