Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives
Abstract
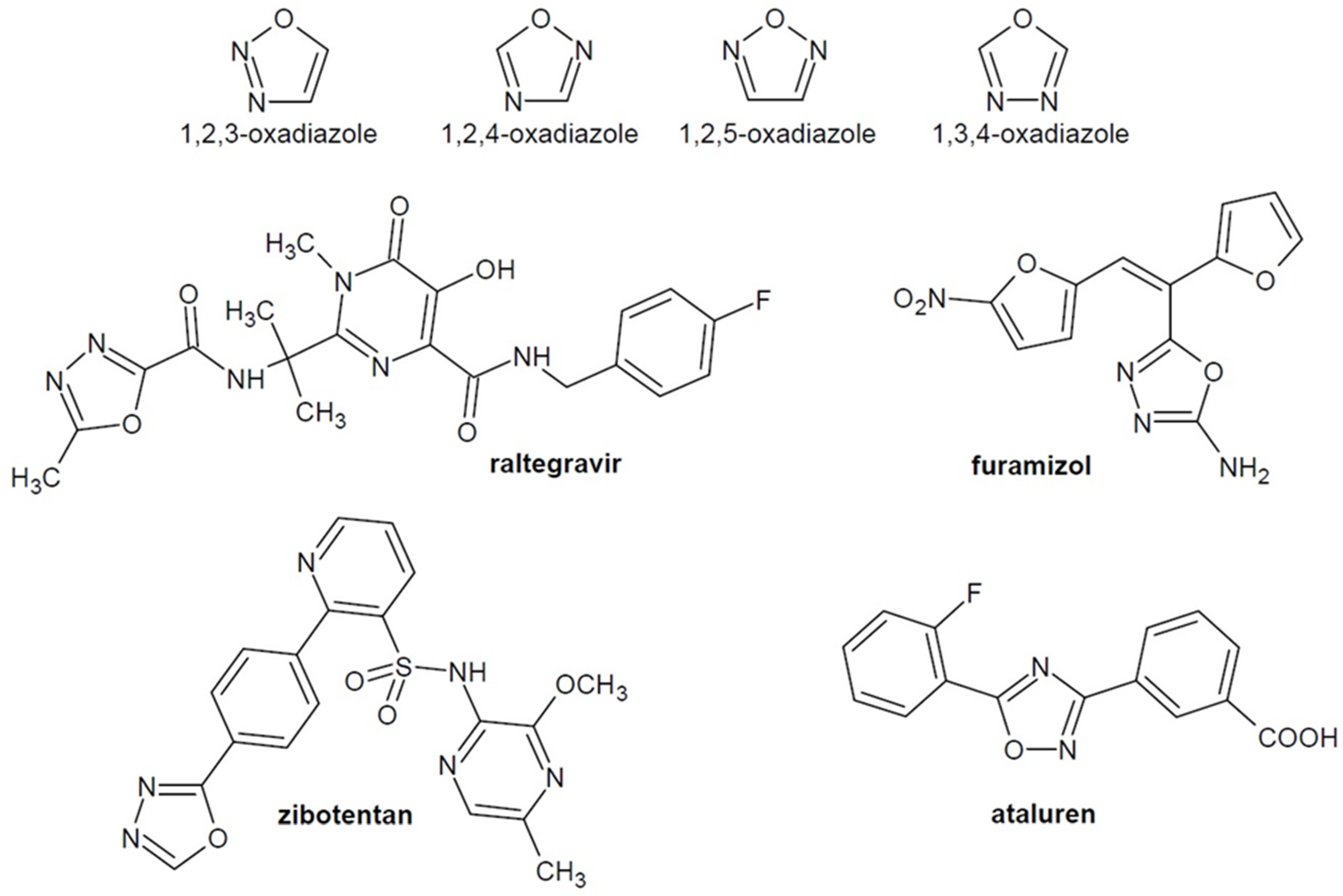
:1. Introduction
2. Antibacterial Activity of 1,3,4-Oxadiazole Derivatives
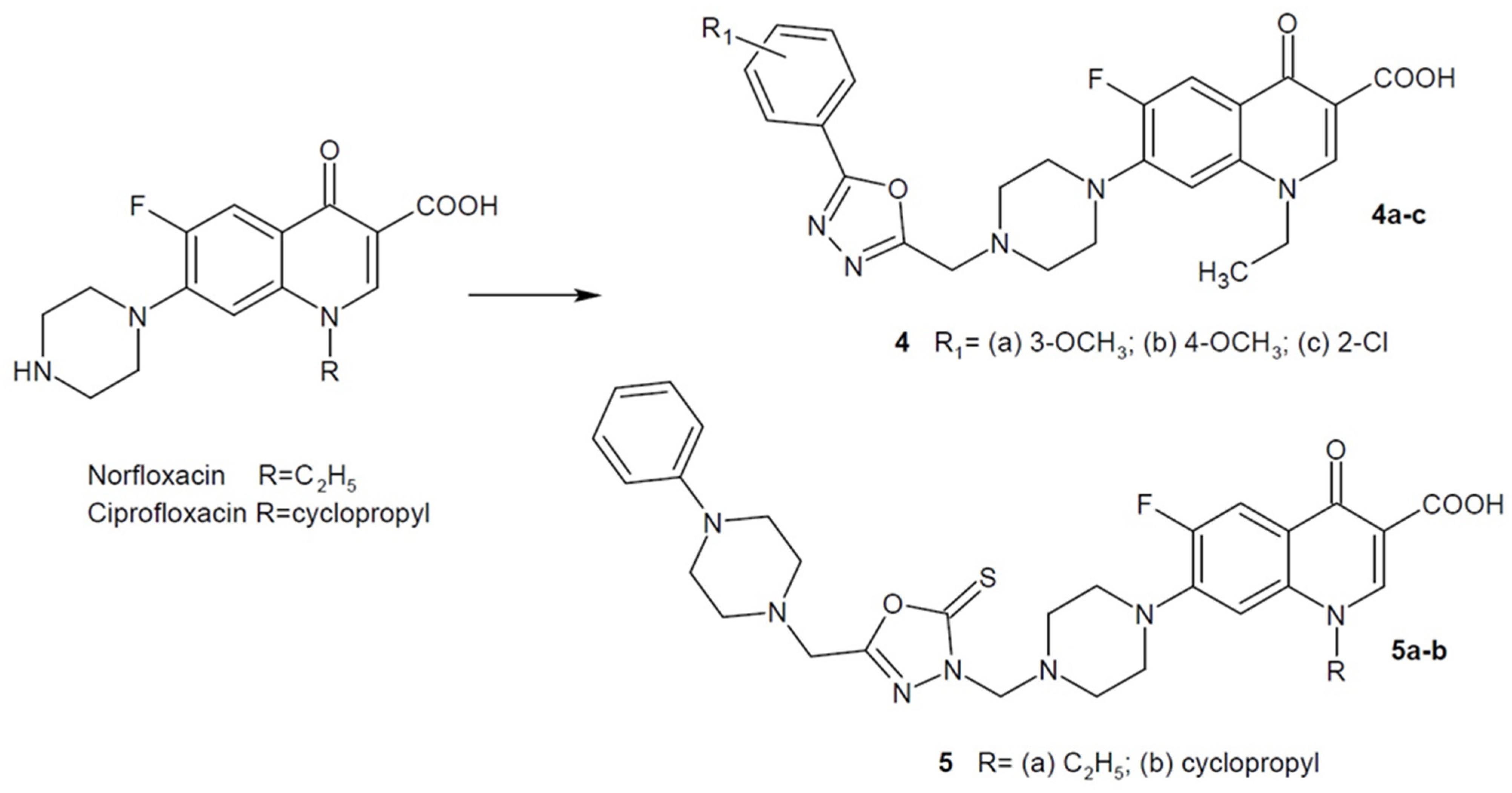
2.1. 1,3,4-Oxadiazole Hybrids of Quinolone Antibacterial Drugs
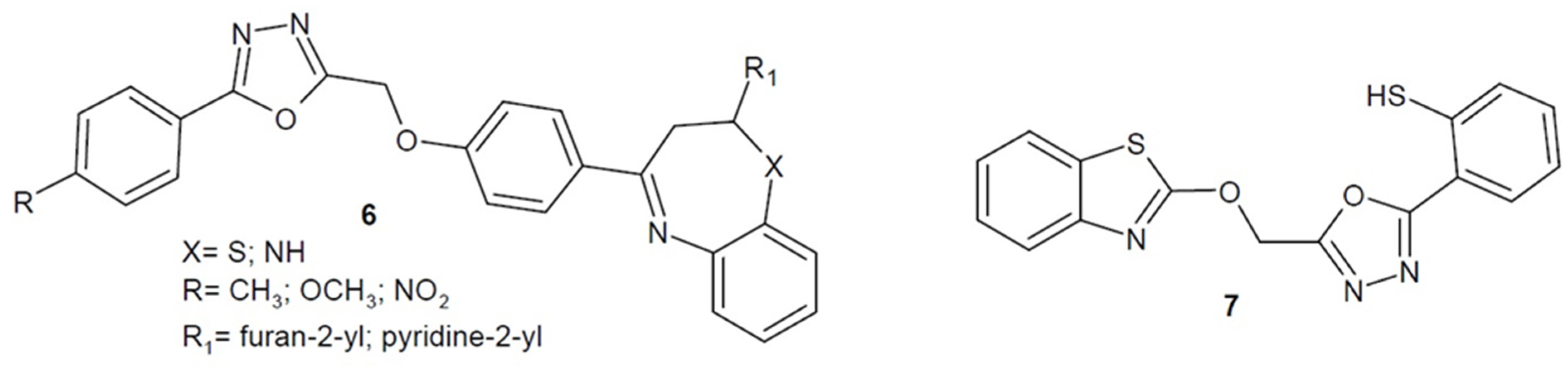
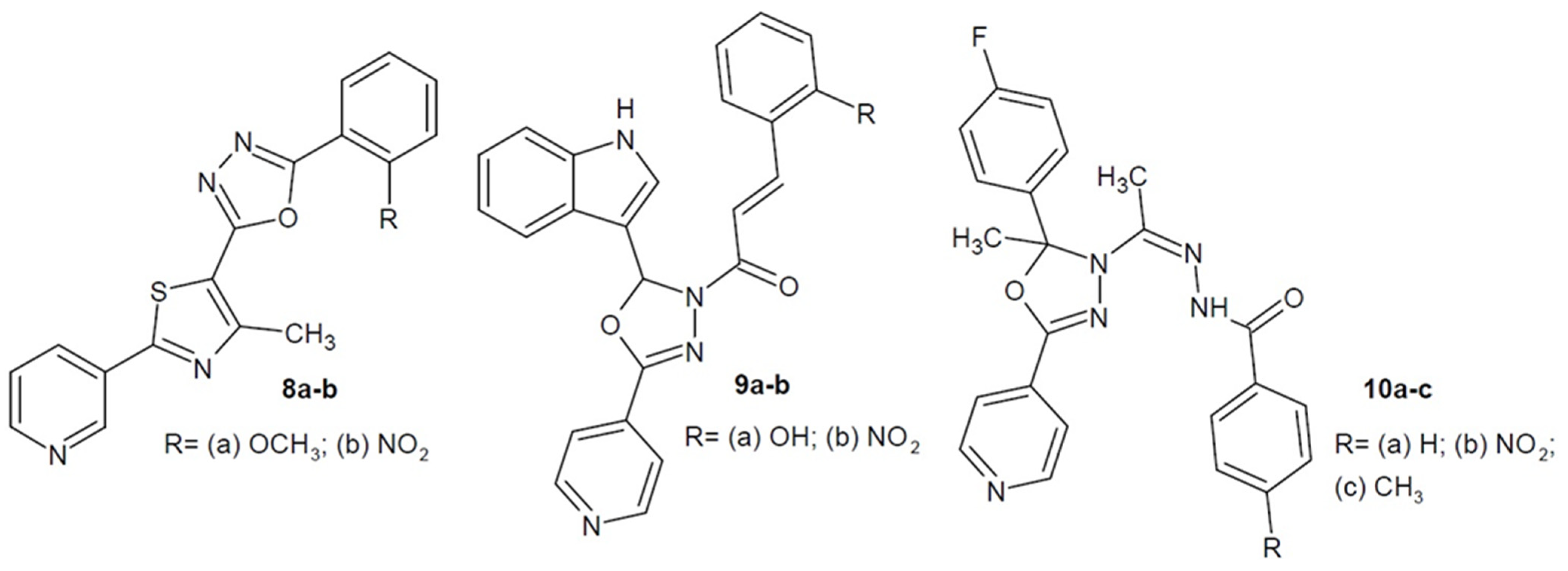
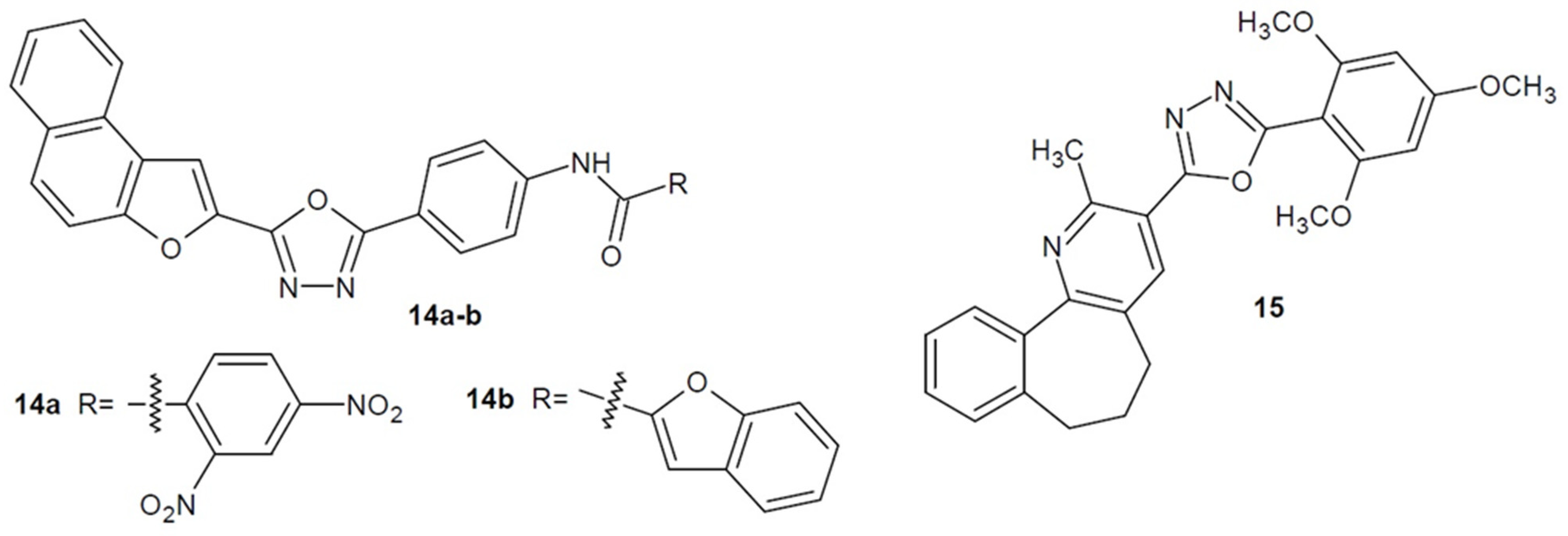
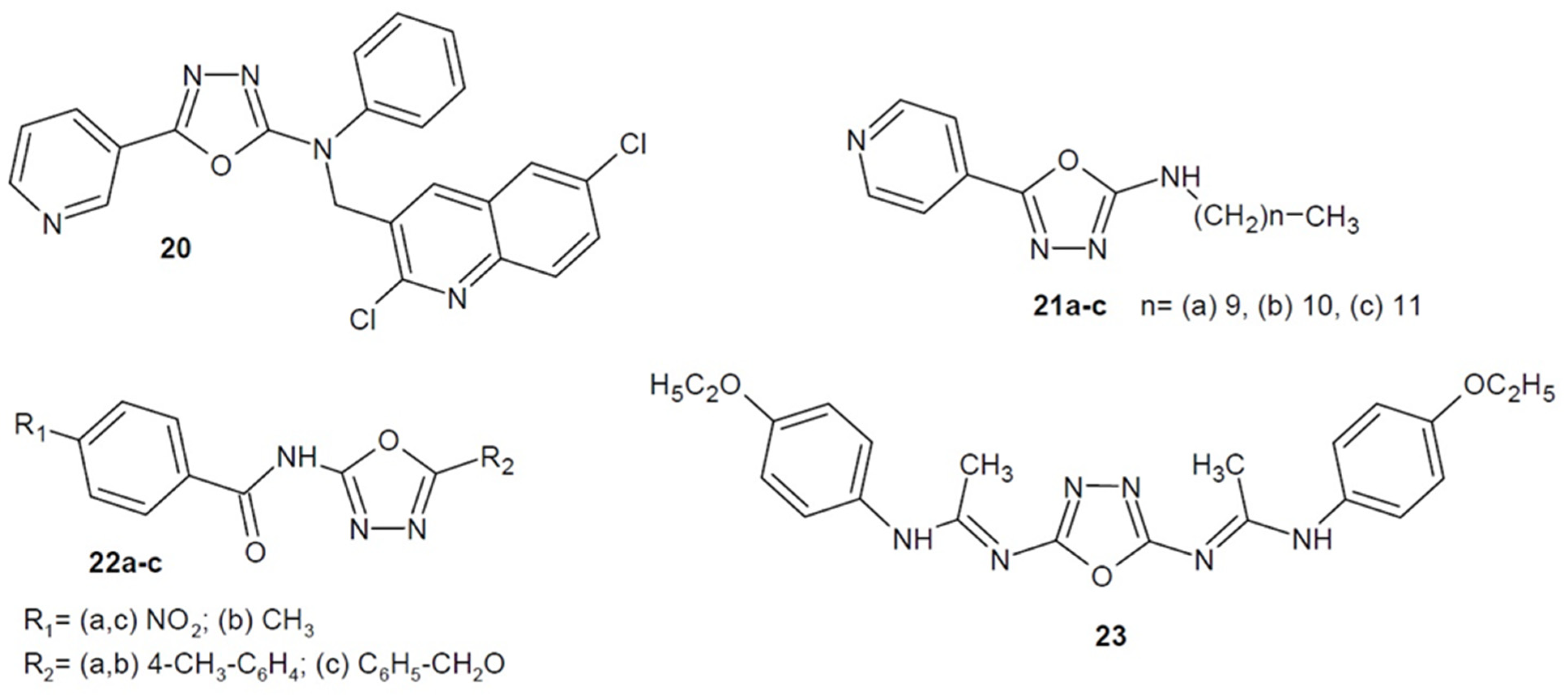
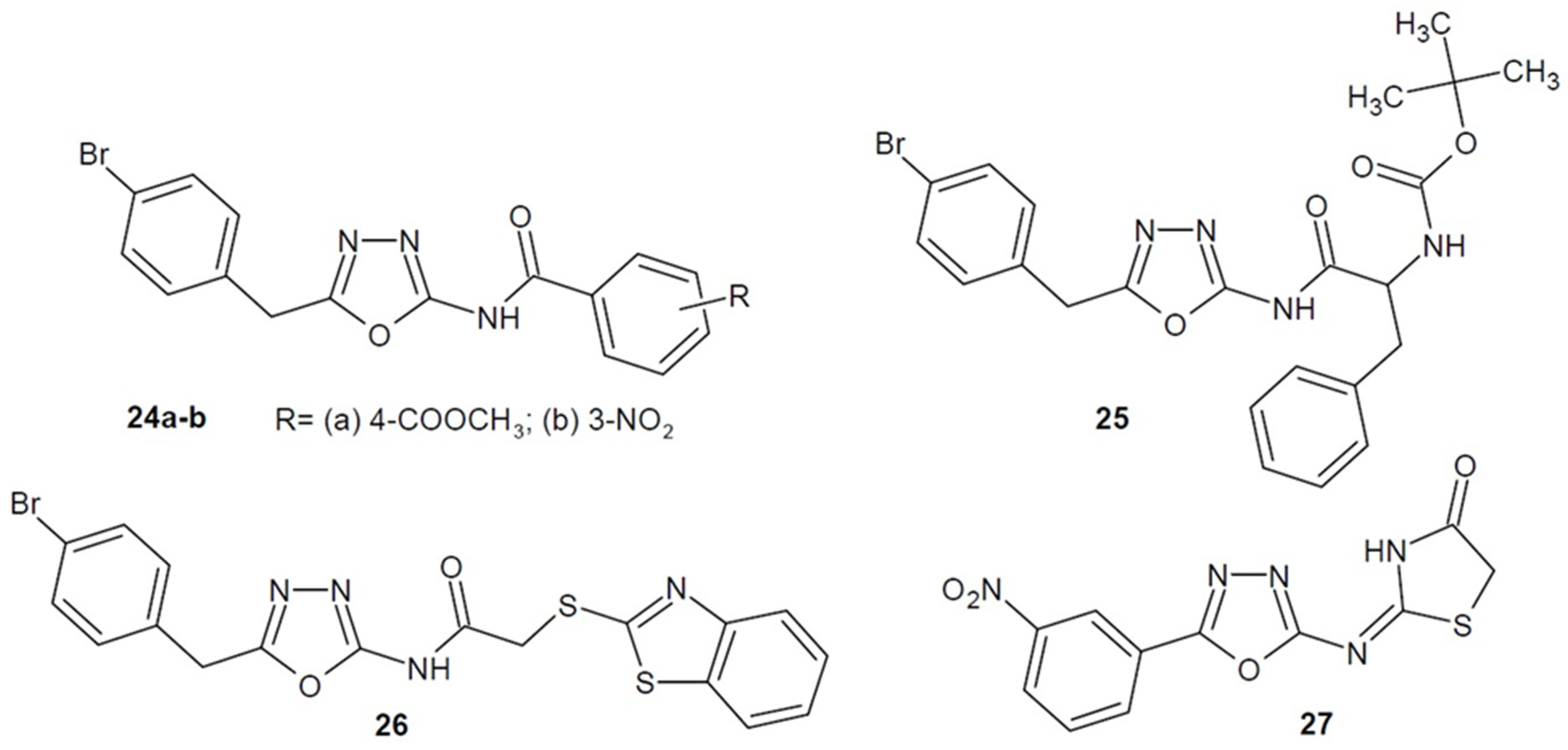
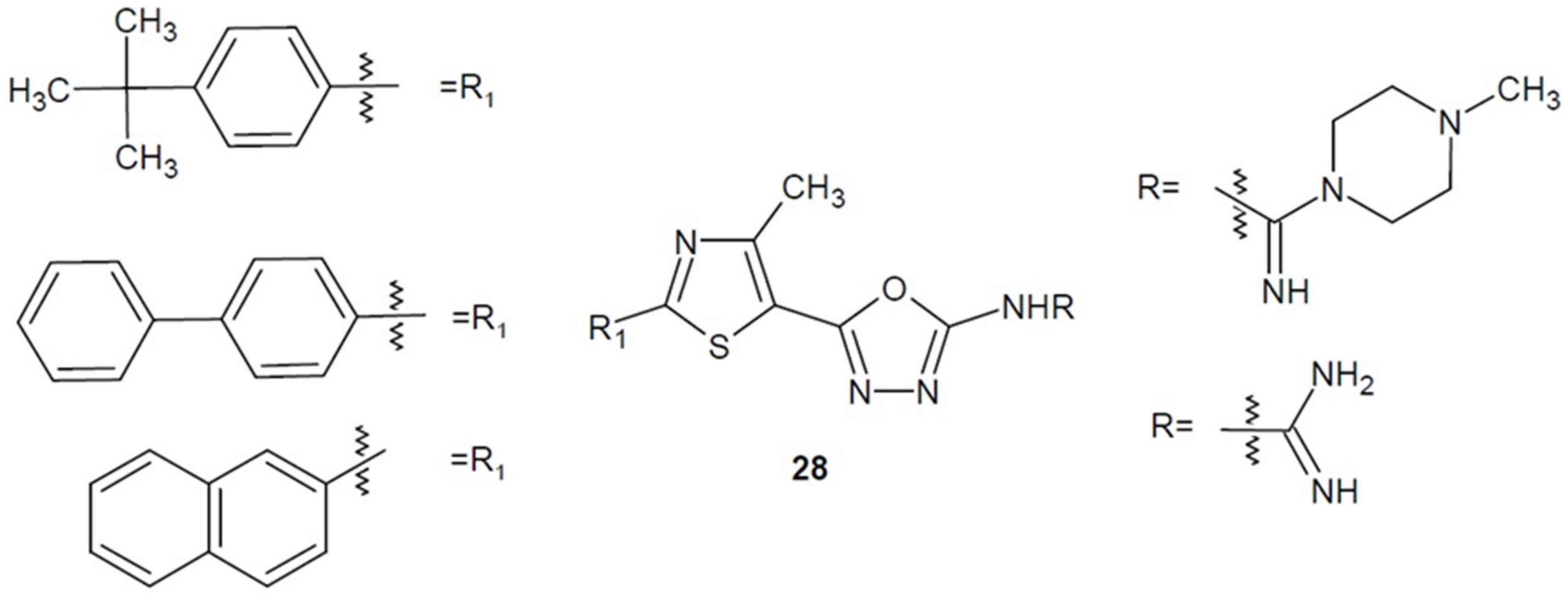
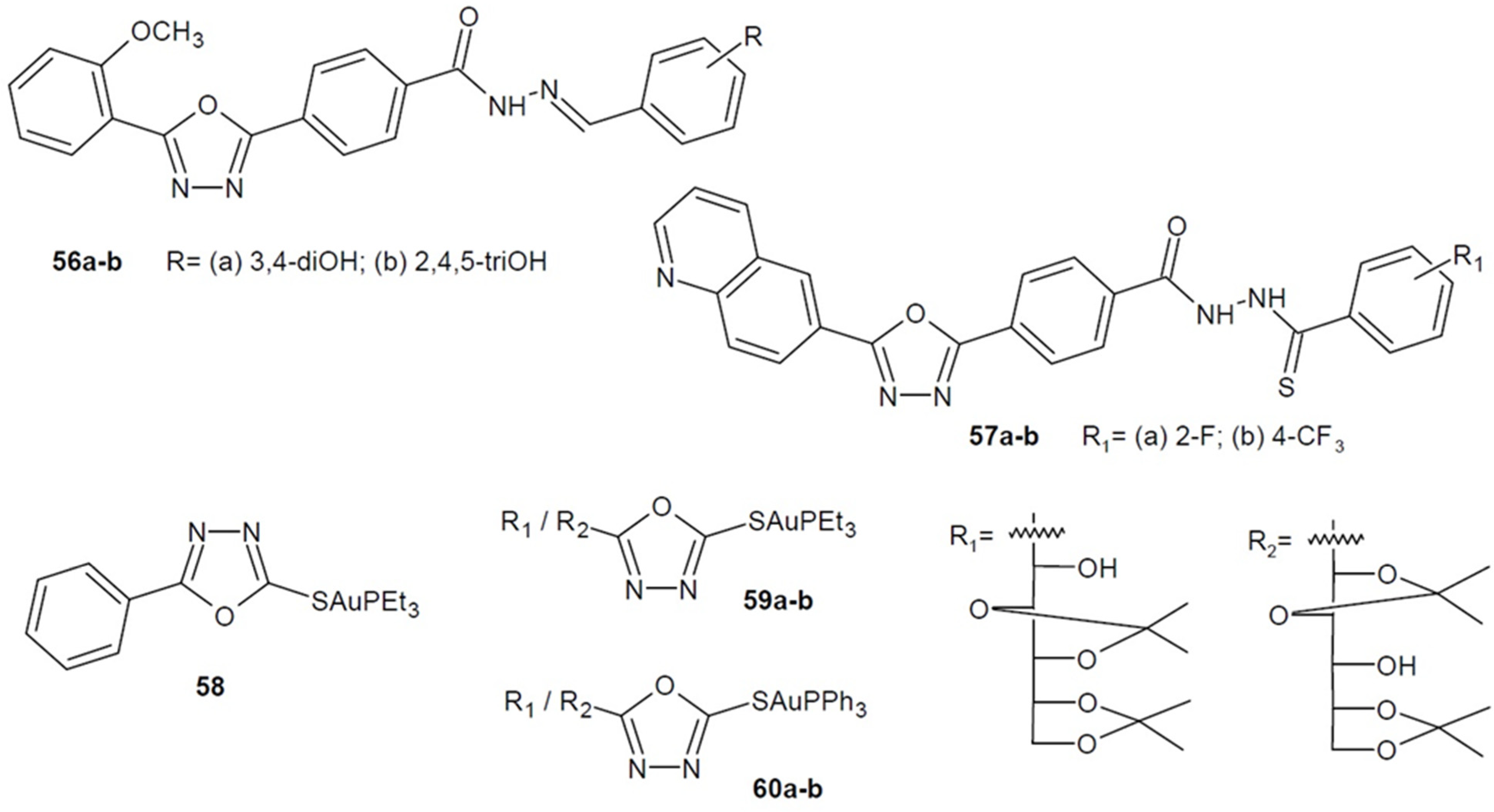
2.2. Antibacterial Activity of Aryl/Heteroaryl Derivatives of 1,3,4-Oxadiazole
2.3. Antibacterial Activity of Amino Derivatives of 1,3,4-Oxadiazole
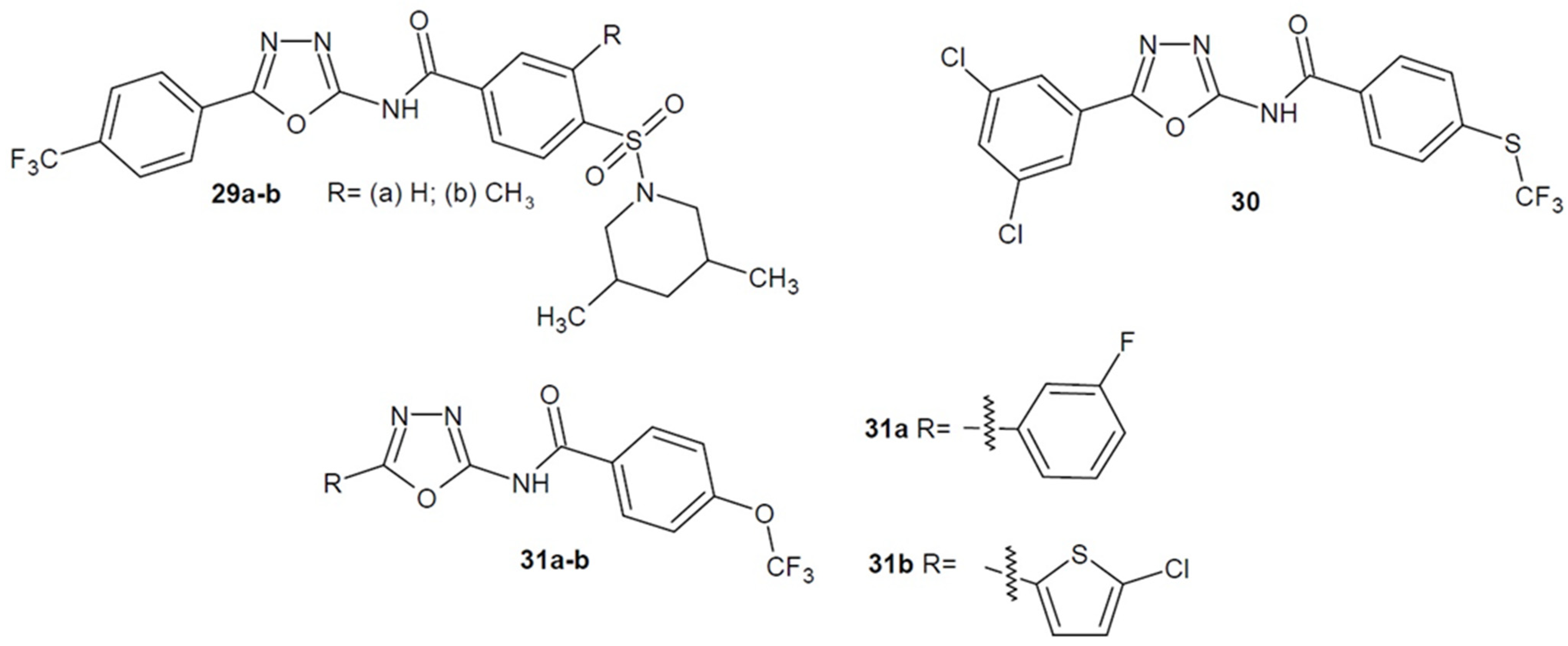
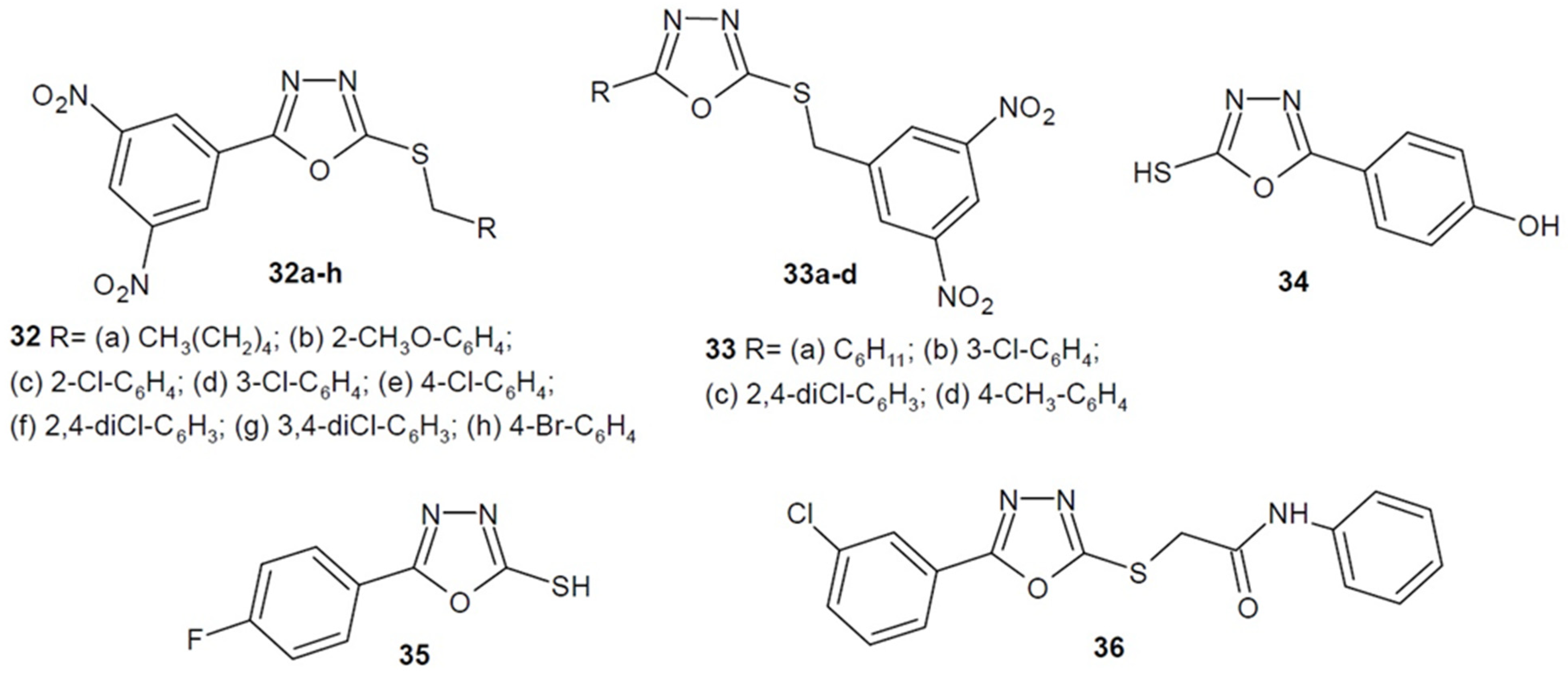
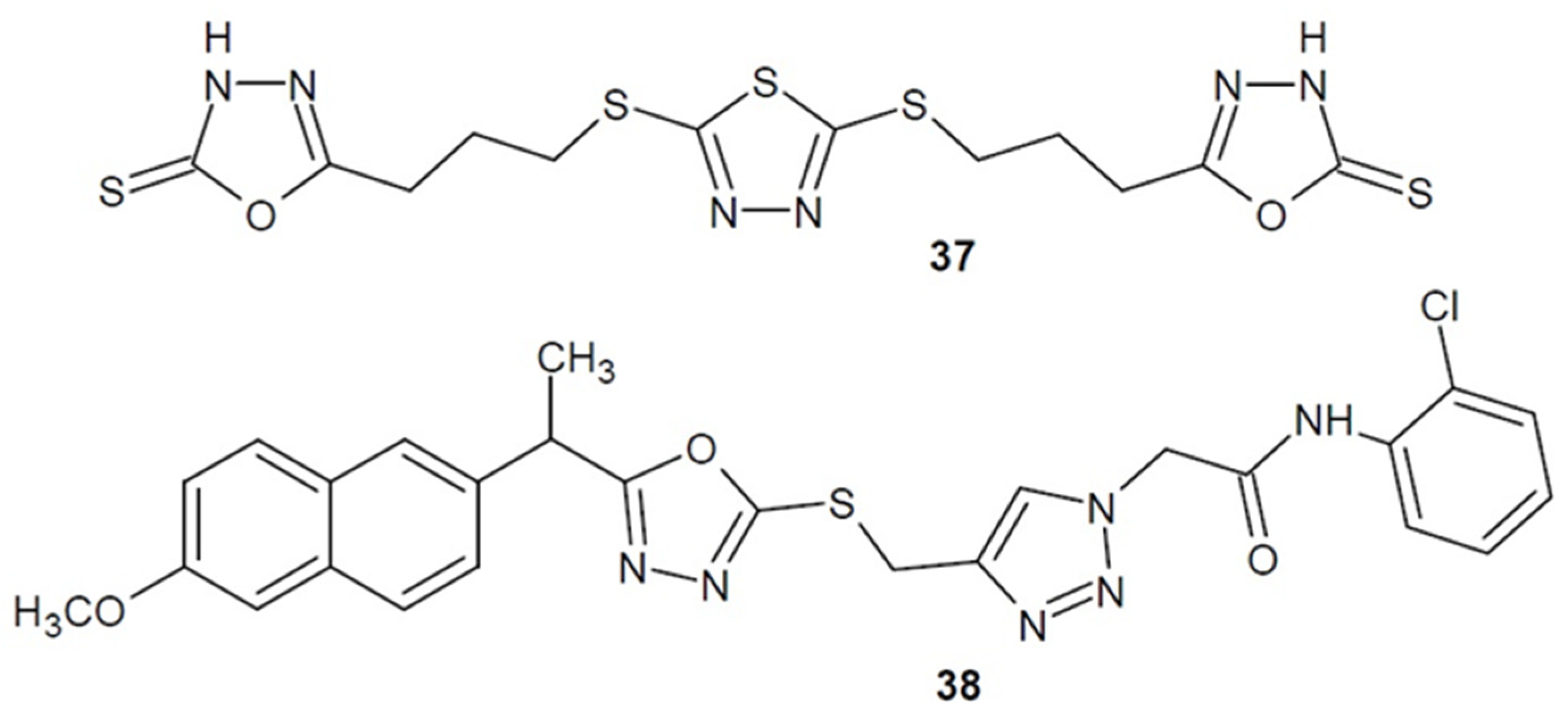
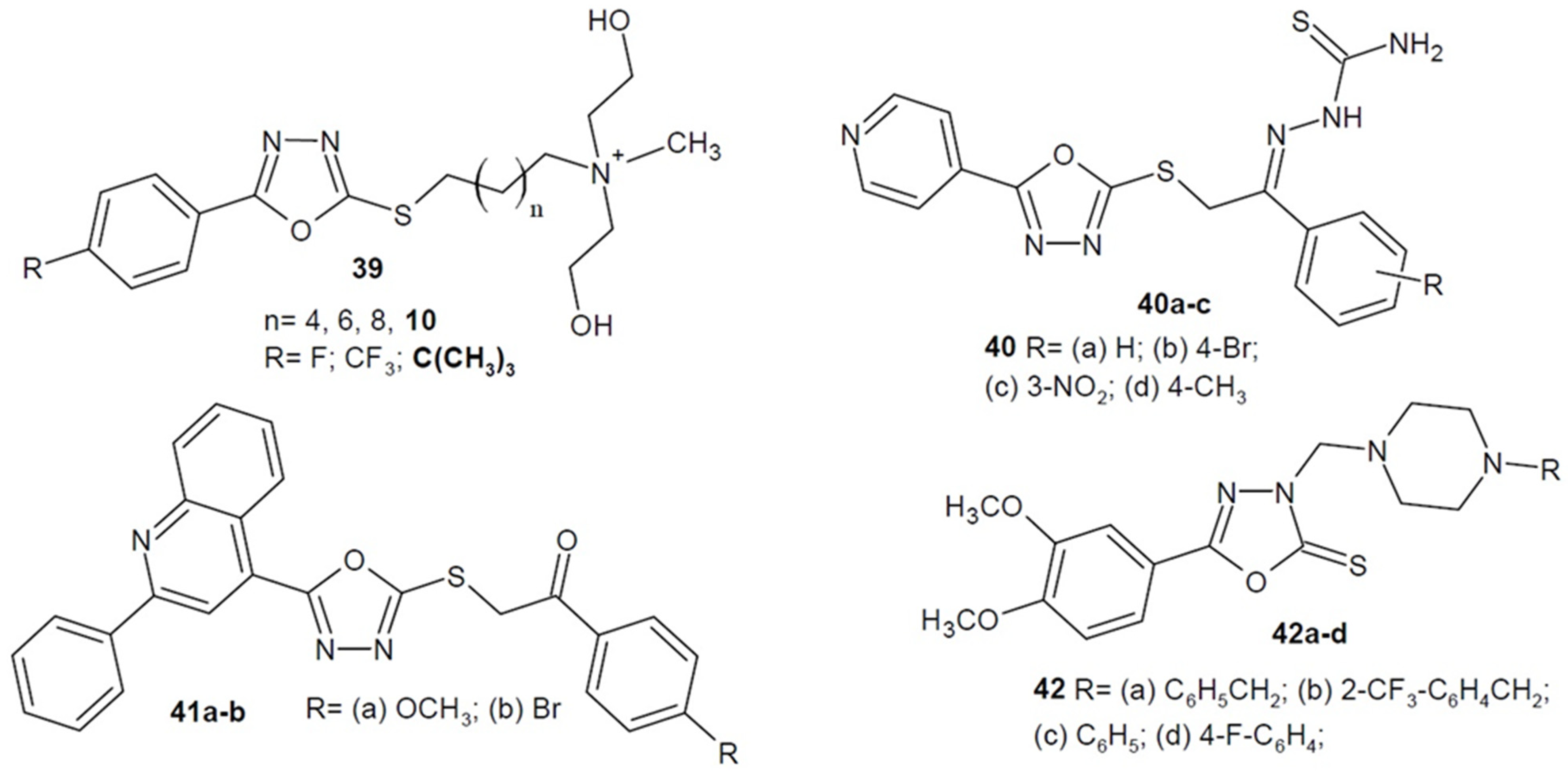
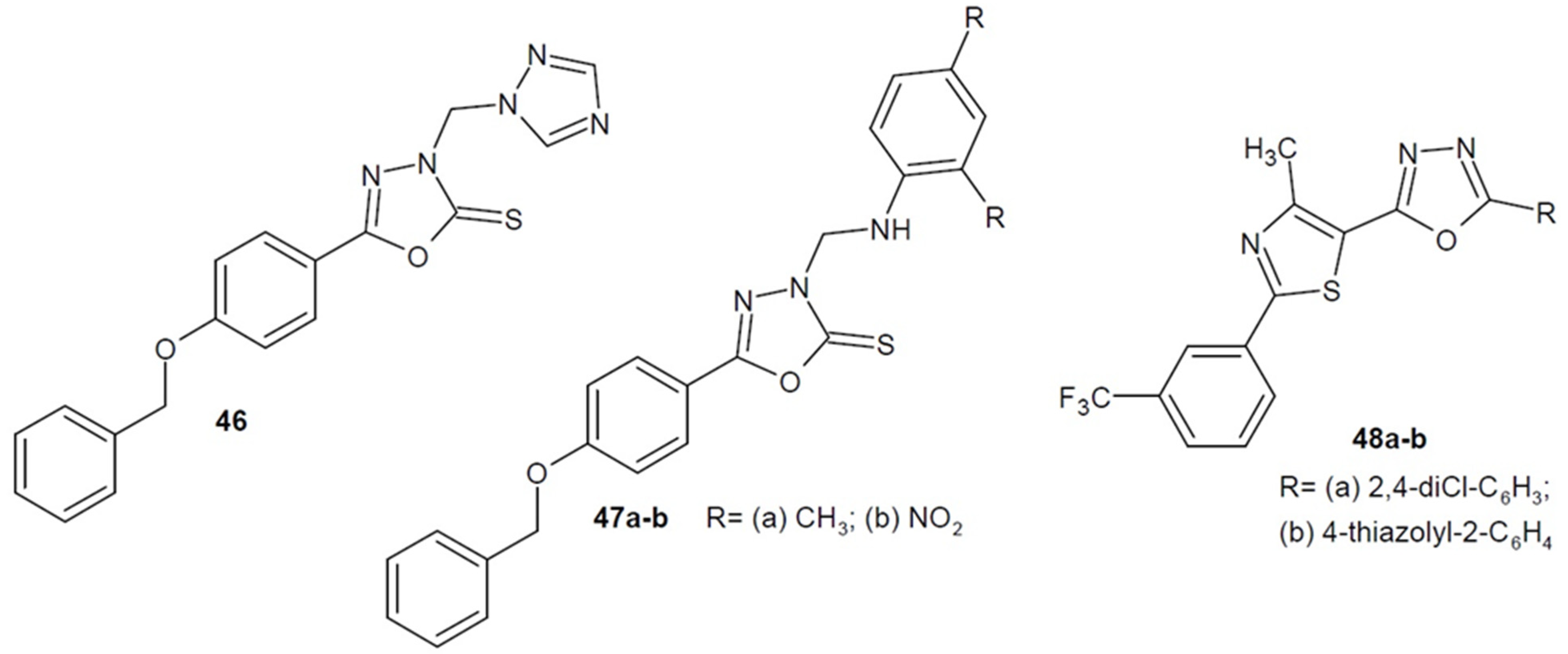
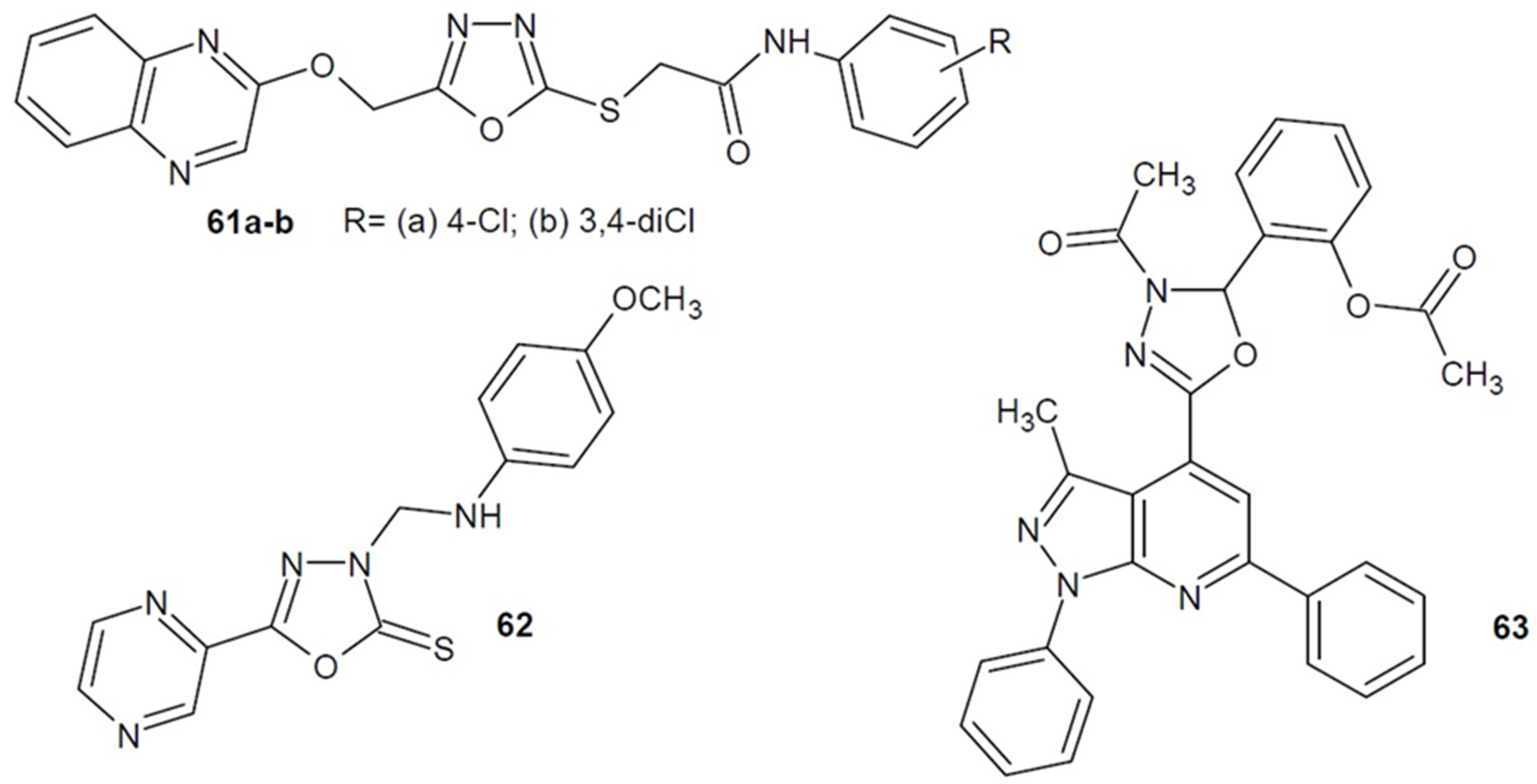
2.4. Antibacterial Activity of 1,3,4-Oxadiazole-2-Thiones/Thiols and their S-Substituted Derivatives
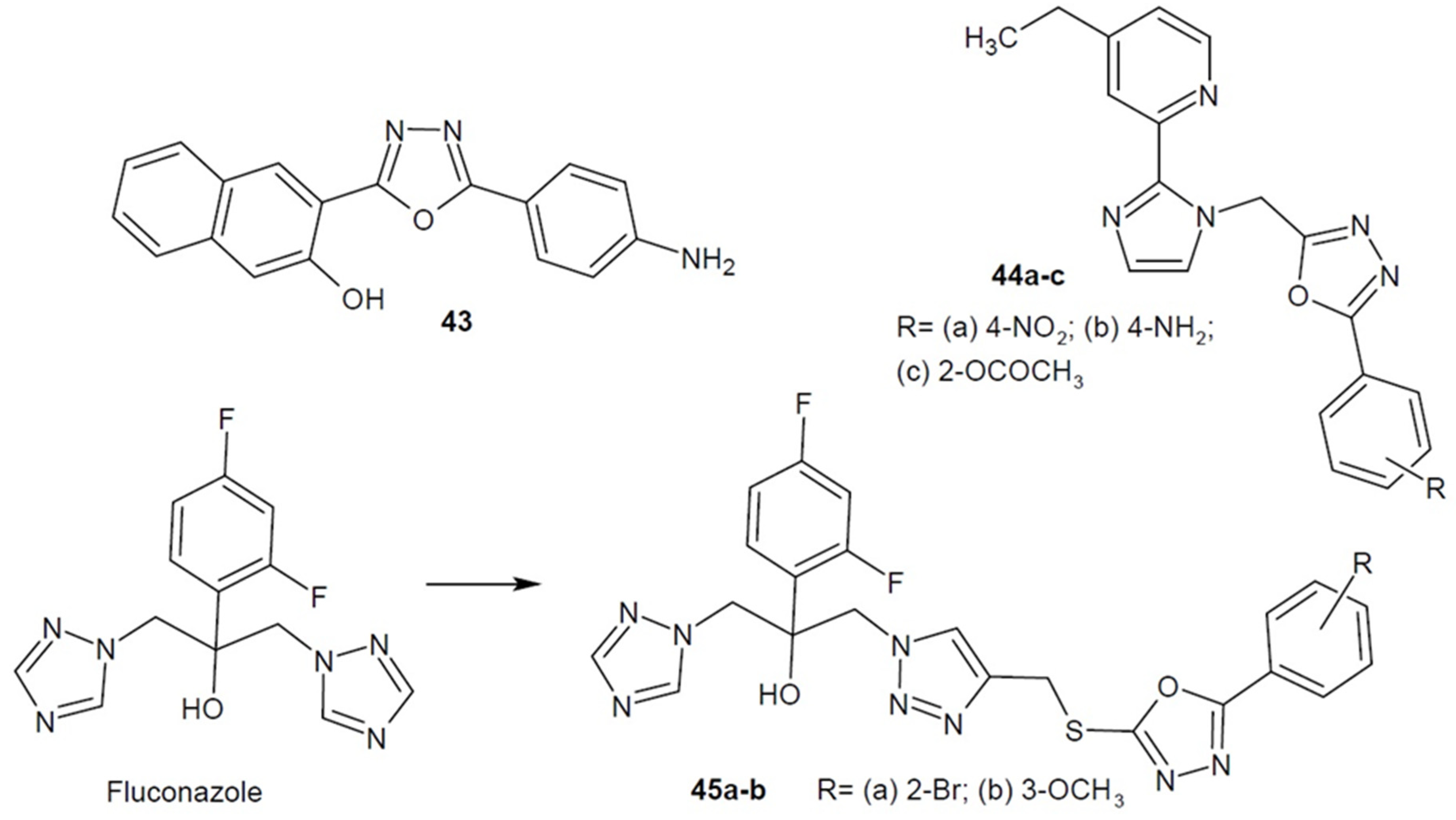
3. Antifungal Activity of 1,3,4-Oxadiazole Derivatives
4. Antiprotozoal Activity of 1,3,4-Oxadiazole Derivatives
4.1. Antimalarial Activity of 1,3,4-Oxadiazole Derivatives
4.2. Antileishmanial Activity of 1,3,4-Oxadiazole Derivatives
4.3. Antitrypanocidal Activity of 1,3,4-Oxadiazole Derivatives
5. Antiviral Activity of 1,3,4-Oxadiazole Derivatives
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef] [Green Version]
- Duval, R.E.; Grare, M.; Demoré, B. Fight against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinchliffe, S.; Butcher, A.; Rahman, M.M. The AMR Problem: Demanding Economies, Biological Margins, and Co-Producing Alternative Strategies. Palgrave Commun. 2018, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, H.; Shen, W.; He, P.; Zhou, Z. Effectiveness and Tolerability of Targeted Drugs for the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Network Meta-Analysis of Randomized Controlled Trials. J. Cancer Res. Clin. Oncol. 2018, 1751–1768. [Google Scholar] [CrossRef] [PubMed]
- Siwach, A.; Verma, P.K. Therapeutic Potential of Oxadiazole or Furadiazole Containing Compounds. BMC Chem. 2020. [Google Scholar] [CrossRef]
- Alburquerque-González, B.; Bernabé-García, Á.; Bernabé-García, M.; Ruiz-Sanz, J.; López-Calderón, F.F.; Gonnelli, L.; Banci, L.; Peña-García, J.; Luque, I.; Nicolás, F.J.; et al. The FDA-Approved Antiviral Raltegravir Inhibits Fascin1-Dependent Invasion of Colorectal Tumor Cells In Vitro and In Vivo. Cancers 2021, 13, 861. [Google Scholar] [CrossRef]
- Sheikh, O.; Yokota, T. Developing DMD Therapeutics: A Review of the Effectiveness of Small Molecules, Stop-Codon Readthrough, Dystrophin Gene Replacement, and Exon-Skipping Therapies. Expert Opin. Investig. Drugs 2021, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pinner, A.; Caro, N. Ueber Die Einwirkung von Hydrazin Auf Imidoäther. Berichte Dtsch. Chem. Gesellschaft 1894, 27, 3273–3291. [Google Scholar] [CrossRef] [Green Version]
- Rupe, H.; Labhardt, H. Eine Neue Synthese von Phenyloxytriazolen. Berichte Dtsch. Chem. Gesellschaft 1900, 33, 233–246. [Google Scholar] [CrossRef]
- Stollé, R. Ueber Die Ueberführung Der Secundären Säurehydrazide in Derivate Des Furodiazols, Pyrrodiazols Und Thiodiazols. Berichte Dtsch. Chem. Gesellschaft 1899, 32, 797–798. [Google Scholar] [CrossRef] [Green Version]
- Hetzheim, A.; Möckel, K. Recent Advances in 1,3,4-Oxadiazole Chemistry. Adv. Heterocycl. Chem. 1967, 7, 183–224. [Google Scholar] [CrossRef]
- Nesynov, E.P.; Grekov, A.P. The chemistry of 1,3,4-oxadiazole. Russ. Chem. Rev. 1964, 33, 508. [Google Scholar] [CrossRef]
- Patel, K.D.; Prajapati, S.M.; Panchal, S.N.; Patel, H.D. Review of Synthesis of 1,3,4-Oxadiazole Derivatives. Synth. Commun. 2014, 44, 1859–1875. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; de Athayde-Filho, P.F. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef] [Green Version]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.G.; Das, N.; Shengule, S.A.; Srivastava, R.S.; Shrivastava, S.K. Synthesis, Characterization, Evaluation and Molecular Dynamics Studies of 5, 6-Diphenyl-1,2,4-Triazin-3(2 H)-One Derivatives Bearing 5-Substituted 1,3,4-Oxadiazole as Potential Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2015, 101, 81–95. [Google Scholar] [CrossRef]
- Gulnaz, A.R.; Mohammed, Y.H.E.; Khanum, S.A. Design, Synthesis and Molecular Docking of Benzophenone Conjugated with Oxadiazole Sulphur Bridge Pyrazole Pharmacophores as Anti Inflammatory and Analgesic Agents. Bioorg. Chem. 2019. [Google Scholar] [CrossRef]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.M.; Kaoud, T.S.; Ahmed, A.S.F.F. New 1,3,4-Oxadiazole/Oxime Hybrids: Design, Synthesis, Anti-Inflammatory, COX Inhibitory Activities and Ulcerogenic Liability. Bioorg. Chem. 2017, 74, 15–29. [Google Scholar] [CrossRef]
- Jayashankar, B.; Lokanath Rai, K.M.; Baskaran, N.; Sathish, H.S. Synthesis and Pharmacological Evaluation of 1,3,4-Oxadiazole Bearing Bis(Heterocycle) Derivatives as Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2009, 44, 3898–3902. [Google Scholar] [CrossRef]
- Kaur, J.; Soto-Velasquez, M.; Ding, Z.; Ghanbarpour, A.; Lill, M.A.; van Rijn, R.M.; Watts, V.J.; Flaherty, D.P. Optimization of a 1,3,4-Oxadiazole Series for Inhibition of Ca2+/Calmodulin-Stimulated Activity of Adenylyl Cyclases 1 and 8 for the Treatment of Chronic Pain. Eur. J. Med. Chem. 2019, 162, 568–585. [Google Scholar] [CrossRef]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of Benzimidazole-Linked-1,3,4-Oxadiazole Carboxamides as GSK-3β Inhibitors with in Vivo Antidepressant Activity. Bioorg. Chem. 2018, 77, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, P.; Sharma, J.; Upadhyay, A.; Kumar, N. Synthesis and Evaluation of Substituted Diphenyl-1,3,4-Oxadiazole Derivatives for Central Nervous System Depressant Activity. Org. Med. Chem. Lett. 2012, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadagiri, B.; Gurrala, S.; Bantu, R.; Nagarapu, L.; Polepalli, S.; Srujana, G.; Jain, N. Synthesis and Evaluation of Benzosuberone Embedded with 1,3,4-Oxadiazole, 1,3,4-Thiadiazole and 1,2,4-Triazole Moieties as New Potential Anti Proliferative Agents. Bioorg. Med. Chem. Lett. 2015, 25, 2220–2224. [Google Scholar] [CrossRef]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyma, C.P.; Balakrishna, K.; Peethambar, K.S.; Vijesh, M.A. Synthesis, Characterization, Antidiabetic and Antioxidant Activity of 1,3,4-Oxadiazole Derivatives Bearing 6-Methyl Pyridine Moiety. Scholars Research Library Der Pharma Chemica 2015, 7, 137–145. [Google Scholar]
- Nazreen, S.; Alam, M.S.; Hamid, H.; Yar, M.S.; Shafi, S.; Dhulap, A.; Alam, P.; Pasha, M.A.Q.; Bano, S.; Alam, M.M.; et al. Design, Synthesis, in Silico Molecular Docking and Biological Evaluation of Novel Oxadiazole Based Thiazolidine-2,4-Diones Bis-Heterocycles as PPAR-γ Agonists. Eur. J. Med. Chem. 2014, 87, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Şahin, G.; Palaska, E.; Ekizoğlu, M.; Özalp, M. Synthesis and Antimicrobial Activity of Some 1,3,4-Oxadiazole Derivatives. Farmaco 2002, 57, 539–542. [Google Scholar] [CrossRef]
- Ali, M.A.; Shaharyar, M. Oxadiazole Mannich Bases: Synthesis and Antimycobacterial Activity. Bioorg. Med. Chem. Lett. 2007, 17, 3314–3316. [Google Scholar] [CrossRef]
- Rane, R.A.; Bangalore, P.; Borhade, S.D.; Khandare, P.K. Synthesis and Evaluation of Novel 4-Nitropyrrole-Based 1,3,4-Oxadiazole Derivatives as Antimicrobial and Anti-Tubercular Agents. Eur. J. Med. Chem. 2013, 70, 49–58. [Google Scholar] [CrossRef]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of Novel Sulfonamide-1,2,4-Triazoles, 1,3,4-Thiadiazoles and 1,3,4-Oxadiazoles, as Potential Antibacterial and Antifungal Agents. Biological Evaluation and Conformational Analysis Studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef]
- Xu, W.; He, J.; He, M.; Han, F.; Chen, X.; Pan, Z.; Wang, J.; Tong, M. Synthesis and Antifungal Activity of Novel Sulfone Derivatives Containing 1,3,4-Oxadiazole Moieties. Molecules 2011, 16, 9129–9141. [Google Scholar] [CrossRef]
- Johns, B.A.; Weatherhead, J.G.; Allen, S.H.; Thompson, J.B.; Garvey, E.P.; Foster, S.A.; Jeffrey, J.L.; Miller, W.H. 1,3,4-Oxadiazole Substituted Naphthyridines as HIV-1 Integrase Inhibitors. Part 2: SAR of the C5 Position. Bioorg. Med. Chem. Lett. 2009, 19, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, W.A.; El-Essawy, F.A.; Ali, O.M.; Nasr, B.S.; Abdalla, M.M.; Abdel-Rahman, A.A.H. Anti-HIV Activity of New Substituted 1,3,4-Oxadiazole Derivatives and Their Acyclic Nucleoside Analogues. Zeitschrift Naturforsch. Sect. C J. Biosci. 2010, 64, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Peraman, R.; Varma, R.V.; Reddy, Y.P. Re-Engineering Nalidixic Acid’s Chemical Scaffold: A Step towards the Development of Novel Anti-Tubercular and Anti-Bacterial Leads for Resistant Pathogens. Bioorg. Med. Chem. Lett. 2015, 25, 4314–4319. [Google Scholar] [CrossRef]
- Omar, F.A.; Abelrasoul, M.; Sheha, M.M.; Hassan, H.Y.; Ibrahiem, Y.M. Synthesis, Antibacterial Activity and Molecular Docking of Substituted Naphthyridines as Potential DNA Gyrase Inhibitors. ChemistrySelect 2018, 3, 2604–2612. [Google Scholar] [CrossRef]
- Hofny, H.A.; Mohamed, M.F.A.; Gomaa, H.A.M.; Abdel-Aziz, S.A.; Youssif, B.G.M.; El-koussi, N.A.; Aboraia, A.S. Design, Synthesis, and Antibacterial Evaluation of New Quinoline-1,3,4-Oxadiazole and Quinoline-1,2,4-Triazole Hybrids as Potential Inhibitors of DNA Gyrase and Topoisomerase IV. Bioorg. Chem. 2021, 112, 104920. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and Synthesis of New Norfloxacin-1,3,4-Oxadiazole Hybrids as Antibacterial Agents against Methicillin-Resistant Staphylococcus Aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966. [Google Scholar] [CrossRef] [PubMed]
- Mermer, A.; Faiz, O.; Demirbas, A.; Demirbas, N.; Alagumuthu, M.; Arumugam, S. Piperazine-Azole-Fluoroquinolone Hybrids: Conventional and Microwave Irradiated Synthesis, Biological Activity Screening and Molecular Docking Studies. Bioorg. Chem. 2019, 85, 308–318. [Google Scholar] [CrossRef]
- Navin, P.; Sarvil, P.; Amit, P.; Divyesh, P.; Dhansukh, R.; Moo-Puc, R.; Rivera, G. Synthesis and Biological Evaluation of Newer 1,3,4-Oxadiazoles Incorporated with Benzothiazepine and Benzodiazepine Moieties. Zeitschrift Naturforsch. Sect. C J. Biosci. 2017, 72, 133–146. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Alam, M.M.; Nazreen, S. In Silico ADME Predictions and in Vitro Antibacterial Evaluation of 2-Hydroxy Benzothiazole-Based 1,3,4-Oxadiazole Derivatives. Turkish J. Chem. 2020, 44, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, S.T.; Deshmukh, A.R.; Bhosle, M.R.; Khedkar, V.M.; Nawale, L.U.; Sarkar, D.; Mane, R.A. Synthesis and Antitubercular Activity of New 1,3,4-Oxadiazoles Bearing Pyridyl and Thiazolyl Scaffolds. Bioorg. Med. Chem. Lett. 2016, 26, 3646–3651. [Google Scholar] [CrossRef]
- Desai, N.C.; Somani, H.; Trivedi, A.; Bhatt, K.; Nawale, L.; Khedkar, V.M.; Jha, P.C.; Sarkar, D. Synthesis, Biological Evaluation and Molecular Docking Study of Some Novel Indole and Pyridine Based 1,3,4-Oxadiazole Derivatives as Potential Antitubercular Agents. Bioorg. Med. Chem. Lett. 2016, 26, 1776–1783. [Google Scholar] [CrossRef]
- Desai, N.C.; Trivedi, A.; Somani, H.; Jadeja, K.A.; Vaja, D.; Nawale, L.; Khedkar, V.M.; Sarkar, D. Synthesis, Biological Evaluation, and Molecular Docking Study of Pyridine Clubbed 1,3,4-Oxadiazoles as Potential Antituberculars. Synth. Commun. 2018, 48, 524–540. [Google Scholar] [CrossRef]
- Mansoori, M.H.; Khatik, G.L.; Mishra, V. Synthesis and Pharmacological Evaluation of Pyridinyl-1,3,4-Oxadiazolyl-Ethanone Derivatives as Antimicrobial, Antifungal and Antitubercular Agents. Med. Chem. Res. 2018, 27, 744–755. [Google Scholar] [CrossRef]
- Shingare, R.M.; Patil, Y.S.; Sangshetti, J.N.; Patil, R.B.; Rajani, D.P.; Madje, B.R. Synthesis, Biological Evaluation and Docking Study of Some Novel Isoxazole Clubbed 1,3,4-Oxadiazoles Derivatives. Med. Chem. Res. 2018, 27, 1283–1291. [Google Scholar] [CrossRef]
- Das, R.; Mehta, D.K. Evaluation and Docking Study of Pyrazine Containing 1,3,4-Oxadiazoles Clubbed with Substituted Azetidin-2-One: A New Class of Potential Antimicrobial and Antitubercular. Drug Res. 2021, 71, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sindhe, M.A.; Bodke, Y.D.; Kenchappa, R.; Telkar, S.; Chandrashekar, A. Synthesis of a Series of Novel 2,5-Disubstituted-1,3,4-Oxadiazole Derivatives as Potential Antioxidant and Antibacterial Agents. J. Chem. Biol. 2016, 9, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Sajja, Y.; Vanguru, S.; Vulupala, H.R.; Nagarapu, L.; Perumal, Y.; Sriram, D.; Nanubolu, J.B. Design, Synthesis, and in Vitro Antituberculosis Activity of Benzo[6,7]Cyclohepta[1,2- b ]Pyridine-1,3,4-Oxadiazole Derivatives. Chem. Biol. Drug Des. 2017, 90, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Gholap, S.; Tambe, M.; Nawale, L.; Sarkar, D.; Sangshetti, J.; Damale, M. Design, Synthesis, and Pharmacological Evaluation of Fluorinated Azoles as Anti-Tubercular Agents. Arch. Pharm. 2018, 351, 1700294. [Google Scholar] [CrossRef]
- Triloknadh, S.; Venkata Rao, C.; Nagaraju, K.; Hari Krishna, N.; Venkata Ramaiah, C.; Rajendra, W.; Trinath, D.; Suneetha, Y. Design, Synthesis, Neuroprotective, Antibacterial Activities and Docking Studies of Novel Thieno[2,3-d]Pyrimidine-Alkyne Mannich Base and Oxadiazole Hybrids. Bioorg. Med. Chem. Lett. 2018, 28, 1663–1669. [Google Scholar] [CrossRef]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Hordyjewska, A.; Malm, A.; Wujec, M. Novel 3-Acetyl-2,5-Disubstituted-1,3,4-Oxadiazolines: Synthesis and Biological Activity. Molecules 2020, 25, 5844. [Google Scholar] [CrossRef]
- Ladani, G.G.; Patel, M.P. Novel 1,3,4-Oxadiazole Motifs Bearing a Quinoline Nucleus: Synthesis, Characterization and Biological Evaluation of Their Antimicrobial, Antitubercular, Antimalarial and Cytotoxic Activities. New J. Chem. 2015, 39, 9848–9857. [Google Scholar] [CrossRef]
- Vosátka, R.; Krátký, M.; Švarcová, M.; Janoušek, J.; Stolaříková, J.; Madacki, J.; Huszár, S.; Mikušová, K.; Korduláková, J.; Trejtnar, F.; et al. New Lipophilic Isoniazid Derivatives and Their 1,3,4-Oxadiazole Analogues: Synthesis, Antimycobacterial Activity and Investigation of Their Mechanism of Action. Eur. J. Med. Chem. 2018, 151, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wen, G.; Li, J.; Zhang, W.; Wu, S. A Useful Synthesis of 2-Acylamino-1,3,4-Oxadiazoles from Acylthiosemicarbazides Using Potassium Iodate and the Discovery of New Antibacterial Compounds. Molecules 2019, 24, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hkiri, S.; Hafidh, A.; Cavalier, J.; Touil, S.; Samarat, A. Design, Synthesis, Antimicrobial Evaluation, and Molecular Docking Studies of Novel Symmetrical 2,5-difunctionalized 1,3,4-oxadiazoles. J. Heterocycl. Chem. 2020, 57, 1044–1054. [Google Scholar] [CrossRef]
- Salama, E.E. Synthesis of New 2-Amino-1,3,4-Oxadiazole Derivatives with Anti-Salmonella Typhi Activity Evaluation. BMC Chem. 2020, 14, s13065. [Google Scholar] [CrossRef]
- Hagras, M.; Hegazy, Y.A.; Elkabbany, A.H.; Mohammad, H.; Ghiaty, A.; Abdelghany, T.M.; Seleem, M.N.; Mayhoub, A.S. Biphenylthiazole Antibiotics with an Oxadiazole Linker: An Approach to Improve Physicochemical Properties and Oral Bioavailability. Eur. J. Med. Chem. 2018, 143, 1448–1456. [Google Scholar] [CrossRef]
- Kotb, A.; Abutaleb, N.S.; Hagras, M.; Bayoumi, A.; Moustafa, M.M.; Ghiaty, A.; Seleem, M.N.; Mayhoub, A.S. Tert -Butylphenylthiazoles with an Oxadiazole Linker: A Novel Orally Bioavailable Class of Antibiotics Exhibiting Antibiofilm Activity. RSC Adv. 2019, 9, 6770–6778. [Google Scholar] [CrossRef] [Green Version]
- Hannoun, M.H.; Hagras, M.; Kotb, A.; El-Attar, A.A.M.M.; Abulkhair, H.S. Synthesis and Antibacterial Evaluation of a Novel Library of 2-(Thiazol-5-Yl)-1,3,4-Oxadiazole Derivatives against Methicillin-Resistant Staphylococcus Aureus (MRSA). Bioorg. Chem. 2020, 94, 103364. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Naclerio, G.A.; Mohammad, H.; Dayal, N.; Abutaleb, N.S.; Seleem, M.N.; Sintim, H.O. N-(1,3,4-Oxadiazol-2-Yl)Benzamide Analogs, Bacteriostatic Agents against Methicillin- and Vancomycin-Resistant Bacteria. Eur. J. Med. Chem. 2018, 155, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, G.A.; Karanja, C.W.; Opoku-Temeng, C.; Sintim, H.O. Antibacterial Small Molecules That Potently Inhibit Staphylococcus Aureus Lipoteichoic Acid Biosynthesis. ChemMedChem 2019, 14, 1000–1004. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Abutaleb, N.S.; Li, D.; Seleem, M.N.; Sintim, H.O. Ultrapotent Inhibitor of Clostridioides Difficile Growth, Which Suppresses Recurrence in Vivo. J. Med. Chem. 2020, 63, 11934–11944. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, G.A.; Onyedibe, K.I.; Sintim, H.O. Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. Aureus and E. Faecalis Growth and Biofilm Formation. Molecules 2020, 25, 2277. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Abutaleb, N.S.; Alhashimi, M.; Seleem, M.N.; Sintim, H.O. N-(1,3,4-Oxadiazol-2-Yl)Benzamides as Antibacterial Agents against Neisseria Gonorrhoeae. Int. J. Mol. Sci. 2021, 22, 2427. [Google Scholar] [CrossRef]
- Karabanovich, G.; Zemanová, J.; Smutný, T.; Székely, R.; Šarkan, M.; Centárová, I.; Vocat, A.; Pávková, I.; Čonka, P.; Němeček, J.; et al. Development of 3,5-Dinitrobenzylsulfanyl-1,3,4-Oxadiazoles and Thiadiazoles as Selective Antitubercular Agents Active Against Replicating and Nonreplicating Mycobacterium Tuberculosis. J. Med. Chem. 2016, 59, 2362–2380. [Google Scholar] [CrossRef]
- Karabanovich, G.; Němeček, J.; Valášková, L.; Carazo, A.; Konečná, K.; Stolaříková, J.; Hrabálek, A.; Pavliš, O.; Pávek, P.; Vávrová, K.; et al. S-Substituted 3,5-Dinitrophenyl 1,3,4-Oxadiazole-2-Thiols and Tetrazole-5-Thiols as Highly Efficient Antitubercular Agents. Eur. J. Med. Chem. 2017, 126, 369–383. [Google Scholar] [CrossRef]
- Makane, V.B.; Krishna, V.S.; Krishna, E.V.; Shukla, M.; Mahizhaveni, B.; Misra, S.; Chopra, S.; Sriram, D.; Azger Dusthackeer, V.N.; Rode, H.B. Novel 1,3,4-Oxadiazoles as Antitubercular Agents with Limited Activity against Drug-Resistant Tuberculosis. Future Med. Chem. 2019, 11, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, E.; Beyzaei, H.; Aryan, R.; Moradi, A. Ultrasound-Assisted, Low-Solvent and Acid/Base-Free Synthesis of 5-Substituted 1,3,4-Oxadiazole-2-Thiols as Potent Antimicrobial and Antioxidant Agents. Mol. Divers. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Ur, R.; Khan, S.G.; Bokhari, T.H.; Anjum, F.; Akhter, N.; Rasool, S.; Ali Shah, S.A.; Shahid, M.; Arshad, A. Synthesis, Characterization, Antibacterial, Hemolytic and Thrombolytic Activity Evaluation of 5-(3-Chlorophenyl)-2-((N-(Substituted)-2-Acetamoyl)Sulfanyl)-1,3,4-Oxadiazole Derivatives. Pak. J. Pharm. Sci. 2020, 33, 871–876. [Google Scholar]
- Rezki, N.; Al-Yahyawi, A.M.; Bardaweel, S.K.; Al-Blewi, F.F.; Aouad, M.R. Synthesis of Novel 2,5-Disubstituted-1,3,4-Thiadiazoles Clubbed 1,2,4-Triazole, 1,3,4-Thiadiazole, 1,3,4-Oxadiazole and/or Schiff Base as Potential Antimicrobial and Antiproliferative Agents. Molecules 2015, 20, 16048–16067. [Google Scholar] [CrossRef] [Green Version]
- Neeraja, P.; Srinivas, S.; Mukkanti, K.; Dubey, P.K.; Pal, S. 1H-1,2,3-Triazolyl-Substituted 1,3,4-Oxadiazole Derivatives Containing Structural Features of Ibuprofen/Naproxen: Their Synthesis and Antibacterial Evaluation. Bioorg. Med. Chem. Lett. 2016, 26, 5212–5217. [Google Scholar] [CrossRef]
- Wang, C.-H.; Xie, X.-R.; Liu, W.-S.; Hou, G.-G.; Sun, J.-F.; Zhao, F.; Cong, W.; Li, H.-J.; Xin, W.-Y. Quaternary Ammonium Salts Substituted by 5-Phenyl-1,3,4-Oxadiazole-2-Thiol as Novel Antibacterial Agents with Low Cytotoxicity. Chem. Biol. Drug Des. 2017, 90, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cong, W.; Zhao, F.; Li, H.; Xin, W.; Hou, G.; Wang, C. Synthesis, Physiochemical Property and Antimicrobial Activity of Novel Quaternary Ammonium Salts. J. Enzyme Inhib. Med. Chem. 2018, 33, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambhore, A.N.; Kamble, S.S.; Kadam, S.N.; Kamble, R.D.; Hebade, M.J.; Hese, S.V.; Gaikwad, M.V.; Meshram, R.J.; Gacche, R.N.; Dawane, B.S. Design, Synthesis and in Silico Study of Pyridine Based 1,3,4-Oxadiazole Embedded Hydrazinecarbothioamide Derivatives as Potent Anti-Tubercular Agent. Comput. Biol. Chem. 2019, 80, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Mohamed, A.A.B.; Tawfik, S.S.; Hassan, H.M.; El-Emam, A.A. 1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities. Molecules 2021, 26, 2110. [Google Scholar] [CrossRef]
- Gavarkar, P.S.; Somani, R.R. Synthesis of Novel Azole Heterocycles with Their Antitubercular and Antifungal Evaluation. Int. J. Chem. Sci. 2015, 13, 432–440. [Google Scholar]
- Wani, M.Y.; Ahmad, A.; Shiekh, R.A.; Al-Ghamdi, K.J.; Sobral, A.J.F.N. Imidazole Clubbed 1,3,4-Oxadiazole Derivatives as Potential Antifungal Agents. Bioorg. Med. Chem. 2015, 23, 4172–4180. [Google Scholar] [CrossRef]
- Liao, J.; Yang, F.; Zhang, L.; Chai, X.; Zhao, Q.; Yu, S.; Zou, Y.; Meng, Q.; Wu, Q. Synthesis and Biological Evaluation of Novel Fluconazole Analogues Bearing 1,3,4-Oxadiazole Moiety as Potent Antifungal Agents. Arch. Pharm. Res. 2015, 38, 470–479. [Google Scholar] [CrossRef]
- Nimbalkar, U.; Tupe, S.; Seijas Vazquez, J.; Khan, F.; Sangshetti, J.; Nikalje, A. Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-Substituted Phenyl)-3-((Phenylamino)Methyl)-1,3,4-Oxadiazole-2(3H)-Thiones. Molecules 2016, 21, 484. [Google Scholar] [CrossRef] [Green Version]
- Stoica, C.I.; Marc, G.; Pirnau, A.; Vlase, L.; Araniciu, C.; Oniga, S.; Palage, M.; Oniga, O. Thiazolyl-Oxadiazole Derivatives Targeting Lanosterol 14α-Demethylase as Potential Antifungal Agents: Design, Synthesis and Molecular Docking Studies. Farmacia 2016, 64, 390–397. [Google Scholar]
- Frost, J.R.; Scully, C.C.G.; Yudin, A.K. Oxadiazole Grafts in Peptide Macrocycles. Nat. Chem. 2016, 8, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Revie, N.M.; Robbins, N.; Whitesell, L.; Frost, J.R.; Appavoo, S.D.; Yudin, A.K.; Cowen, L.E. Oxadiazole-Containing Macrocyclic Peptides Potentiate Azole Activity against Pathogenic Candida Species. mSphere 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Levent, S.; Kaya, Ç.B.; Sağlık, B.; Osmaniye, D.; Acar, Ç.U.; Atlı, Ö.; Özkay, Y.; Kaplancıklı, Z. Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity. Molecules 2017, 22, 2004. [Google Scholar] [CrossRef] [Green Version]
- Karaburun, A.Ç.; Çavuşo, G.B.K.; Çevik, U.A.; Osmaniye, D.; Sa Glık, B.N.; Levent, S.; Özkay, Y.; Atlı, Ö.; Koparal, A.S.; Kaplancıklı, Z.A. Synthesis and Antifungal Potential of Some Novel Benzimidazole-1,3,4-Oxadiazole Compounds. Molecules 2019, 24, 191. [Google Scholar] [CrossRef] [Green Version]
- Bordei Telehoiu, A.T.; Nuță, D.C.; Căproiu, M.T.; Dumitrascu, F.; Zarafu, I.; Ioniță, P.; Bădiceanu, C.D.; Avram, S.; Chifiriuc, M.C.; Bleotu, C.; et al. Design, Synthesis and In Vitro Characterization of Novel Antimicrobial Agents Based on 6-Chloro-9H-Carbazol Derivatives and 1,3,4-Oxadiazole Scaffolds. Molecules 2020, 25, 266. [Google Scholar] [CrossRef] [Green Version]
- Radini, I.; Elsheikh, T.; El-Telbani, E.; Khidre, R. New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents. Molecules 2016, 21, 909. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, S.S.; Thakor, P.; Doshi, H.; Ray, A. 1,2,4-Triazole and 1,3,4-Oxadiazole Analogues: Synthesis, MO Studies, in Silico Molecular Docking Studies, Antimalarial as DHFR Inhibitor and Antimicrobial Activities. Bioorg. Med. Chem. 2017, 25, 4064–4075. [Google Scholar] [CrossRef]
- Verma, G.; Chashoo, G.; Ali, A.; Khan, M.F.; Akhtar, W.; Ali, I.; Akhtar, M.; Alam, M.M.; Shaquiquzzaman, M. Synthesis of Pyrazole Acrylic Acid Based Oxadiazole and Amide Derivatives as Antimalarial and Anticancer Agents. Bioorg. Chem. 2018, 77, 106–124. [Google Scholar] [CrossRef]
- Verma, G.; Khan, M.F.; Mohan Nainwal, L.; Ishaq, M.; Akhter, M.; Bakht, A.; Anwer, T.; Afrin, F.; Islamuddin, M.; Husain, I.; et al. Targeting Malaria and Leishmaniasis: Synthesis and Pharmacological Evaluation of Novel Pyrazole-1,3,4-Oxadiazole Hybrids. Part II. Bioorg. Chem. 2019, 89, 102986. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Bova, F.; Zappalà, M.; Grasso, S.; Micale, N. Falcipain-2 Inhibitors. Med. Res. Rev. 2010, 30, 136–167. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Ali, M.; Rashid, U.; Imran, S.; Uddin, N.; Khan, K.M. Molecular Hybridization Conceded Exceptionally Potent Quinolinyl-Oxadiazole Hybrids through Phenyl Linked Thiosemicarbazide Antileishmanial Scaffolds: In Silico Validation and SAR Studies. Bioorg. Chem. 2017, 71, 192–200. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Anouar, E.H.; Selvaraj, M.; Jamil, W.; Ali, M.; Kashif, S.M.; Rahim, F.; Khan, K.M.; et al. Synthesis and Molecular Modelling Studies of Phenyl Linked Oxadiazole-Phenylhydrazone Hybrids as Potent Antileishmanial Agents. Eur. J. Med. Chem. 2017, 126, 1021–1033. [Google Scholar] [CrossRef]
- Chaves, J.D.S.; Tunes, L.G.; Chris, C.H.; Francisco, T.M.; Corrêa, C.C.; Murta, S.M.F.; Monte-Neto, R.L.; Silva, H.; Fontes, A.P.S.; de Almeida, M.V. Novel Gold(I) Complexes with 5-Phenyl-1,3,4-Oxadiazole-2-Thione and Phosphine as Potential Anticancer and Antileishmanial Agents. Eur. J. Med. Chem. 2017, 127, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.V.; Costa, D.d.S.; Tunes, L.G.; Monte-Neto, R.L.d.; Grazul, R.M.; Almeida, M.V.; Silva, H. Anticancer and Antileishmanial in Vitro Activity of Gold(I) Complexes with 1,3,4-oxadiazole-2(3H)-thione Ligands Derived from Δ-D-gluconolactone. Chem. Biol. Drug Des. 2021, 97, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B.; Patel, J.N.; Purohit, A.C.; Patel, V.M.; Rajani, D.P.; Moo-Puc, R.; Lopez-Cedillo, J.C.; Nogueda-Torres, B.; Rivera, G. In Vitro and in Vivo Assessment of Newer Quinoxaline–oxadiazole Hybrids as Antimicrobial and Antiprotozoal Agents. Int. J. Antimicrob. Agents 2017, 50, 413–418. [Google Scholar] [CrossRef]
- Shaykoon, M.S.; Marzouk, A.A.; Soltan, O.M.; Wanas, A.S.; Radwan, M.M.; Gouda, A.M.; Youssif, B.G.M.; Abdel-Aziz, M. Design, Synthesis and Antitrypanosomal Activity of Heteroaryl-Based 1,2,4-Triazole and 1,3,4-Oxadiazole Derivatives. Bioorg. Chem. 2020, 100, 103933. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.L.S.; Soares, J.C.A.V.; Portapilla, G.B.; Providello, M.V.; Lima, C.H.S.; Muri, E.M.F.; de Albuquerque, S.; Dias, L.R.S. Trypanocidal Activity of New 1,6-Diphenyl-1H-Pyrazolo[3,4-b]Pyridine Derivatives: Synthesis, in Vitro and in Vivo Studies. Bioorg. Med. Chem. 2021, 29, 115855. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, F.; Eydoux, C.; Querat, G.; De Lamballerie, X.; Canard, B.; Alvarez, K.; Guillemot, J.C.; Barral, K. Novel 2-Phenyl-5-[(E)-2-(Thiophen-2-Yl)Ethenyl]-1,3,4-Oxadiazole and 3-Phenyl-5-[(E)-2-(Thiophen-2-Yl)Ethenyl]-1,2,4-Oxadiazole Derivatives as Dengue Virus Inhibitors Targeting NS5 Polymerase. Eur. J. Med. Chem. 2016, 109, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.S.; Farahat, A.A.; El-Sayed, M.; Tantawy, A.S.; Bagato, O.; Ali, M.A. Synthesis and Anti-Influenza Activity of Novel Thiadiazole, Oxadiazole and Triazole Based Scaffolds. Lett. Drug Des. Discov. 2018, 15, 363–374. [Google Scholar] [CrossRef]
- Albratty, M.; El-Sharkawy, K.A.; Alhazmi, H.A. Synthesis and Evaluation of Some New 1,3,4-Oxadiazoles Bearing Thiophene, Thiazole, Coumarin, Pyridine and Pyridazine Derivatives as Antiviral Agents. Acta Pharm. 2019, 69, 261–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mansouri, A.E.; Maatallah, M.; Ait Benhassou, H.; Moumen, A.; Mehdi, A.; Snoeck, R.; Andrei, G.; Zahouily, M.; Lazrek, H.B. Design, Synthesis, Chemical Characterization, Biological Evaluation, and Docking Study of New 1,3,4-Oxadiazole Homonucleoside Analogs. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.S.; Khan, B.A.; Hameed, S.; Batool, F.; Saleem, H.N.; Mughal, E.U.; Saeed, M. Synthesis and Evaluation of Novel S-Benzyl- and S-Alkylphthalimide- Oxadiazole -Benzenesulfonamide Hybrids as Inhibitors of Dengue Virus Protease. Bioorg. Chem. 2020, 96, 103567. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. https://doi.org/10.3390/ijms22136979
Glomb T, Świątek P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. International Journal of Molecular Sciences. 2021; 22(13):6979. https://doi.org/10.3390/ijms22136979
Chicago/Turabian StyleGlomb, Teresa, and Piotr Świątek. 2021. "Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives" International Journal of Molecular Sciences 22, no. 13: 6979. https://doi.org/10.3390/ijms22136979
APA StyleGlomb, T., & Świątek, P. (2021). Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. International Journal of Molecular Sciences, 22(13), 6979. https://doi.org/10.3390/ijms22136979





