Neuroinflammation: A Signature or a Cause of Epilepsy?
Abstract
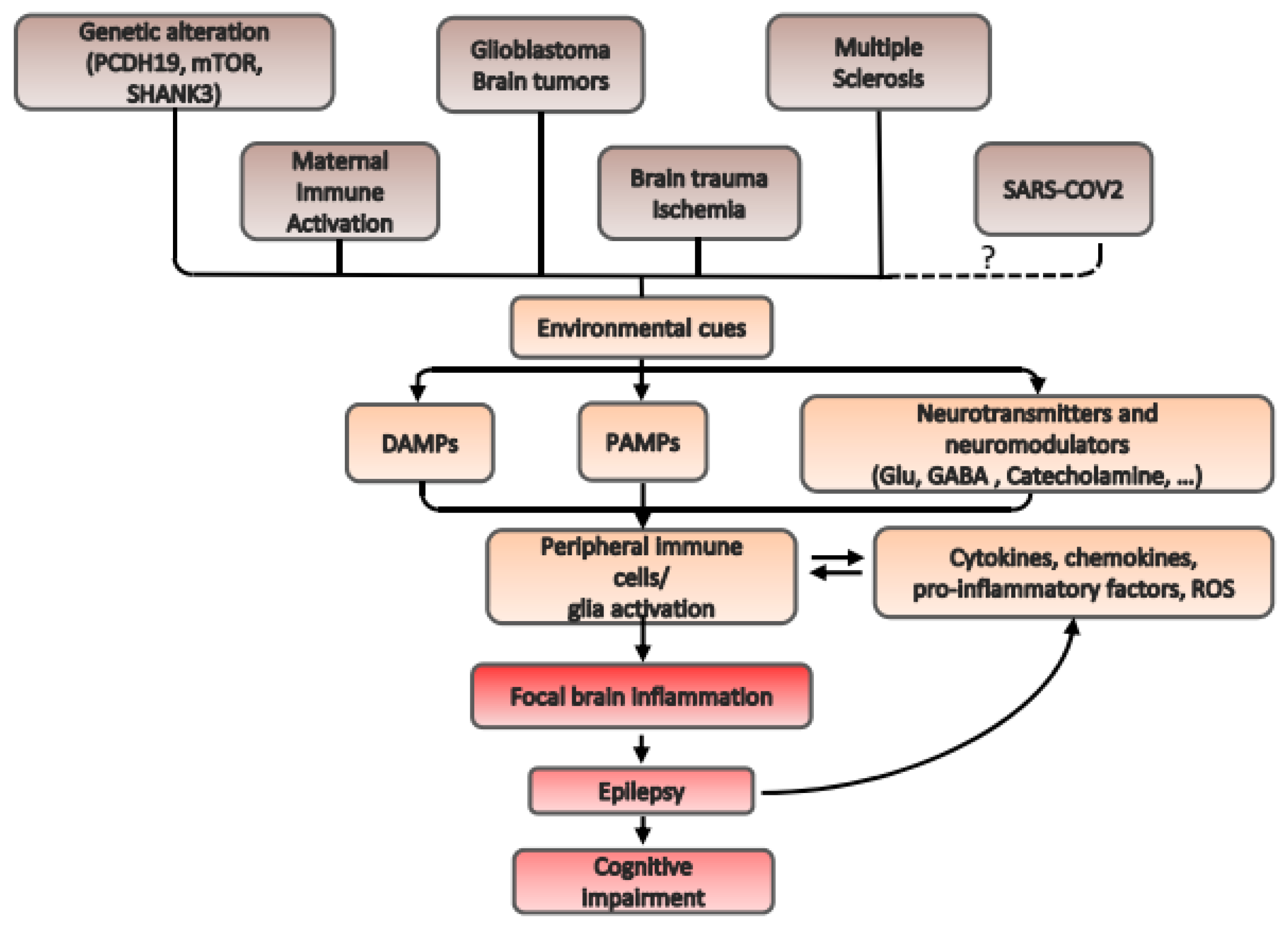
1. Introduction
2. Mechanisms of Neuroinflammation in Some Exemplificative Pathologies Related to Different Forms of Epilepsy
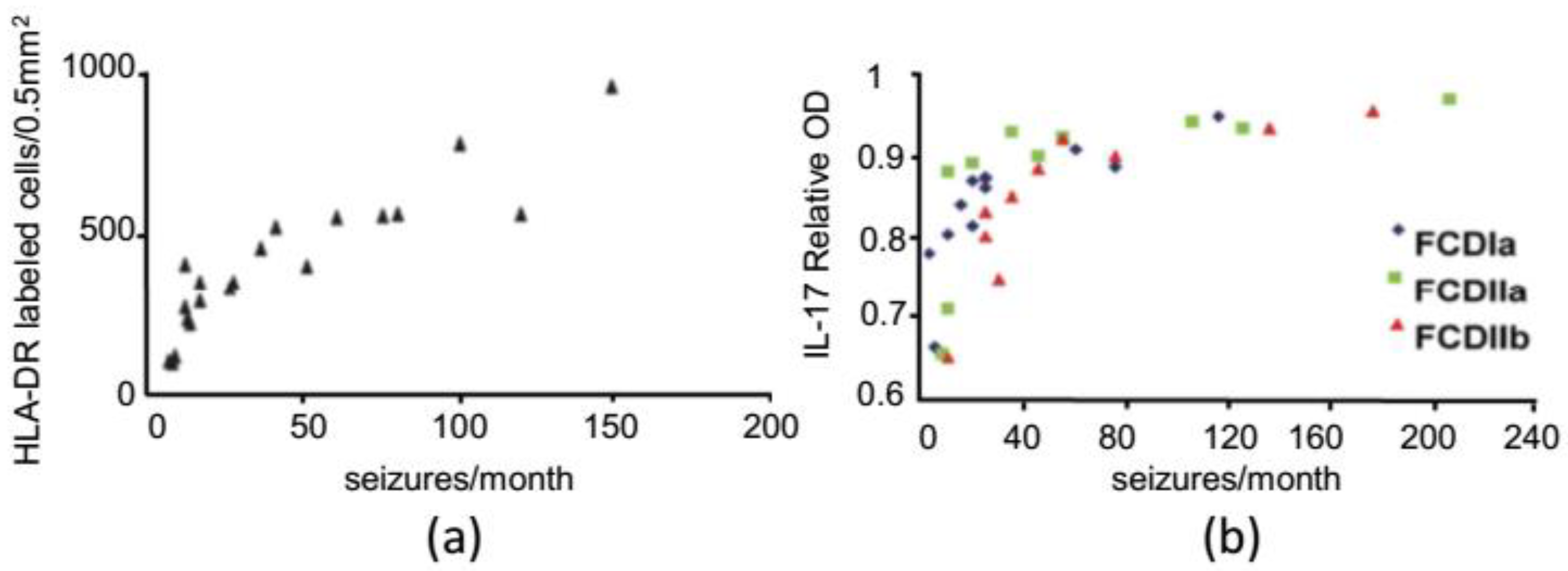
2.1. Structural Epilepsy: Focal Cortical Dysplasia
2.2. Genetic Epilepsy: PCDH19 Epilepsy
2.3. Brain Tumors: Glioblastoma Multiforme
2.4. Epilepsy Caused by Infection: Maternal Immune Activation
2.5. Autoimmune Neurodegenerative Disease: Multiple Sclerosis
2.6. A New Infection Associated to Altered Brain Excitability with Unknown Etiology: SARS-CoV-2
2.7. Alterations in Gut Microbiota in Epilepsy and in Autism Spectrum Disorders with Co-Morbidity with Epilepsy
3. An Overview on Therapies Affecting Neuroinflammation with Possible Outcome in Epilepsy
3.1. Inhibition of Immune Response
- – Corticosteroids and especially adrenocorticotropic hormone (ACTH) have been used in various forms of childhood epilepsy, as in the case of PCDH19 female epilepsy ([47]; see the previous paragraph);
- – Antagonists of Toll-like receptor have been efficaciously used in convulsive epilepsy, as in the case of resveratrol, an anti-inflammatory stilbenoid. The application of resveratrol reduced the frequency of spontaneous seizures in KA-treated rats [154]. This effect was associated with a reduction in neuronal cell loss and an inhibition of mossy fiber sprouting in the hippocampus;
- – Inhibition of the prostaglandin E2-PGE2- receptor subtype is neuroprotective in a pilocarpine model of SE [155];
- – Immunosuppressants, such as cyclosporine A, FK-506 (also known as tacrolimus), and rapamycin inhibiting T-lymphocyte activation, can stop seizures [156]. Indeed, daily systemic injection of cyclosporine A or FK-506 during electrical amygdala kindling prevented the acquisition of severe chronic seizures in rats [157]. However, long-term protection from crisis failed after drug withdrawal, showing limited anticonvulsant capacities [158]. Moreover, these data are quite controversial, since other authors showed opposite effects [159];
- – In status epilepticus, minocycline represents a promising candidate for the anti-inflammatory treatment of epilepsy [162,163]. Despite often being referred to as an inhibitor of microglial activation, minocycline also affects—either directly or indirectly—other cell types, such as neurons, astrocytes, and oligodendrocytes. A similar drug, Minozac, blocks the production of pro-inflammatory cytokines and prevents the cognitive degenerative phenotype associated in a mouse ‘two-hit’ model of epilepsy [164]. Interestingly, an IL-1β inhibitor, VX-765, being used in psoriasis therapy, completed phase 2 clinical trials in 60 people with treatment-resistant partial-onset epilepsy [165].
3.2. Antibody Antagonists
3.3. Probiotics
3.4. Cannabinoids
3.5. Inhibitors of Voltage-Gated Potassium Channels Kv1.3
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary between Brain and Periphery. Antioxid. Redox Signal. 2020, 33, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, J.A.A.; Qurie, A. Interleukin; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, G.; Zhang, F. Role of Peripheral Immune Cells-Mediated Inflammation on the Process of Neurodegenerative Diseases. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflamm. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.S.; Kohane, I.S.; Cai, T.; Gorman, M.P.; Mandl, K.D. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014, 71, 569–574. [Google Scholar] [CrossRef]
- Geis, C.; Planagumà, J.; Carreño, M.; Graus, F.; Dalmau, J. Autoimmune seizures and epilepsy. J. Clin. Investig. 2019, 129, 926–940. [Google Scholar] [CrossRef]
- Barker-Haliski, M.L.; Löscher, W.; White, H.S.; Galanopoulou, A.S. Neuroinflammation in epileptogenesis: Insights and translational perspectives from new models of epilepsy. Epilepsia 2017, 58, 39–47. [Google Scholar] [CrossRef]
- Meng, F.; Yao, L. The role of inflammation in epileptogenesis. Acta Epileptol. 2020, 2, 15. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Goddard, G.V. Development of epileptic seizures through brain stimulation at low intensity. Nature 1967, 214, 1020–1021. [Google Scholar] [CrossRef]
- Pitkänen, A.; Löscher, W.; Vezzani, A.; Becker, A.J.; Simonato, M.; Lukasiuk, K.; Gröhn, O.; Bankstahl, J.P.; Friedman, A.; Aronica, E.; et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016, 15, 843–856. [Google Scholar] [CrossRef]
- Ravizza, T.; Vezzani, A. Pharmacological targeting of brain inflammation in epilepsy: Therapeutic perspectives from experimental and clinical studies. Epilepsia Open 2018, 3, 133–142. [Google Scholar] [CrossRef]
- Kim, I.; Mlsna, L.M.; Yoon, S.; Le, B.; Yu, S.; Xu, D.; Koh, S. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain Behav. 2015, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.L.; Kielian, T. Toll-like receptors in health and disease in the brain: Mechanisms and therapeutic potential. Clin. Sci. 2011, 121, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Chao, Z.-Y.; Feng, D.-Y. Role of Toll-like receptor/MYD88 signaling in neurodegenerative diseases. Rev. Neurosci. 2015, 26, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Manfredi, A.A. Dangers in and out. Science 2009, 323, 1683–1684. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gu, Y.; Zhou, R.; Wang, M.; Guo, Y.; Chen, Y.; Ma, J.; Xiao, F.; Wang, X.; Tian, X. Serum Exosomal Proteins F9 and TSP-1 as Potential Diagnostic Biomarkers for Newly Diagnosed Epilepsy. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B. The Pentraxins 1975–2018: Serendipity, Diagnostics and Drugs. Front. Immunol. 2018, 9, 2382. [Google Scholar] [CrossRef]
- Ummenthum, K.; Peferoen, L.A.N.; Finardi, A.; Baker, D.; Pryce, G.; Mantovani, A.; Bsibsi, M.; Bottazzi, B.; Peferoen-Baert, R.; van der Valk, P.; et al. Pentraxin-3 is upregulated in the central nervous system during MS and EAE, but does not modulate experimental neurological disease. Eur. J. Immunol. 2016, 46, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Bronisz, E.; Kurkowska-Jastrzȩbska, I. Matrix Metalloproteinase 9 in Epilepsy: The Role of Neuroinflammation in Seizure Development. Mediators Inflamm. 2016, 2016. [Google Scholar] [CrossRef]
- Dwir, D.; Giangreco, B.; Xin, L.; Tenenbaum, L.; Cabungcal, J.H.; Steullet, P.; Goupil, A.; Cleusix, M.; Jenni, R.; Chtarto, A.; et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: A reverse translation study in schizophrenia patients. Mol. Psychiatry 2020, 25, 2889–2904. [Google Scholar] [CrossRef]
- De Vivo, L.; Landi, S.; Panniello, M.; Baroncelli, L.; Chierzi, S.; Mariotti, L.; Spolidoro, M.; Pizzorusso, T.; Maffei, L.; Ratto, G.M. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat. Commun. 2013, 4, 1484. [Google Scholar] [CrossRef]
- Faini, G.; Aguirre, A.; Landi, S.; Lamers, D.; Pizzorusso, T.; Ratto, G.M.; Deleuze, C.; Bacci, A. Perineuronal nets control visual input via thalamic recruitment of cortical PV interneurons. Elife 2018, 7, e41520. [Google Scholar] [CrossRef]
- Gong, X.; Hu, H.; Qiao, Y.; Xu, P.; Yang, M.; Dang, R.; Han, W.; Guo, Y.; Chen, D.; Jiang, P. The Involvement of Renin-Angiotensin System in Lipopolysaccharide-Induced Behavioral Changes, Neuroinflammation, and Disturbed Insulin Signaling. Front. Pharmacol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Guerrini, R.; Duchowny, M.; Jayakar, P.; Krsek, P.; Kahane, P.; Tassi, L.; Melani, F.; Polster, T.; Andre, V.M.; Cepeda, C.; et al. Diagnostic methods and treatment options for focal cortical dysplasia. Epilepsia 2015, 56, 1669–1686. [Google Scholar] [CrossRef]
- Guerrini, R.; Dobyns, W.B. Malformations of cortical development: Clinical features and genetic causes. Lancet Neurol. 2014, 13, 710–726. [Google Scholar] [CrossRef]
- D’Gama, A.M.; Walsh, C.A. Somatic mosaicism and neurodevelopmental disease. Nat. Neurosci. 2018, 21, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Boer, K.; Spliet, W.G.M.; Van Rijen, P.C.; Redeker, S.; Troost, D.; Aronica, E. Evidence of activated microglia in focal cortical dysplasia. J. Neuroimmunol. 2006, 173, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B. Focal cortical dysplasia. Semin. Neurol. 2015, 35, 201–208. [Google Scholar] [CrossRef]
- Sun, F.J.; Zhang, C.Q.; Chen, X.; Wei, Y.J.; Li, S.; Liu, S.Y.; Zang, Z.-l.; He, J.J.; Guo, W.; Yang, H. Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. J. Neuroinflamm. 2016, 13. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Liu, M.; Wang, H.; Dong, Y.; Ji, T.; Liu, X.; Jiang, Y.; Cai, L.; Wu, Y. Upregulation of HMGB1-TLR4 inflammatory pathway in focal cortical dysplasia type II. J. Neuroinflamm. 2018, 15. [Google Scholar] [CrossRef]
- He, J.J.; Li, S.; Shu, H.F.; Yu, S.X.; Liu, S.Y.; Yin, Q.; Yang, H. The interleukin 17 system in cortical lesions in focal cortical dysplasias. J. Neuropathol. Exp. Neurol. 2013, 72, 152–163. [Google Scholar] [CrossRef]
- Berg, A.T.; Rychlik, K.; Levy, S.R.; Testa, F.M. Complete remission of childhood-onset epilepsy: Stability and prediction over two decades. Brain 2014, 137, 3213–3222. [Google Scholar] [CrossRef]
- Wong, M. Animal models of focal cortical dysplasia and tuberous sclerosis complex: Recent progress toward clinical applications. Epilepsia 2009, 50, 34–44. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Wen, J.H.; Claycomb, K.; Huang, Y.; Harrsch, F.A.; Naegele, J.R.; Hyder, F.; Buchanan, G.F.; Bordey, A. Convulsive seizures from experimental focal cortical dysplasia occur independently of cell misplacement. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ribierre, T.; Deleuze, C.; Bacq, A.; Baldassari, S.; Marsan, E.; Chipaux, M.; Muraca, G.; Roussel, D.; Navarro, V.; Leguern, E.; et al. Second-hit mosaic mutation in mTORC1 repressor DEPDC5 causes focal cortical dysplasia-associated epilepsy. J. Clin. Investig. 2018, 128, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Trovato, F.; Parra, R.; Pracucci, E.; Landi, S.; Cozzolino, O.; Nardi, G.; Cruciani, F.; Pillai, V.; Mosti, L.; Cwetsch, A.W.; et al. Modelling genetic mosaicism of neurodevelopmental disorders in vivo by a Cre-amplifying fluorescent reporter. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Dibbens, L.M.; Tarpey, P.S.; Hynes, K.; Bayly, M.A.; Scheffer, I.E.; Smith, R.; Bomar, J.; Sutton, E.; Vandeleur, L.; Shoubridge, C.; et al. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat. Genet. 2008, 40, 776–781. [Google Scholar] [CrossRef]
- Depienne, C.; Bouteiller, D.; Keren, B.; Cheuret, E.; Poirier, K.; Trouillard, O.; Benyahia, B.; Quelin, C.; Carpentier, W.; Julia, S.; et al. Correction: Sporadic Infantile Epileptic Encephalopathy Caused by Mutations in PCDH19 Resembles Dravet Syndrome but Mainly Affects Females. PLoS Genet. 2009, 5. [Google Scholar] [CrossRef]
- Higurashi, N.; Takahashi, Y.; Kashimada, A.; Sugawara, Y.; Sakuma, H.; Tomonoh, Y.; Inoue, T.; Hoshina, M.; Satomi, R.; Ohfu, M.; et al. Immediate suppression of seizure clusters by corticosteroids in PCDH19 female epilepsy. Seizure 2015, 27, 1–5. [Google Scholar] [CrossRef]
- Bertani, G.; Spagnoli, C.; Iodice, A.; Salerno, G.G.; Frattini, D.; Fusco, C. Steroids efficacy in the acute management of seizure clusters in one case of PCDH19 female epilepsy. Seizure 2015, 32, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Chung, H.J. A Case of Effective Treatment of a Patient with PCDH 19- Related Epilepsy using Corticosteroid. J. Korean Child Neurol. Soc. 2018, 26, 32–37. [Google Scholar] [CrossRef]
- Marchi, N.; Granata, T.; Freri, E.; Ciusani, E.; Ragona, F.; Puvenna, V.; Teng, Q.; Alexopolous, A.; Janigro, D. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS ONE 2011, 6, e18200. [Google Scholar] [CrossRef]
- Cooper, S.R.; Jontes, J.D.; Sotomayor, M. Structural determinants of adhesion by protocadherin-19 and implications for its role in epilepsy. Elife 2016, 5. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro. Oncol. 2019, 21, V1–V100. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Arimappamagan, A.; Somasundaram, K.; Thennarasu, K.; Peddagangannagari, S.; Srinivasan, H.; Shailaja, B.C.; Samuel, C.; Patric, I.R.P.; Shukla, S.; Thota, B.; et al. A Fourteen Gene GBM Prognostic Signature Identifies Association of Immune Response Pathway and Mesenchymal Subtype with High Risk Group. PLoS ONE 2013, 8, e62042. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lv, X.; Lu, C.; Ye, X.; Chen, X.; Fu, J.; Luo, C.; Zhao, Y. Prognostic factors of patients with Gliomas—An analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer 2020, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Ertürk Çetin, Ö.; İşler, C.; Uzan, M.; Özkara, Ç. Epilepsy-related brain tumors. Seizure 2017, 44, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, S.; Spliet, W.G.M.; Geurts, M.; Van Hecke, W.; Seute, T.; Snijders, T.J.; Bours, V.; Bell, E.H.; Chakravarti, A.; Robe, P.A. Epilepsy Associates with Decreased HIF-1α/STAT5b Signaling in Glioblastoma. Cancers 2019, 11, 41. [Google Scholar] [CrossRef]
- Tremblay, P.; Beaudet, M.J.; Tremblay, E.; Rueda, N.; Thomas, T.; Vallières, L. Matrix metalloproteinase 2 attenuates brain tumour growth, while promoting macrophage recruitment and vascular repair. J. Pathol. 2011, 224, 222–233. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the immune system in glioblastoma. Br. J. Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, X.; Zhang, J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2012, 60, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Bertics, P.J. Eosinophils in glioblastoma biology. J. Neuroinflamm. 2012, 9. [Google Scholar] [CrossRef]
- Sowers, J.L.; Johnson, K.M.; Conrad, C.; Patterson, J.T.; Sowers, L.C. The role of inflammation in brain cancer. Adv. Exp. Med. Biol. 2014, 816, 75–105. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.R.; Gupta, A.S.; Mockenhaupt, K.; Brown, L.S.N.; Biswas, D.D.; Kordula, T. RelB acts as a molecular switch driving chronic inflammation in glioblastoma multiforme. Oncogenesis 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Meini, A.; Sticozzi, C.; Massai, L.; Palmi, M. A nitric oxide/Ca 2+/calmodulin/ERK1/2 mitogen-activated protein kinase pathway is involved in the mitogenic effect of IL-1β in human astrocytoma cells. Br. J. Pharmacol. 2008, 153, 1706–1717. [Google Scholar] [CrossRef]
- Yeung, Y.T.; McDonald, K.L.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: Implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [CrossRef]
- Tanabe, K.; Kozawa, O.; Iida, H. Midazolam suppresses interleukin-1β-induced interleukin-6 release from rat glial cells. J. Neuroinflamm. 2011, 8, 68. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Sasayama, T.; Tanaka, K.; Koma, Y.-I.; Nishihara, M.; Tanaka, H.; Nakamizo, S.; Nagashima, H.; Maeyama, M.; Fujita, Y.; et al. Tumor-associated macrophage related interleukin-6 in cerebrospinal fluid as a prognostic marker for glioblastoma. J. Clin. Neurosci. 2019, 68, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Coelho-Santos, V.; Santos, L.; Fontes-Ribeiro, C.; Silva, A.P.; Gomes, C.M.F. The interplay between glioblastoma and microglia cells leads to endothelial cell monolayer dysfunction via the interleukin-6-induced JAK2/STAT3 pathway. J. Cell. Physiol. 2019, 234, 19750–19760. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Booth, S.; Price, S. Corticosteroid-use in primary and secondary brain tumour patients: A review. J. Neurooncol. 2012, 106, 449–459. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Binder, D.K.; Manley, G.T.; Krishna, S.; Verkman, A.S. Molecular mechanisms of brain tumor edema. Neuroscience 2004, 129, 1009–1018. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; de Sampaio e Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Solek, C.M.; Farooqi, N.; Verly, M.; Lim, T.K.; Ruthazer, E.S. Maternal immune activation in neurodevelopmental disorders. Dev. Dyn. 2018, 247, 588–619. [Google Scholar] [CrossRef]
- Glass, H.C.; Pham, T.N.; Danielsen, B.; Towner, D.; Glidden, D.; Wu, Y.W. Antenatal and Intrapartum Risk Factors for Seizures in Term Newborns: A Population-Based Study, California 1998–2002. J. Pediatr. 2009, 154. [Google Scholar] [CrossRef]
- Samuelsson, A.M.; Jennische, E.; Hansson, H.A.; Holmäng, A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABAA dysregulation and impaired spatial learning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1345–1356. [Google Scholar] [CrossRef]
- Zeraati, M.; Najdi, N.; Mosaferi, B.; Salari, A.A. Environmental enrichment alters neurobehavioral development following maternal immune activation in mice offspring with epilepsy. Behav. Brain Res. 2021, 399. [Google Scholar] [CrossRef]
- Washington, J.; Kumar, U.; Medel-Matus, J.S.; Shin, D.; Sankar, R.; Mazarati, A. Cytokine-dependent bidirectional connection between impaired social behavior and susceptibility to seizures associated with maternal immune activation in mice. Epilepsy Behav. 2015, 50, 40–45. [Google Scholar] [CrossRef][Green Version]
- Pineda, E.; Shin, D.; You, S.J.; Auvin, S.; Sankar, R.; Mazarati, A. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann. Neurol. 2013, 74, 11–19. [Google Scholar] [CrossRef]
- Corradini, I.; Focchi, E.; Rasile, M.; Morini, R.; Desiato, G.; Tomasoni, R.; Lizier, M.; Ghirardini, E.; Fesce, R.; Morone, D.; et al. Maternal Immune Activation Delays Excitatory-to-Inhibitory Gamma-Aminobutyric Acid Switch in Offspring. Biol. Psychiatry 2018, 83, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Baranzini, S.E.; Oksenberg, J.R. The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years. Trends Genet. 2017, 33, 960–970. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef]
- Lapato, A.S.; Thompson, S.M.; Parra, K.; Tiwari-Woodruff, S.K. Astrocyte Glutamate Uptake and Water Homeostasis Are Dysregulated in the Hippocampus of Multiple Sclerosis Patients with Seizures. ASN Neuro 2020, 12, 1759091420979604. [Google Scholar] [CrossRef]
- Dagiasi, I.; Vall, V.; Kumlien, E.; Burman, J.; Zelano, J. Treatment of epilepsy in multiple sclerosis. Seizure 2018, 58, 47–51. [Google Scholar] [CrossRef]
- Chou, I.J.; Kuo, C.F.; Tanasescu, R.; Tench, C.R.; Tiley, C.G.; Constantinescu, C.S.; Whitehouse, W.P. Epilepsy and associated mortality in patients with multiple sclerosis. Eur. J. Neurol. 2019, 26, 342–e23. [Google Scholar] [CrossRef]
- Langenbruch, L.; Krämer, J.; Güler, S.; Möddel, G.; Geßner, S.; Melzer, N.; Elger, C.E.; Wiendl, H.; Budde, T.; Meuth, S.G.; et al. Seizures and epilepsy in multiple sclerosis: Epidemiology and prognosis in a large tertiary referral center. J. Neurol. 2019, 266, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Poser, C.M.; Brinar, V. V Epilepsy and multiple sclerosis. Epilepsy Behav. 2003, 4, 6–12. [Google Scholar] [CrossRef]
- Pack, A. Is There a Relationship Between Multiple Sclerosis and Epilepsy? If So What Does It Tell Us About Epileptogenesis? Epilepsy Curr. 2018, 18, 95–96. [Google Scholar] [CrossRef]
- Neuß, F.; von Podewils, F.; Wang, Z.I.; Süße, M.; Zettl, U.K.; Grothe, M. Epileptic seizures in multiple sclerosis: Prevalence, competing causes and diagnostic accuracy. J. Neurol. 2021, 268, 1721–1727. [Google Scholar] [CrossRef]
- DePaula-Silva, A.B.; Hanak, T.J.; Libbey, J.E.; Fujinami, R.S. Theiler’s murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: Models for multiple sclerosis and epilepsy. J. Neuroimmunol. 2017, 308, 30–42. [Google Scholar] [CrossRef]
- Mahamud, Z.; Burman, J.; Zelano, J. Prognostic impact of epilepsy in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 38, 101497. [Google Scholar] [CrossRef]
- Steardo, L.; Steardo, L.; Zorec, R.; Verkhratsky, A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020, 229. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef]
- Azhideh, A. COVID-19 Neurological Manifestations. Int. Clin. Neurosci. J. 2020, 7, 54. [Google Scholar] [CrossRef]
- Niazkar, H.R.; Zibaee, B.; Nasimi, A.; Bahri, N. The neurological manifestations of COVID-19: A review article. Neurol. Sci. 2020, 41, 1667–1671. [Google Scholar] [CrossRef]
- Najjar, S.; Najjar, A.; Chong, D.J.; Pramanik, B.K.; Kirsch, C.; Kuzniecky, R.I.; Pacia, S.V.; Azhar, S. Central nervous system complications associated with SARS-CoV-2 infection: Integrative concepts of pathophysiology and case reports. J. Neuroinflamm. 2020, 17, 17. [Google Scholar] [CrossRef]
- Murray, R.S.; Brown, B.; Brain, D.; Cabirac, G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992, 31, 525–533. [Google Scholar] [CrossRef]
- Hung, E.C.W.; Chim, S.S.C.; Chan, P.K.S.; Tong, Y.K.; Ng, E.K.O.; Chiu, R.W.K.; Leung, C.B.; Sung, J.J.Y.; Tam, J.S.; Lo, Y.M.D. Detection of SARS Coronavirus RNA in the Cerebrospinal Fluid of a Patient with Severe Acute Respiratory Syndrome. Clin. Chem. 2003, 49, 2108–2109. [Google Scholar] [CrossRef]
- Morfopoulou, S.; Brown, J.R.; Davies, E.G.; Anderson, G.; Virasami, A.; Qasim, W.; Chong, W.K.; Hubank, M.; Plagnol, V.; Desforges, M.; et al. Human Coronavirus OC43 Associated with Fatal Encephalitis. N. Engl. J. Med. 2016, 375, 497–498. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Edner, N.; Albert, J.; Ternhag, A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect. Dis. 2020, 52, 419–422. [Google Scholar] [CrossRef]
- Skinner, D.; Marro, B.S.; Lane, T.E. Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease. Viral Immunol. 2019, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Castro, P.J.; Estivill-Torrús, G.; Cabezudo-García, P.; Reyes-Bueno, J.A.; Ciano Petersen, N.; Aguilar-Castillo, M.J.; Suárez-Pérez, J.; Jiménez-Hernández, M.D.; Moya-Molina, M.; Oliver-Martos, B.; et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurologia 2020, 35, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2020. [Google Scholar] [CrossRef]
- Kubota, T.; Gajera, P.K.; Kuroda, N. Meta-analysis of EEG findings in patients with COVID-19. Epilepsy Behav. 2021, 115, 107682. [Google Scholar] [CrossRef]
- Lu, L.; Xiong, W.; Liu, D.; Liu, J.; Yang, D.; Li, N.; Mu, J.; Guo, J.; Li, W.; Wang, G.; et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia 2020, 61, e49–e53. [Google Scholar] [CrossRef]
- Hwang, S.T.; Ballout, A.A.; Mirza, U.; Sonti, A.N.; Husain, A.; Kirsch, C.; Kuzniecky, R.; Najjar, S. Acute Seizures Occurring in Association With SARS-CoV-2. Front. Neurol. 2020, 11, 576329. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Simani, L.; Shahisavandi, M.; Barzegar, Z. COVID-19, de novo seizures, and epilepsy: A systematic review. Neurol. Sci. 2021, 42, 415–431. [Google Scholar] [CrossRef]
- D’Orsi, G.; Mazzeo, F.; Ravidà, D.; Di Claudio, M.T.; Sabetta, A.; Lalla, A.; Sbrizzi, S.; Avolio, C. The effect of quarantine due to Covid-19 pandemic on seizure frequency in 102 adult people with epilepsy from Apulia and Basilicata regions, Southern Italy. Clin. Neurol. Neurosurg. 2021, 203, 106592. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, F.; Mohammadkhanizadeh, A.; Mohammadi, E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult. Scler. Relat. Disord. 2020, 46, 102535. [Google Scholar] [CrossRef]
- Novi, G.; Rossi, T.; Pedemonte, E.; Saitta, L.; Rolla, C.; Roccatagliata, L.; Inglese, M.; Farinini, D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef]
- Alberti, P.; Beretta, S.; Piatti, M.; Karantzoulis, A.; Piatti, M.L.; Santoro, P.; Viganò, M.; Giovannelli, G.; Pirro, F.; Montisano, D.A.; et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. NeuroInflamm. 2020, 7. [Google Scholar] [CrossRef]
- Bossù, P.; Toppi, E.; Sterbini, V.; Spalletta, G. Implication of aging related chronic neuroinflammation on covid-19 pandemic. J. Pers. Med. 2020, 10, 102. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Liu, X.; Zhang, J.; Liu, G. Crosstalk between the Ketogenic Diet and Epilepsy: From the Perspective of Gut Microbiota. Mediat. Inflamm. 2019, 2019, 8373060. [Google Scholar] [CrossRef]
- De Caro, C.; Iannone, L.F.; Citraro, R.; Striano, P.; De Sarro, G.; Constanti, A.; Cryan, J.F.; Russo, E. Can we “seize” the gut microbiota to treat epilepsy? Neurosci. Biobehav. Rev. 2019, 107, 750–764. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Lum, G.R.; Olson, C.A.; Hsiao, E.Y. Emerging roles for the intestinal microbiome in epilepsy. Neurobiol. Dis. 2020, 135, 104576. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. May the Force Be With You: The Light and Dark Sides of the Microbiota-Gut-Brain Axis in Neuropsychiatry. CNS Drugs 2016, 30, 1019–1041. [Google Scholar] [CrossRef]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Wang, B.; Ahmadi, S.; Burnham, C.A.D.; Tarr, P.I.; Warner, B.B.; Dantas, G. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat. Microbiol. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain. Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hagan, C.C.; Graham, J.M.E.; Wilkinson, P.O.; Midgley, N.; Suckling, J.; Sahakian, B.J.; Goodyer, I.M. Neurodevelopment and ages of onset in depressive disorders. Lancet Psychiatry 2015, 2, 1112–1116. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Tatti, R.; Haley, M.S.; Swanson, O.K.; Tselha, T.; Maffei, A. Neurophysiology and Regulation of the Balance between Excitation and Inhibition in Neocortical Circuits. Biol. Psychiatry 2017, 81, 821–831. [Google Scholar] [CrossRef]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered intestinal morphology and microbiota composition in the autism spectrum disorders associated SHANK3 mouse model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Sauer, A.K.; Hagmeyer, S.; Mangus, K.; Linta, L.; Liebau, S.; Bockmann, J.; Huguet, G.; Bourgeron, T.; Boeckers, T.M.; et al. Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain. Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Möhle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C (hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Medel-Matus, J.-S.; Shin, D.; Dorfman, E.; Sankar, R.; Mazarati, A. Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open 2018, 3, 290–294. [Google Scholar] [CrossRef]
- Şafak, B.; Altunan, B.; Topçu, B.; Eren Topkaya, A. The gut microbiome in epilepsy. Microb. Pathog. 2020, 139, 103853. [Google Scholar] [CrossRef]
- Dahlin, M.; Prast-Nielsen, S. The gut microbiome and epilepsy. EBioMedicine 2019, 44, 741–746. [Google Scholar] [CrossRef]
- Aronica, E.; Bauer, S.; Bozzi, Y.; Caleo, M.; Dingledine, R.; Gorter, J.A.; Henshall, D.C.; Kaufer, D.; Koh, S.; Löscher, W.; et al. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 2017, 58, 27–38. [Google Scholar] [CrossRef]
- Kobylarek, D.; Iwanowski, P.; Lewandowska, Z.; Limphaibool, N.; Szafranek, S.; Labrzycka, A.; Kozubski, W. Advances in the potential biomarkers of epilepsy. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Sulis Sato, S.; Artoni, P.; Landi, S.; Cozzolino, O.; Parra, R.; Pracucci, E.; Trovato, F.; Szczurkowska, J.; Luin, S.; Arosio, D.; et al. Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E8770–E8779. [Google Scholar] [CrossRef]
- Vitaliti, G.; Pavone, P.; Mahmood, F.; Nunnari, G.; Falsaperla, R. Targeting inflammation as a therapeutic strategy for drug-resistant epilepsies: An update of new immune-modulating approaches. Hum. Vaccines Immunother. 2014, 10, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Sasaki, K.; Ichimaru, T.; Nakazato, S.; Hamasaki, Y. Increased IL-1β Production From dsRNA-stimulated Leukocytes in Febrile Seizures. Pediatr. Neurol. 2006, 35, 102–106. [Google Scholar] [CrossRef]
- Jiang, J.; Ganesh, T.; Du, Y.; Quan, Y.; Serrano, G.; Qui, M.; Speigel, I.; Rojas, A.; Lelutiu, N.; Dingledine, R. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc. Natl. Acad. Sci. USA 2012, 109, 3149–3154. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. Fk-506, A Novel Immunosuppressant Isolated From A Streptomyces Ii. Immunosuppressive Effect of Fk-506 In Vitro. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Chwiej, J.; Janeczko, K.; Marciszko, M.; Czyzycki, M.; Rickers, K.; Setkowicz, Z. Neuroprotective action of FK-506 (tacrolimus) after seizures induced with pilocarpine: Quantitative and topographic elemental analysis of brain tissue. J. Biol. Inorg. Chem. 2010, 15, 283–289. [Google Scholar] [CrossRef]
- Setkowicz, Z.; Ciarach, M. Neuroprotectants FK-506 and cyclosporin A ameliorate the course of pilocarpine-induced seizures. Epilepsy Res. 2007, 73, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Omura, S.; Ohashi, Y.; Kawai, M.; Iwata, Y.; Tani, K.; Sekine, Y.; Takei, N.; Mori, N. Fk506 facilitates chemical kindling induced by pentylenetetrazole in rats. Epilepsy Res. 2001, 46, 279–282. [Google Scholar] [CrossRef]
- Villani, F.; Avanzini, G. The use of immunoglobulins in the treatment of human epilepsy. Neurol. Sci. 2002, 23, S33–S37. [Google Scholar] [CrossRef]
- Tan, T.H.-L.; Perucca, P.; O’Brien, T.J.; Kwan, P.; Monif, M. Inflammation, ictogenesis, and epileptogenesis: An exploration through human disease. Epilepsia 2021, 62, 303–324. [Google Scholar] [CrossRef]
- Möller, T.; Bard, F.; Bhattacharya, A.; Biber, K.; Campbell, B.; Dale, E.; Eder, C.; Gan, L.; Garden, G.A.; Hughes, Z.A.; et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 2016, 64, 1788–1794. [Google Scholar] [CrossRef]
- Bialer, M.; Johannessen, S.I.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Thirteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIII). Epilepsia 2017, 58, 181–221. [Google Scholar] [CrossRef]
- Chrzaszcz, M.; Venkatesan, C.; Dragisic, T.; Watterson, D.M.; Wainwright, M.S. Minozac treatment prevents increased seizure susceptibility in a mouse “two-hit” model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures. J. Neurotrauma 2010, 27, 1283–1295. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Iori, V.; Wright, C.I.; French, J.; Vezzani, A. Interleukin-1β Biosynthesis Inhibition Reduces Acute Seizures and Drug Resistant Chronic Epileptic Activity in Mice. Neurotherapeutics 2011, 8, 304–315. [Google Scholar] [CrossRef]
- Reichert, J.M. Marketed therapeutic antibodies compendium. MAbs 2012, 4, 413–415. [Google Scholar] [CrossRef]
- Bakpa, O.D.; Reuber, M.; Irani, S.R. Antibody-associated epilepsies: Clinical features, evidence for immunotherapies and future research questions. Seizure 2016, 41, 26–41. [Google Scholar] [CrossRef]
- DeSena, A.D.; Do, T.; Schulert, G.S. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflamm. 2018, 15, 38. [Google Scholar] [CrossRef]
- Cantarín-Extremera, V.; Jiménez-Legido, M.; Duat-Rodríguez, A.; García-Fernández, M.; Ortiz-Cabrera, N.V.; Ruiz-Falcó-Rojas, M.L.; González-Gutiérrez-Solana, L. Tocilizumab in pediatric refractory status epilepticus and acute epilepsy: Experience in two patients. J. Neuroimmunol. 2020, 340, 577142. [Google Scholar] [CrossRef]
- Tognini, P. Gut microbiota: A potential regulator of neurodevelopment. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Eguílaz, M.; Ramón-Trapero, J.L.; Pérez-Martínez, L.; Blanco, J.R. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: A pilot study. Benef. Microbes 2018, 9, 875–881. [Google Scholar] [CrossRef]
- Cheung, K.A.K.; Peiris, H.; Wallace, G.; Holland, O.J.; Mitchell, M.D. The interplay between the endocannabinoid system, epilepsy and cannabinoids. Int. J. Mol. Sci. 2019, 20, 6079. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Guo, J.; Zhang, Z.; Zhang, S.; Zhu, Y.; Cheng, J.; Yu, L.; Ji, Y.; Tao, J. Kv1.3 Channel as a Key Therapeutic Target for Neuroinflammatory Diseases: State of the Art and Beyond. Front. Neurosci. 2019, 13, 1393. [Google Scholar] [CrossRef]
- Koh, S. Role of Neuroinflammation in Evolution of Childhood Epilepsy. J. Child Neurol. 2018, 33, 64–72. [Google Scholar] [CrossRef]
- Veggiotti, P.; Pera, M.C.; Teutonico, F.; Brazzo, D.; Balottin, U.; Tassinari, C.A. Therapy of encephalopathy with status epilepticus during sleep (ESES/CSWS syndrome): An update. Epileptic Disord. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Kelley, S.A.; Hartman, A.L. Metabolic Treatments for Intractable Epilepsy. Semin. Pediatr. Neurol. 2011, 18, 179–185. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pracucci, E.; Pillai, V.; Lamers, D.; Parra, R.; Landi, S. Neuroinflammation: A Signature or a Cause of Epilepsy? Int. J. Mol. Sci. 2021, 22, 6981. https://doi.org/10.3390/ijms22136981
Pracucci E, Pillai V, Lamers D, Parra R, Landi S. Neuroinflammation: A Signature or a Cause of Epilepsy? International Journal of Molecular Sciences. 2021; 22(13):6981. https://doi.org/10.3390/ijms22136981
Chicago/Turabian StylePracucci, Enrico, Vinoshene Pillai, Didi Lamers, Riccardo Parra, and Silvia Landi. 2021. "Neuroinflammation: A Signature or a Cause of Epilepsy?" International Journal of Molecular Sciences 22, no. 13: 6981. https://doi.org/10.3390/ijms22136981
APA StylePracucci, E., Pillai, V., Lamers, D., Parra, R., & Landi, S. (2021). Neuroinflammation: A Signature or a Cause of Epilepsy? International Journal of Molecular Sciences, 22(13), 6981. https://doi.org/10.3390/ijms22136981





