In Vitro Osteoinductivity Assay of Hydroxylapatite Scaffolds, Obtained with Biomorphic Transformation Processes, Assessed Using Human Adipose Stem Cell Cultures
Abstract
:1. Introduction
2. Results
2.1. Microstructural Analysis
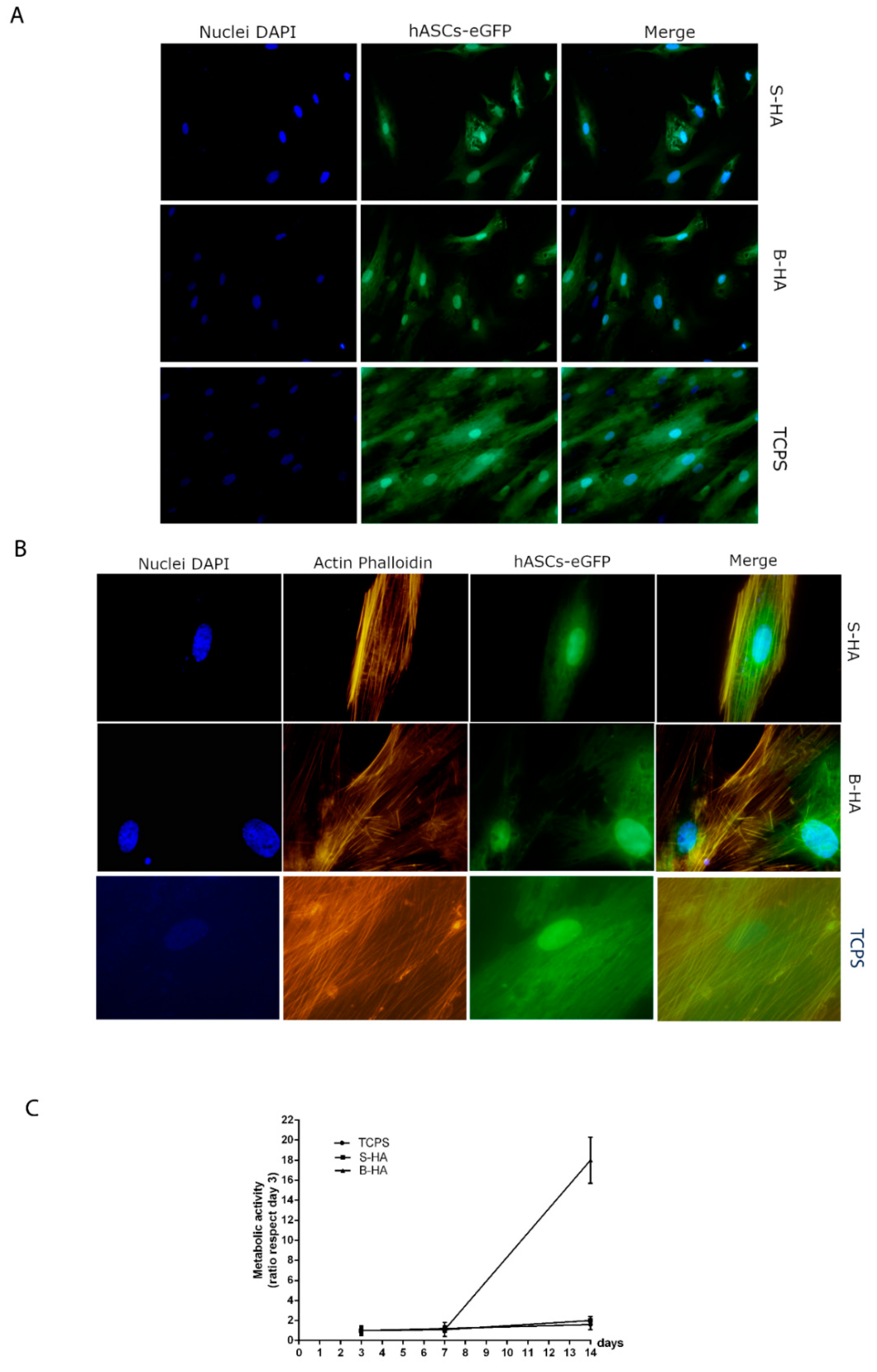
2.2. Biocompatibility Analysis of Biomorphic HA Scaffold Employing hASCs
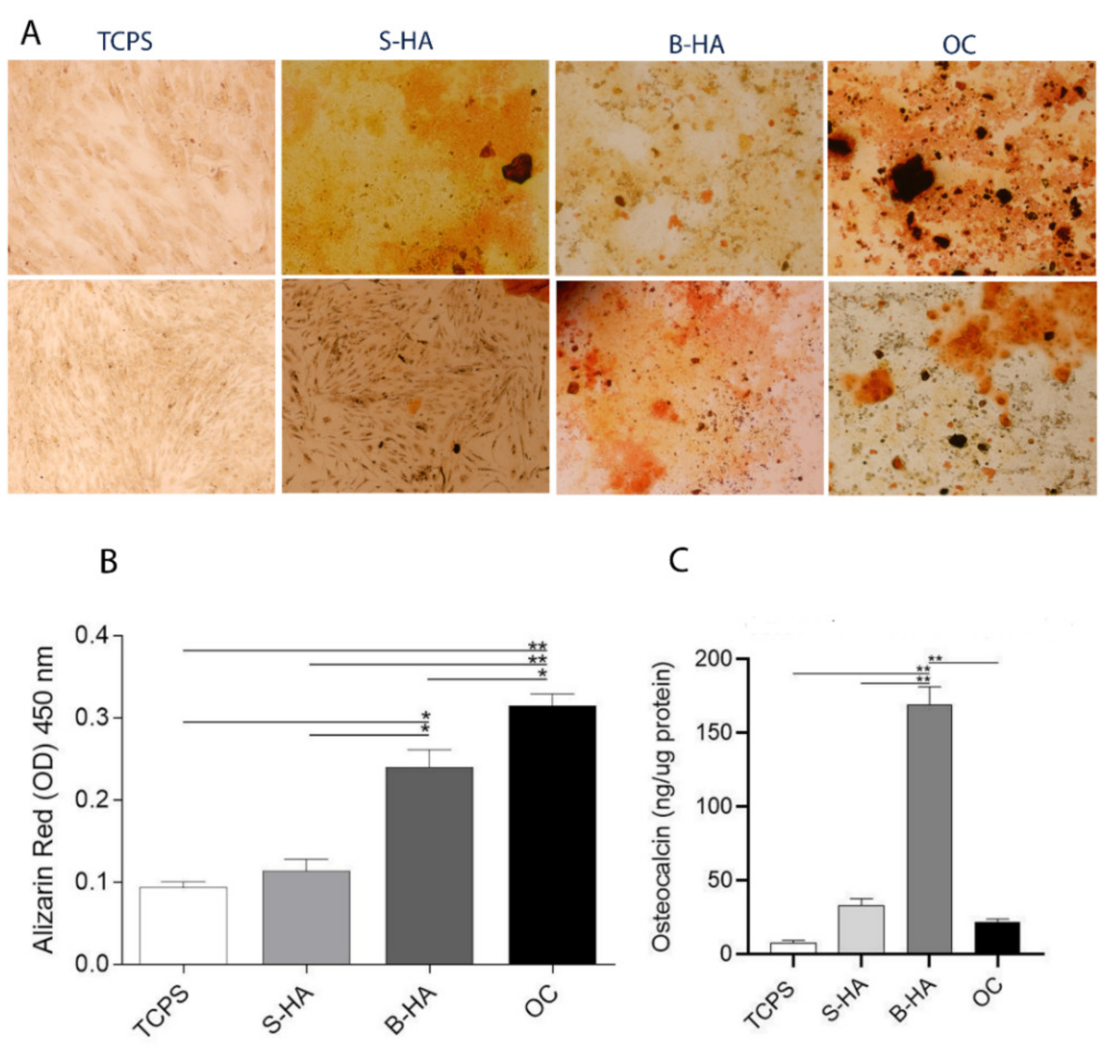
2.3. Matrix Mineralization and Osteocalcin Expression Protein in hASCs
2.4. Osteogenic Gene Expression in hASCs
3. Discussion
4. Materials and Methods
4.1. Biomaterials
4.2. Microstructural Analysis
4.3. Cell Culture and Scaffold Seeding
4.4. Cell Morphology
4.5. Cytoskeleton Architecture Evaluation
4.6. Cell Proliferation Assay
4.7. Osteogenic Differentiation and Matrix Mineralization in hASCs
4.8. Osteogenesis RT2PCR Array
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Habibovic, P. Strategic Directions in Osteoinduction and Biomimetics. Tissue Eng. Part A. 2017, 23, 1295–1296. [Google Scholar] [CrossRef]
- Pacelli, S.; Basu, S.; Whitlow, J.; Chakravarti, A.; Acosta, F.; Varshney, A.; Modaresi, S.; Berkland, C.; Paul, A. Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug Deliv. Rev. 2017, 120, 50–70. [Google Scholar] [CrossRef]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Homma, Y.; Zimmermann, G.; Hernigou, P. Cellular therapies for the treatment of non-union: The past, present and future. Injury 2013, 44 (Suppl. 1), S46–S49. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampieri, A.; Sandri, M.; Iafisco, M.; Panseri, S.; Montesi, M.; Adamiano, A.; Dapporto, M.; Campodoni, E.; Dozio, S.M.; Degli Esposti, L.; et al. Nanotechnological approach and bio-inspired materials to face degenerative diseases in aging. Aging Clin. Exp. Res. 2019, 33, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Ruffini, A.; Ballardini, A.; Montesi, M.; Panseri, S.; Salamanna, F.; Fini, M.; Sprio, S. Heterogeneous chemistry in the 3-D state: An original approach to generate bioactive, mechanically-competent bone scaffolds. Biomater. Sci. 2019, 7, 307–321. [Google Scholar] [CrossRef]
- Spennato, P.; Canella, V.; Aliberti, F.; Russo, C.; Ruggiero, C.; Nataloni, A.; Lombardo, M.; Cinalli, G. Hydroxylapatite ceramic implants for cranioplasty in children: A retrospective evaluation of clinical outcome and osteointegration. Child’s Nerv. Syst. 2020, 36, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous hydroxylapatite for artificial bone applications. Sci. Technol. Adv. Mater. 2007, 8, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Fricia, M.; Passanisi, M.; Salamanna, F.; Parrilli, A.; Giavaresi, G.; Fini, M. Osteointegration in Custom-made Porous Hydroxylapatite Cranial Implants: From Reconstructive Surgery to Regenerative Medicine. World Neurosurg. 2015, 84, 591.e11–591e16. [Google Scholar] [CrossRef]
- De Siqueira, L.; de Paula, C.G.; Gouveia, R.F.; Motisuke, M.; de Sousa Triches, E. Evaluation of the sintering temperature on the mechanical behavior of beta-tricalcium phosphate/calcium silicate scaffolds obtained by gelcasting method. J. Mech. Behav. Biomed. Mater. 2019, 90, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Diez-Escudero, A.; Espanol, M.; Bonany, M.; Sadowska, J.M.; Guillem-Marti, J.; Ohman-Magi, C.; Persson, C.; Manzanares, M.C.; Franch, J.; et al. Impact of Biomimicry in the Design of Osteoinductive Bone Substitutes: Nanoscale Matters. ACS Appl. Mater. Interfaces 2019, 11, 8818–8830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Bigoni, D.; Cavuoto, R.; Misseroni, D.; Paggi, M.; Ruffini, A.; Sprio, S.; Tampieri, A. Ceramics with the signature of wood: A mechanical insight. Mater. Today Bio 2020, 5, 100032. [Google Scholar] [CrossRef]
- Sprio, S.; Ruffini, A.; Dapporto, M.; Tampieri, A. New biomimetic strategies for regeneration of load bearing bones. In Bio-inspired Regenerative Medicine: Materials, Processes and Clinical Applications; Tampieri, A., Sprio, S., Eds.; PAN Stanford Publishing: Singapore, 2016; ISBN 978-981-4669-14-6. [Google Scholar]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Montecino, M.; Carrasco, M.E.; Nardocci, G. Epigenetic Control of Osteogenic Lineage Commitment. Front. Cell Dev. Biol. 2020, 8, 611197. [Google Scholar] [CrossRef]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Preti, L.; Mazzoni, E.; Iaquinta, M.R.; Martini, F.; Tognon, M.; Pugno, N.M.; Restivo, E.; Visai, L.; et al. Enhancement of the Biological and Mechanical Performances of Sintered Hydroxylapatite by Multiple Ions Doping. Front. Mater. 2020, 7, 224. [Google Scholar] [CrossRef]
- Barbanti Brodano, G.; Mazzoni, E.; Tognon, M.; Griffoni, C.; Manfrini, M. Human mesenchymal stem cells and biomaterials interaction: A promising synergy to improve spine fusion. Eur. Spine J. 2012, 21 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, Y.; Suzuki, K.; Koshikawa, M.; Ando, M.; Iida, J. Necessity of enzymatic activity of alkaline phosphatase for mineralization of osteoblastic cells. Jpn. J. Pharmacol. 2002, 88, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.M.; Lawrence, A.R.; Stottmann, R.W.; Bachiller, D.; Klingensmith, J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 2002, 129, 4975–4987. [Google Scholar] [CrossRef]
- Itoh, Y.; Saitoh, M.; Miyazawa, K. Smad3-STAT3 crosstalk in pathophysiological contexts. Acta Biochim. Biophys. Sin. 2018, 50, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Teng, B.H.; Wang, L.N.; Li, K.; Xu, C.; Ma, X.L.; Zhang, Y.; Kong, D.L.; Wang, L.Y.; Zhao, Y.H. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-beta3 for chondrogenic differentiation of adipose-derived stem cells. Int. J. Nanomed. 2017, 12, 6721–6733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qiao, Z.; Yu, F.; Hu, H.; Huang, Y.; Xiang, Q.; Zhang, Q.; Yang, Y.; Zhao, Y. Transforming Growth Factor-beta3/Chitosan Sponge (TGF-beta3/CS) Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. Int. J. Mol. Sci. 2019, 20, 4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, H.; Rothrauff, B.B.; Alexander, P.G.; Lin, H.; Gottardi, R.; Fu, F.H.; Tuan, R.S. In Vitro Repair of Meniscal Radial Tear with Hydrogels Seeded with Adipose Stem Cells and TGF-beta3. Am. J. Sports Med. 2018, 46, 2402–2413. [Google Scholar] [CrossRef]
- Lee, H.L.; Yu, B.; Deng, P.; Wang, C.Y.; Hong, C. Transforming Growth Factor-beta-Induced KDM4B Promotes Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2016, 34, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-beta Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef]
- Deng, M.; Mei, T.; Hou, T.; Luo, K.; Luo, F.; Yang, A.; Yu, B.; Pang, H.; Dong, S.; Xu, J. TGFbeta3 recruits endogenous mesenchymal stem cells to initiate bone regeneration. Stem Cell Res Ther. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripamonti, U.; Ramoshebi, L.N.; Teare, J.; Renton, L.; Ferretti, C. The induction of endochondral bone formation by transforming growth factor-β3: Experimental studies in the non-human primate Papio ursinus. J. Cell. Mol. Med. 2008, 12, 1029–1048. [Google Scholar] [CrossRef]
- Ripamonti, U.; Duarte, R.; Ferretti, C. Re-evaluating the induction of bone formation in primates. Biomaterials 2014, 35, 9407–9422. [Google Scholar] [CrossRef]
- Del Angel-Mosqueda, C.; Gutierrez-Puente, Y.; Lopez-Lozano, A.P.; Romero-Zavaleta, R.E.; Mendiola-Jimenez, A.; Medina-De la Garza, C.E.; Marquez, M.M.; De la Garza-Ramos, M.A. Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro. Head Face Med. 2015, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Hojo, H.; Ohba, S.; Yano, F.; Saito, T.; Ikeda, T.; Nakajima, K.; Komiyama, Y.; Nakagata, N.; Suzuki, K.; Takato, T.; et al. Gli1 protein participates in Hedgehog-mediated specification of osteoblast lineage during endochondral ossification. J. Biol. Chem. 2012, 287, 17860–17869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, B.; Liu, G.; Xing, L.; Tian, F. Research progress of hedgehog signaling pathway in regulating bone formation and osteogenic differentiation of bone mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2016, 30, 1545–1550. [Google Scholar] [PubMed]
- Milner, J.M.; Cawston, T.E. Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 363–375. [Google Scholar] [CrossRef]
- Sasano, Y.; Zhu, J.X.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxylapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef] [Green Version]
- Hippler, M.; Lemma, E.D.; Bertels, S.; Blasco, E.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. 3D Scaffolds to Study Basic Cell Biology. Adv. Mater. 2019, 31, e1808110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfrini, M.; Di Bona, C.; Canella, A.; Lucarelli, E.; Pellati, A.; D’Agostino, A.; Barbanti-Brodano, G.; Tognon, M. Mesenchymal stem cells from patients to assay bone graft substitutes. J Cell. Physiol. 2013, 228, 1229–1237. [Google Scholar] [CrossRef]
- Cunha, C.; Sprio, S.; Panseri, S.; Dapporto, M.; Marcacci, M.; Tampieri, A. High biocompatibility and improved osteogenic potential of novel Ca-P/titania composite scaffolds designed for regeneration of load-bearing segmental bone defects. J. Biomed. Mater. Res. A 2013, 101, 1612–1619. [Google Scholar] [CrossRef]
- Mazzoni, E.; D’Agostino, A.; Iaquinta, M.R.; Bononi, I.; Trevisiol, L.; Rotondo, J.C.; Patergnani, S.; Giorgi, C.; Gunson, M.J.; Arnett, G.W.; et al. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl. Med. 2020, 9, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Mebarki, M.; Coquelin, L.; Layrolle, P.; Battaglia, S.; Tossou, M.; Hernigou, P.; Rouard, H.; Chevallier, N. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater. 2017, 59, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, J.K.; Sakata, S.; Liang, I.; Park, W.; Hajjar, R.J.; Lebeche, D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell. Cardiol. 2008, 45, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoni, E.; D’Agostino, A.; Manfrini, M.; Maniero, S.; Puozzo, A.; Bassi, E.; Marsico, S.; Fortini, C.; Trevisiol, L.; Patergnani, S.; et al. Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J. 2017, 31, 4555–4565. [Google Scholar] [CrossRef] [PubMed] [Green Version]

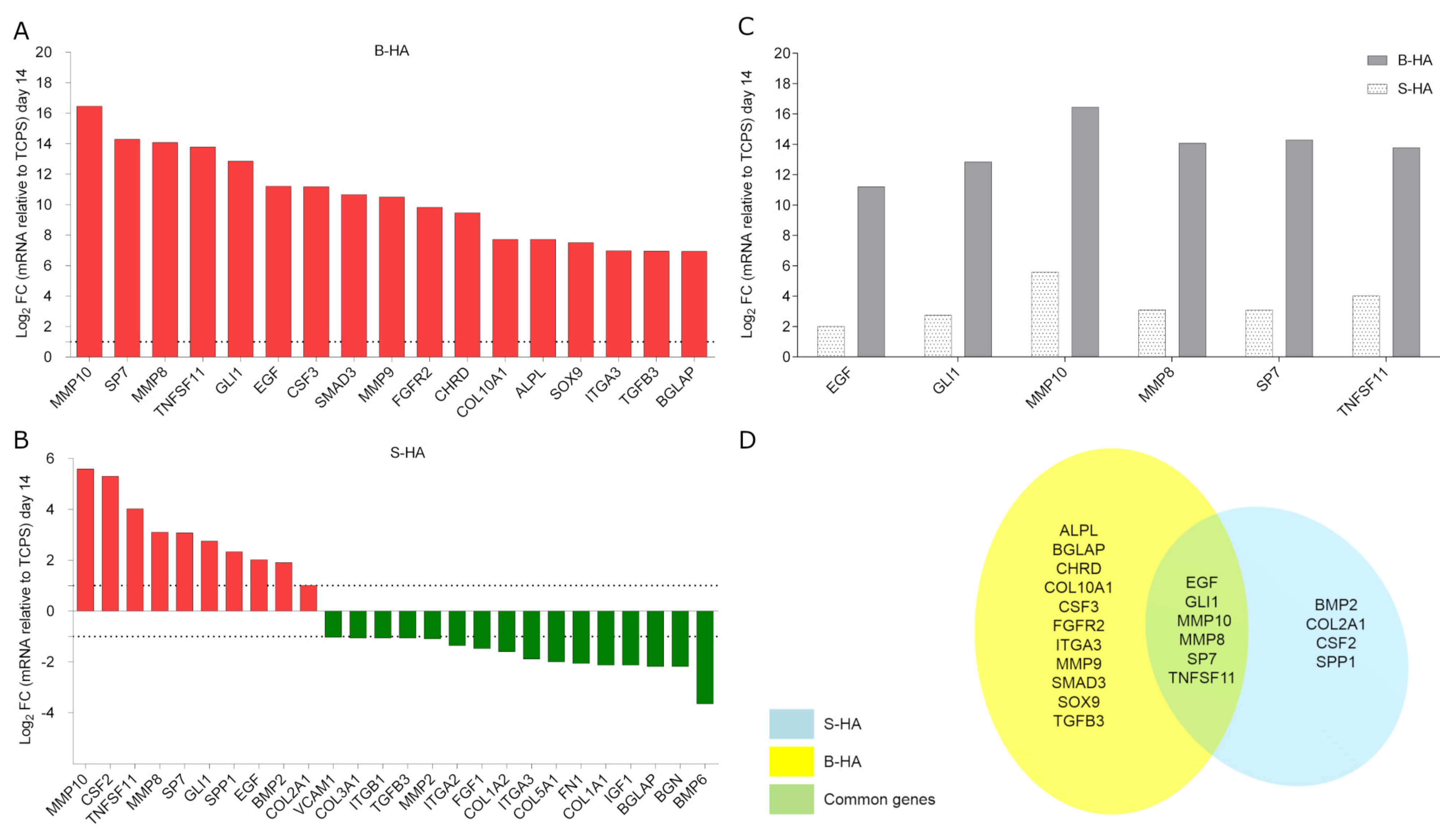
| Common De-Regulated Genes | |||
|---|---|---|---|
| Number | Acronym | Fold-Change (Log2 FC) | |
| S-HA | B-HA | ||
| 1 | BGLAP | −2.18 | +6.94 |
| 2 | EGF | +2.01 | +11.21 |
| 3 | GLI1 | +2.75 | +12.85 |
| 4 | ITGA3 | −1.89 | +6.97 |
| 5 | MMP10 | +5.59 | +16.46 |
| 6 | MMP8 | +3.10 | +14.08 |
| 7 | SP7 | +3.08 | +14.29 |
| 8 | TGFB3 | −1.06 | +6.95 |
| 9 | TNFSF11 | +4.02 | +13.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iaquinta, M.R.; Torreggiani, E.; Mazziotta, C.; Ruffini, A.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F.; Mazzoni, E. In Vitro Osteoinductivity Assay of Hydroxylapatite Scaffolds, Obtained with Biomorphic Transformation Processes, Assessed Using Human Adipose Stem Cell Cultures. Int. J. Mol. Sci. 2021, 22, 7092. https://doi.org/10.3390/ijms22137092
Iaquinta MR, Torreggiani E, Mazziotta C, Ruffini A, Sprio S, Tampieri A, Tognon M, Martini F, Mazzoni E. In Vitro Osteoinductivity Assay of Hydroxylapatite Scaffolds, Obtained with Biomorphic Transformation Processes, Assessed Using Human Adipose Stem Cell Cultures. International Journal of Molecular Sciences. 2021; 22(13):7092. https://doi.org/10.3390/ijms22137092
Chicago/Turabian StyleIaquinta, Maria Rosa, Elena Torreggiani, Chiara Mazziotta, Andrea Ruffini, Simone Sprio, Anna Tampieri, Mauro Tognon, Fernanda Martini, and Elisa Mazzoni. 2021. "In Vitro Osteoinductivity Assay of Hydroxylapatite Scaffolds, Obtained with Biomorphic Transformation Processes, Assessed Using Human Adipose Stem Cell Cultures" International Journal of Molecular Sciences 22, no. 13: 7092. https://doi.org/10.3390/ijms22137092
APA StyleIaquinta, M. R., Torreggiani, E., Mazziotta, C., Ruffini, A., Sprio, S., Tampieri, A., Tognon, M., Martini, F., & Mazzoni, E. (2021). In Vitro Osteoinductivity Assay of Hydroxylapatite Scaffolds, Obtained with Biomorphic Transformation Processes, Assessed Using Human Adipose Stem Cell Cultures. International Journal of Molecular Sciences, 22(13), 7092. https://doi.org/10.3390/ijms22137092








