Abstract
The prevalence of NAFLD (non-alcoholic fatty liver disease) is a rapidly increasing problem, affecting a huge population around the globe. However, CVDs (cardiovascular diseases) are the most common cause of mortality in NAFLD patients. Atherogenic dyslipidemia, characterized by plasma hypertriglyceridemia, increased small dense LDL (low-density lipoprotein) particles, and decreased HDL-C (high-density lipoprotein cholesterol) levels, is often observed in NAFLD patients. In this review, we summarize recent genetic evidence, proving the diverse nature of metabolic pathways involved in NAFLD pathogenesis. Analysis of available genetic data suggests that the altered operation of fatty-acid β-oxidation in liver mitochondria is the key process, connecting NAFLD-mediated dyslipidemia and elevated CVD risk. In addition, we discuss several NAFLD-associated genes with documented anti-atherosclerotic or cardioprotective effects, and current pharmaceutical strategies focused on both NAFLD treatment and reduction of CVD risk.
1. Introduction
The prevalence of NAFLD is a rapidly increasing problem, affecting 25–45% of the adult population worldwide and up to 70% in T2DM (type 2 diabetes mellitus) and obesity patient groups [1]. It is known that NAFLD is associated with many co-morbidities (such as hypertension, obesity, MetS (metabolic syndrome) and hyperlipidemia). NASH (non-alcoholic steatohepatitis) a more severe form, affects 2–7% of adults and could further progress to cirrhosis form or HCC (hepatocellular carcinoma). Despite the liver-related complication, CVD is a common cause of death among NAFLD patients [2]. However, it is important to note, while most probably all types of NAFLD are associated with elevated CVD risk, the strongest links were defined for NASH and advanced stages of fibrosis [3,4].
The exact mechanism responsible for the connection between NAFLD and CVD is not proven. However, during recent years, several “drivers” were proposed to be responsible for NAFLD progression and accelerating atherogenesis: dyslipidemia, chronic inflammation, and endothelial dysfunction [5]. The accompanying expansion of adipose tissue initiates a pro-inflammatory cascade with the NF-kB (nuclear factor kappa B) and JNK (c-Jun N-terminal kinase) pathways. Further NAFLD complications may include IR (insulin resistance) (hepatic or system-wide level), increased production of inflammatory cytokines (IL-6 (Interleukin 6), C-reactive protein, TNF-α (tumour necrosis factor-alpha), and others), synthesis of procoagulant factors (factor VIII, endothelin, TGF-β (transforming growth factor-beta), fibrinogen, and others) and hepatokines, dysregulated glucose, and lipid metabolism [6,7].
Atherosclerotic neo-intimal plaques develop in large arteries and drive adverse CV events (such as stroke and myocardial infarction). Atherosclerosis is a decades-lasting chronic disease, during which inflammation, calcification, fibrosis, and lipid-deposition change the composition of atherosclerotic plaques [8]. Several methods have been used to detect plaque features and evaluate CVD risk: invasive, used mostly on more advanced stages (angiography, optical coherence tomography, or intravascular ultrasonography); and non-invasive, more often used for initial diagnostics (positron emission and computer tomography, measurement of carotid intima-media thickness, and others) (reviewed in [9]).
Results of many cross-sectional studies, meta-analyses and systematic reviews suggest that NAFLD increases the risk of atherosclerosis and favours the development of unstable plaques [10,11,12,13]. In addition, genetic evidence suggests that NAFLD-mediated dyslipidemia is a crucial factor of elevated CVD risk [14]. While many genetic polymorphism sites and mutations are associated with both CVD [15,16] and NAFLD [17], some NAFLD favouring SNPs (single-nucleotide polymorphisms) have been described as decreasing CVD risk [18,19,20]. Other research, however, found no such protection [21,22]. For readers interested in immuno-inflammatory aspects and the role of bile acid and cholesterol metabolism in NAFLD–CVD relations, we recommend recent excellent reviews [23,24,25,26,27].
Association between Liver and Heart Disease
Several mechanisms that explain the close connection between CVD and NAFLD have been suggested. It is known that IR plays a crucial role in NAFLD and NASH pathogenesis [28]. In addition, IR affects many physiological processes and causes hyperglycemia and dyslipidemia, activating low-grade chronic inflammation, ectopic lipid accumulation, OS (oxidative stress), and endothelial dysfunction [29]. An elevated level of serum ferritin, the main iron-storing protein, also is common for NAFLD patients, and is associated with IR [30]. Altogether, these events create a so-called CVD-favouring pro-atherogenic environment [31]. Combined with altered immune-cell populations, defined in NASH patients [23], this suggests an immune and chronic inflammatory link between IR, CVD, and DM [32]. Fetuin-A, a glycoprotein secreted from adipose tissue and liver, stimulates the production of inflammatory cytokines from adipocytes and macrophages, and serves as a biomarker of several chronic inflammatory diseases. The level of fetuin-A (fatty-acid carrier) also is increased in NAFLD/NASH patients [33]. It was recently shown that fetuin-A could inhibit insulin receptor tyrosine kinase in muscle and liver and cause IR [34]. The level of fetuin-A was linked to hypertriglyceridemia, and there was no significant association with risk of ischaemic stroke and other CVD [35], while other research suggests it as a valuable factor for chronic heart failure diagnostics [36].
Different extents of dyslipidemia often present in NAFLD/NASH patients (decreased level of HDL-C, and increased levels of LDL particles and TG (triglycerides)), and serve as an important non-invasive marker for NAFLD diagnostics [27,37]. However, such a lipid profile is known as atherogenic and was also linked with the severity of cardiometabolic risk [38,39]. The liver is the central organ responsible for lipid metabolism, among which cholesterol and TG are of the most importance. While low HDL-C is the well-defined marker for NAFLD and a risk factor for CVD, the exact molecular mechanism responsible for this connection is under intensive investigation [40]. One of the best-known functions of HDL is the ability to promote RCT (reverse cholesterol transport), which allows removal of excess cholesterol from the macrophages with further excretion from the body with bile. RCT has attracted much attention as a promising therapeutic target to reduce CVD risk [41]. However, therapeutic improvement of HDL-C level with drugs was not beneficial to lower CVD risk, thus suggesting a more complex relationship between HDL-C and CVD [42].
An elevated level of serum homocysteine is a well-known cause of hepatic oxidative stress and hepatic steatosis [43], correlating with the level of liver dysfunction in NAFLD/NASH patients [44]. Similarly, serum homocysteine serves as an independent factor for CVD [45]. Homocysteine is known to activate Toll-like receptor 4, dysregulate Ca2+ and NO signalling, increase production of ROS, and induce platelet activation and endothelial dysfunction, which eventually cause CVD [46].
Another connection point between NAFLD and CV effects is inflammatory cytokines, which are released by the liver, and cause system inflammation and promote CVD. The main inflammation-mediated triggers leading to CVD are enhanced plaque formation, alteration in vascular tone, coagulation, and endothelial function [47]. Levels of several cytokines (such as IL-1, IL-6, C-reactive protein, and TNFα) known as system inflammation markers are elevated in NAFLD patients [48]. Recent research suggests an association of liver steatosis and fibrosis with diastolic heart dysfunction and impaired myocardial glucose uptake [49]. In addition, hepatic fat content was linked to increased left ventricular filling pressure, which is a precursor of heart failure [50].
Future research should be concentrated on the early detection of the metabolic markers of liver and heart efficiency, ideally before functional and structural abnormalities appear. Thus, individuals with diagnosed NAFLD would have a possibility to prevent complications with the CV system. In this review, we focus on the role of liver lipid homeostasis and mitochondrial β-oxidation in the connection between NAFLD and CVD, associated genetic regulations, and targeted therapies.
2. Liver as a Central Organ for Lipid Metabolism
The liver plays a crucial role in lipid and glucose homeostasis and metabolism. Under the pressure of continuous impaired FA (fatty acid) metabolism, the liver accumulates a significant amount of lipids that lead to NAFLD development. The main feature of NAFLD is an accumulation of hepatic TGs, which could be caused by internal (impaired FAO, VLDL synthesis and export), external (certain genetic background and environmental conditions) or behavioural (exceed FAs from the diet, circulation or adipose tissue, lack of physical activities) triggers [51,52]. An imbalance between imported/exported and synthesized/processed FAs results in hepatic lipid accumulation, hepatosteatosis and IR, which is further stimulating de novo hepatic lipogenesis making this vicious cycle complete [53]. While the exact molecular mechanisms are not completely understood and are under intensive investigation, impaired FAs metabolism and ROS production, leading to chronic inflammation and mitochondria malfunction have been suggested as key events in this disease development [54,55].
2.1. Lipids Homeostasis in the Liver Mitochondria: Fatty-Acid β-Oxidation
Carbohydrates and FAs are the main energy source for cells, and their uptake from the extracellular space and intracellular release is tightly controlled by several hormones, such as glucagon, insulin, noradrenaline, and others. Inside the cell, FAs are esterified, metabolized to the lipid second messengers (such as ceramide, sphingosine, phosphatidylinositol bisphosphate, and others), or transport to the mitochondria for β-oxidation. However, a VLCFA (very-long-chain fatty acid) (FAs with 22 and more carbons) could not be metabolized in the mitochondria, and should be delivered to peroxisomes (reviewed in [56]). After LCFAs (long-chain FAs) are activated by CoA, LCFA-CoA ester is transported into the mitochondrial matrix via the CPT (carnitine palmitoyltransferase) system, which consists of three proteins: CPT1, CPT2, and CACT (acylcarnitine translocase) [57] (Figure 1).
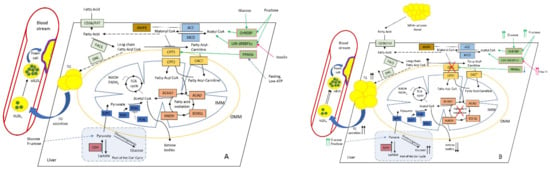
Figure 1.
Altered hepatic lipid metabolism in normal (A) and NAFLD (B) conditions. Increased diet supply of glucose and fructose affects ChREBP (carbohydrate-responsive element-binding protein), SREBP1c (sterol regulatory element-binding protein-1c), and LXR (liver X receptor) TFs, which stimulate malonyl CoA synthesis. PPARα (peroxisome proliferator-activated receptor alpha) normally activates CPT1 under fasting and low-ATP conditions [58]. As a key intermediate, malonyl CoA inhibits CPT1, thus reducing FAO. This leads to the accumulation of long-chain fatty-acid CoA (which could also be delivered from surplus adipose tissue), and stimulates DNL with the subsequent rise in intrahepatic TG and plasma TG levels, further increasing large VLDL1 and the formation of small dense LDL, which favours foam cell formation and ultimately atherosclerosis. During the progression of NAFLD, the production of ketone bodies progressively reduces while hepatic glucose synthesis and output increases, thus further promoting IR and the rise in insulin level [59]. Colour coding as follows: PPARα and other TFs are depicted in pale green; primarily FA-metabolising enzymes (FAT (fatty-acid translocase) and FACS (fatty-acid synthase)) and DNL (de novo lipogenesis) are highlighted in pale blue; malonyl-CoA-metabolising enzymes ACC (acetyl-CoA carboxylase) and MCD (malonyl-CoA decarboxylase) are highlighted in blue; CPT system enzymes (CPT1, CPT2, and CACT) are depicted in light brown; AMPK (AMP-activated protein kinase), the main regulator of CPT system are depicted in brown; FAO enzymes ACAA2 (acetyl-CoA acyltransferase 2), ACAD (acyl-CoA dehydrogenase), ECHS1 (enoyl-CoA hydratase, short chain 1), and HADH (hydroxyacyl-CoA dehydrogenase) are depicted in in pale red; pyruvate metabolism enzymes MPC (mitochondrial pyruvate carrier 1), PDP (pyruvate dehydrogenase phosphatase), PDH (pyruvate dehydrogenase), and PDK (pyruvate dehydrogenase kinase) are depicted in dark blue; and LDH (lactate dehydrogenase) is depicted in red. Abbreviations: IMM and OMM, inner and outer mitochondrial membrane, respectively.
CPT1 could be inhibited by malonyl-CoA derived from glucose metabolism, thus making CPT1 the rate-limiting step in mitochondrial FAO. There are three isoforms of CPT1, which are organ-specific for liver (A), muscle and heart (B), and brain (C). Two more enzymes are involved in the malonyl-CoA metabolism: ACC (acetyl-CoA carboxylase) is responsible for the synthesis of malonyl-CoA, and MCD (malonyl-CoA decarboxylase) is responsible for the degradation of malonyl-CoA [60].
There are two main ACC isoforms, ACC1 and ACC2, which have different tissue expression patterns and functions. ACC1 is localized in the cytoplasm of all cells, but is enriched in lipogenic tissue (such as adipose tissue) [61]. ACC2 is localized in the mitochondria and enriched in oxidative tissue (such as heart and skeletal muscle) [62]. Thus, different tissues have a specific ACC1/ACC2 ratio, which is required to balance FA oxidation and synthesis. ACC1 and ACC2 are both highly expressed in the liver, where processes of both FA oxidation and synthesis are important. However, such a difference in ACC localization and function provides an opportunity to create pharmaceutical drugs for specific inhibition of FA synthesis and stimulation of fatty-acid oxidation, which could be beneficial for some morbidities such as obesity, NAFLD, diabetes, and others [63,64].
AMPK is one of the main regulators of this pathway; it acts via phosphorylation and inhibition of ACCs, thus decreasing expression of FA synthase and supply of intermediates for the FA anabolic pathway. As a secondary and long-term effect, AMPK phosphorylates TFs SREBP1c and ChREBP, thus inhibiting the transcription of subsequent lipogenic genes [65]. Sirtuin proteins (SIRT1 and SIRT3) stimulate AMPK via deacetylation of its upstream activator LKB1 (liver kinase B1) [66]. Nowadays, sirtuins are recognised as crucial regulators of lipid metabolism, providing tissue-specific FAO-promoting activities (in skeletal muscle and liver), lipolysis (in adipose tissue), mitochondrial respiration (BAT (brown adipose tissue)), and food intake (in the hypothalamus) [67].
Mitochondrial FAO in the liver leads to complete oxidation to CO2, or partial when ketone bodies, an exported form of energy-containing molecules, are formed. The data regarding the CPT1A levels of expression and activity and rate of mitochondrial FAO are controversial and greatly depend on the model system used, FFA concentration, and other experimental conditions [68,69]. To explain these differences, several mechanisms have been suggested: (1) the levels of malonyl-CoA are variable and depend on the ratio of ACC/MCD proteins; (2) the physical properties of the mitochondrial membrane could change the sensitivity of CPT1A to malonyl-CoA [70]; and (3) the available pool of FFAs and other lipid intermediates could activate different transcription factors responsible for FA de novo synthesis, uptake, transport, and oxidation [71,72].
The current model explains the relationship between FFAs and FAO as the hormetic effect when a mild or evanescent rise in available FFAs leads to beneficial increased FAO with higher energy output. However, prolonged and significant overflow of FFAs leads to an excessive electron flux in the ETC (electron transport chain), ROS overproduction, and formation of toxic aldehydes, which damage mitochondrial proteins, lipids, and DNA, and cause morphological and functional disturbances [73,74,75]. As a possible strategy to avoid such harmful effects, the liver can switch the balance from complete FA oxidation towards ketone-body production [76]. However, similar to the FAO pathway, this strategy has its limits, and could cause some further complications under prolonged FFA overflow and reduced energy expenditure [77,78].
2.2. Interplay and Co-Regulation with Glucose Metabolism
The modern diet (especially of typical Western style) contains a high amount of simple fructose- and glucose-based saccharides, which are risk factors for the development of several metabolic complications, such as obesity, T2DM, NAFLD, CVD, and others [79,80]. Linoleic acid is a polyunsaturated omega-6 fatty acid, also widely presented in the Western diet and associated with weight gain, obesity, IR, and CVD [81,82,83]. It is known that fructose serves as a substrate for FA synthesis and stimulates the TFs of de novo lipogenesis and triglyceride synthesis, SREBP1c and ChREBP [84]. Simultaneously, fructose decreases FAO via two main mechanisms: increasing the level of hepatic malonyl-CoA, and directly altering the expression of hepatic genes responsible for lipid accumulation and removal [85]. The unique aspect of fructose action is a transient decrease in intracellular levels of phosphate and ATP, which is associated with the uric acid generation and nucleotide turnover. A decreased ATP level induces a series of reactions, including induction of OS, a transient block in protein synthesis, and mitochondrial dysfunction, which turned out to have a key role in fructose-mediated effects [86,87]. Because different CPT1 isoforms have different sensitivities to malonyl-CoA, where liver isoform is 30–100 times less sensitive in comparison to heart and muscle isoforms [88,89], fructose’s malonyl-CoA-independent effect on liver mitochondrial FAO may be more pronounced and promote a higher degree of mitochondrial dysfunction. Additionally, diet-delivered nutrients could regulate mitochondrial functions via post-translational modifications (malonylation, acetylation, succinylation, and others) [90,91,92]. However, the discussion of these modifications is beyond the scope of this manuscript, and we wish to redirect interested readers to the cited papers.
2.3. Role of Perilipin 5 in NAFLD and Atherosclerosis
Plin5 (perilipin 5) is an important member of the perilipin protein family, which is abundant in tissues with very active lipid catabolism, such as the heart, skeletal muscle, brown adipose tissue, and the liver [93]. NAFLD is characterized by increased accumulation of LDs in the liver, as well as increased expression of PLIN5 [94]. Plin5 is known as the main LD forming and coating protein, responsible for restoring hepatic TGs in LDs and inhibition of lipolysis. Not surprisingly, Plin5 overexpression worsens hepatosteatosis [95], and simultaneously blocks stellate-cell activation [96,97], but without adverse effects on IR. On the other hand, PLIN5 deficiency leads to impaired insulin signal transduction and the development of IR [98].
Recent studies have suggested the molecular mechanism explaining these observations. The C-terminal part of the Plin5 (443–63aa) recruits mitochondria to contact LDs. Such LD–mitochondria contact is required for proper supply of FAs to mitochondria, lipid synthesis, and LD expansion [99]. These features refer to the role of mitochondria in the synthesis of TAGs and phospholipid, because enzymes, localized on the outer membrane of mitochondria GPAT1 and 2 (glycerol-3-phosphate acyltransferase 1 and 2) and AGPAT (1-acyl glycerol-3-phosphate acyltransferase) are responsible for the biosynthesis of lysophosphatidic acid and phosphatidic acid, respectively [100]. Mitochondria, associated with LDs, have increased capacities for pyruvate oxidation, electron transport, and ATP synthesis, a reduced β-oxidation capacity, and uniquely low fusion–fission dynamics [101]. Further, Plin5 was shown to limit FA toxicity, clear harmful proteins from the outer mitochondria membrane, and protect against OS [102].
PLIN5 showed implications in the inflammatory response via activation of the NLRP3 (NLR family pyrin domain-containing 3) inflammasome, thus linking it to the NAFLD-to-NASH progression [103]. In addition, a high level of Plin5 was found in HCC biopsy specimens [104]. However, the data regarding PLIN5′s role in HCC development and metastasis are still limited, and further research is required to elucidate the exact function of PLIN5 in HCC.
Interestingly, PLIN5 deficiency was also implicated in the progression of atherosclerosis, as was shown in double knockout mice (ApoE−/−Plin5−/−) that developed more severe atherogenesis (with elevated TG, TC, and LDL-C levels, and reduced HDL-C contents) and accelerated inflammation, apoptosis, lipid accumulation, and OS. Mutant mice had promoted atherogenesis progression, along with an increased entire aorta, aortic arch, and abdominal aorta area [105]. Additionally, PLIN5 is involved in thermoregulation and adaptation to cold stress. A study of LDs and mitochondria isolated from the liver of mice housed at chronic cold stress determined that the mitochondrial TCA cycle and retinol metabolism were enhanced, while oxidative phosphorylation was not affected. Liver adaptation to cold stress conditions involved increased expression of Plin5 and MUPs (major urinary proteins), whereas expression of MPC was dramatically decreased [106]. Previously, the role of thermogenesis in the development of metabolic diseases was associated mostly with adipose tissues (interplay and transition between both white and brown) [107,108]. However, the described cold-adaptation-related role of Plin5 also implies a role of the homeostasis and thermogenesis of liver lipids in NAFLD development [106].
These findings elucidate that PLIN5 is a crucial pleiotropic regulator of hepatic lipid metabolism, thermogenesis, and inflammatory response involved in NAFLD/NASH and atherosclerosis development and progression. PLIN5 is a promising therapeutic target for NAFLD and atherosclerosis, and possibly for some other metabolic diseases. We wish to redirect interested readers to recent reviews for further information about the role of PLIN5 and mitochondria-LD contact sites in the development of NAFLD and other diseases [109,110].
2.4. Role of the Liver Mitochondria in the Development of CVD-Promoting Dyslipidemia
A certain degree of atherogenic dyslipidaemia is present in NAFLD/NASH patients that is characterized by TC/HDL-C, LDL-C/HDL-C, and TG/HDL-C ratios [111,112,113]. Due to the known central role of the liver in the production/clearance of all classes of lipoprotein (HDL) and apolipoproteins (ApoB48 and ApoB100), it makes a solid connection between NAFLD/NASH-associated metabolic dysfunction and elevated CVD risk [114]. In this section, we focus on recent mechanistic insights into links between genes known to protect/cause NAFLD, ameliorate/worsen the NAFLD phenotype, alter liver-specific lipid accumulation, or influence the lipid profile at a system-wide level (Table 1).

Table 1.
Recent results obtained in mouse models and elucidating effects of different genes on molecular aspects of NAFLD pathogenesis and general lipid metabolism.
In general, we could distinguish several groups of genes. The first one combines genes necessary to support mitochondrial functionality (mitochondrial DNA replication, protein synthesis, fission/fusion, integrity, ROS, and bioenergy production). For the second group, we categorized genes participating in fatty-acid β-oxidation (enzymes providing biochemical output and several regulators). The third group has antioxidant genes (SOD, PRX5, and CAT) with known lipid-metabolism and fat-accumulation phenotypes. The next group contains different genes that participate in general lipid metabolism (de novo biosynthesis, uptake, and secretion). The “Supplementation” group combines several diet-intervention studies in which different treatments were used to define their effect on the lipid profile and FAO. The final group contains several genes that participate in the different stages of liver lipid metabolism. Such grouping was very conditional and was done only for the convenience of our discussion. There are many genes implicated in FAO disorders that are not listed here due to the absence of the NAFLD/NASH-related phenotype; however, these genes are relevant for this topic, and we wish to redirect interested readers to the recent study [178].
Analysis of described mutations suggested the central place of liver mitochondrial FAO in the connection between NAFLD and CVD risk. The key points were: (1) increased FAO may alleviate NAFLD symptoms, but cause hypertriglyceridemia and OS damage to the liver; (2) proper antioxidant supply may support FAO by neutralizing excess ROS; (3) complete absence of FAO in liver mitochondria causes resistance to HFD obesity and NAFLD, along with serum dyslipidemia and hepatic OS; (4) proper mitochondria turnover may support effective FAO and ameliorate NAFLD symptoms; (5) complete absence/reduction of FAO enhanced energy expenditure at the system-wide level and suppressed adiposity; (6) with disabled FAO, other mechanisms of FA oxidation are stimulated in other compartments/cells (peroxisomes and microsomes, macrophages, and others). Thus, analysis of collected mutations supported the hypothesis of a hormetic effect [74] when the evanescent rise of input FFAs is beneficial and stimulates FAO, providing higher energy output. Excess or prolonged stimulation of FAO is harmful; the depleted antioxidant pool could not cope with increased ROS production, which caused mitochondrial and liver damage, as well as dyslipidemia with further complications to the CV system. There are several possible pathways that could be responsible for such effects. ROS overproduction may cause lipid peroxidation, which forms a 4-hydroxynonenal-CPT1 adduct, responsible for impaired FAO and lipid removal from hepatocytes. In addition, ROS attack PUFAs, thus initiating lipid peroxidation and the formation of toxic aldehyde by-products (a hydroxy-2-nonenal and malondialdehyde), which have longer half-lives than ROS, and can spread from their site of origin to reach distant intracellular and extracellular targets, thereby amplifying the effects of OS [179].
Under normal physiologic conditions, oxidation of long- and medium-chain FAs is primarily run by the mitochondrial β-oxidation system, with only a minor contribution from the peroxisomal system. VLFAs (very-long-chain fatty acids) are not substrates of CPT-1, and thus cannot enter mitochondria; however, VLFAs are preferential substrates for peroxisomal β-oxidation [180]. Despite the presence in the peroxisome of the full enzymatic machinery to β-oxidize FAs, such oxidation normally is incomplete, and the final products of the peroxisomal β-oxidation are shuttled to mitochondria for complete oxidation to CO2, H2O, and final energy output. Some products of peroxisomal β-oxidation also may be used in other metabolic pathways (for example, by participating in the biosynthesis of the taurine and glycine conjugates, with subsequent export into the biliary ducts) [181].
FAs could also be subjected to ω-oxidation by the action of a microsomal oxidase that uses molecular oxygen, and both an alcohol and aldehyde dehydrogenase to produce dicarboxylic acids. These dicarboxylic acids can be further degraded by peroxisomal β-oxidation to succinate and acetyl-CoA, or completely oxidized after transport into the mitochondrial β-oxidation system [182]. Under physiologic conditions, ω-oxidation is a minor pathway of FA metabolism, but a failure of β-oxidation can result in increased ω-oxidation activity, with a production of excess dicarboxylic acids that are non-specific markers of mitochondrial FAO defects [183].
In the case of complete loss of mitochondrial FAO, the increased levels of serum hepatokines were detected (IGFBP1 (insulin-like growth factor-binding protein 1), GDF15 (growth/differentiation factor 15), and FGF21), suggesting their role in physiological adaptations to the high lipid burden from an HFD [73]. The liver attempts to compensate for the loss of FAO by up-regulating oxidative programming and seeking to increase catabolism in peripheral tissues with help of secreted hepatokines [184]. The primary target for those hepatokines is enhanced energy expenditure from adipose tissue (brown) and stimulation of browning (for white) [185]. Additionally, macrophages are also known to participate in BAT thermogenesis [186].
The important role of macrophages was also supported by recent research, in which macrophage FAO was defined as athero-protective, while inhibition of macrophage FAO may increase foam-cell formation and thereby exacerbate atherosclerosis [187]. Interestingly, FAO inhibition leads to increased ROS levels, probably due to the accumulation of toxic partially metabolized FAO substrates [188]. In this light, NAFLD-associated genes with documented anti-atherosclerotic or cardioprotective effects are intriguing, and we now further discuss some known cases for both metabolic and genetic NAFLD.
3. Cardioprotection
GCN2 (general control nonderepressible 2) is an amino-acid-availability sensor, identified in many organisms. In mammals, the highest level of GCN2 expression was detected in the liver and brain. Limited dietary proteins intake activates GCN2, which phosphorylates eIF2α (eukaryotic initiation factor 2 alpha) to inhibit global protein translation and stimulate de novo amino acid biosynthesis to restore homeostasis [189]. Further downstream targets are ATF4 (activating transcription factor 4) and CHOP (C/EBP homologous protein), which up-regulate autophagy and biosynthesis pathways [190]. Recently it was shown that GCN2 also participates in the regulation of hepatic lipid metabolism. For example, GCN2 deficiency significantly attenuated HFD-induced liver dysfunction, hepatic steatosis, and IR via regulation of lipogenic genes (SREBP-1/PPARγ) and their downstream targets (FASN, CD36, SCD1) [141]. Other research, focused on the combined effect of the GCN2 deficiency and exercise on hepatic steatosis, also defined involvement of the AMPK/SIRT1/PPARα pathway [142].
Interestingly, GCN2 deficiency was also shown to have a cardioprotective effect in diabetic hearts. In particular, GCN2 knockdown reduces OS, cell death, and lipid accumulation via inhibiting eIF2α-ATF4-CHOP signalling with the following reduction of Bcl-2/Bax ratio and UCP2 (uncoupling protein 2) expression [143,144]. Thus, GCN2 target therapy may be a promising strategy in the case of diabetic cardiomyopathy, and also as a treatment to reduce cardiotoxic side effects of popular anti-cancer drugs such as Doxorubicin.
Recent research helped to establish different mechanisms of the CVD risk in the case of NAFLD sub-types (metabolic and genetic). It is known that carriers of many SNP sites and mitochondrial mutations have a higher susceptibility to NAFLD [17]. However, a protective effect against CAD (coronary artery disease) was shown for several such SNPs [18], suggesting that every mutation site could imply a unique mechanism of NAFLD susceptibility/CVD protection [19]. Indeed, recent meta-analysis research showed that NAFLD susceptibility genes do not cause CAD per se [191]. Among NAFLD SNPs carriers, a strong correlation was observed for TC (total cholesterol) and LDL-C with CAD, but not for plasma TG and HDL-C. Overflow of FAs and de novo lipogenesis initiate fat accumulation in the liver and drive further VLDL production, thus shifting the plasma lipid balance into a pro-atherogenic and CVD-favouring environment [40]. However, some NAFLD-associated SNPs have impaired VLDL secretion (TM6SF2 and PNPLA3 (patatin-like phospholipase domain containing 3), and also MTTP and PEMT), thus decreasing plasma lipids and providing a cardioprotective effect [191].
Currently, PNPLA3 I148M is one of the best-studied NAFLD-associated mutations [192]. By itself, the Pnpla3148M variant is not harmful, and experimental mice on a standard diet had a normal level of liver fat [193]. However, the level of liver fat was 2–3 times higher under a high-sucrose diet, with an approximately 40-times-higher level of PNPLA3 protein on hepatic LDs, while Pnpla3 mRNA level was not changed [193]. Further, it was shown that a mutant variant could escape ubiquitination and proteasomal degradation, leading to such significant protein accumulation on LDs [194]. Normally, PNPLA3 acts as a PUFA-specific lipase or transacylase, yielding PUFA-containing PCs (phosphatidylcholines) or DAGs (diacylglycerols) that could be used for PC synthesis [195]. It is important to note that the Pnpla3148M variant and PNPLA3 deficiency had similar effects on liver lipid metabolism, thus preventing TG mobilization and causing lipid accumulation in hepatocytes. In certain conditions it could cause NAFLD development; however, for the cardiovascular system, such TG retention in the liver has a positive system-wide effect on the serum lipids profile, resulting in a lower risk of CVD [196].
TM6SF2 (transmembrane 6 superfamily member 2) is a transmembrane protein localized mainly in the ER and Golgi of enterocytes and hepatocytes. E167K mutations significantly reduce TM6SF2 protein levels, causing high hepatic accumulation of TG but low plasma level of LDL-C, and are thus responsible for a NALFD susceptibility and CVD protection, respectively [191]. TM6SF2 deficiency mimics the NAFLD phenotype, while liver-specific TM6SF2 OE elevates plasma TC and LDL-C levels [197]. ERLIN (ER lipid raft protein 1 and 2) proteins are ER-localized transmembrane glycoproteins that participate in the regulation of the cholesterol biosynthetic pathway by blocking the export of SREBPs from the ER to the Golgi under high-cholesterol conditions [198]. Recent research has found that TM6SF2 could bind and stabilize both ERLINs and APOB, therefore serving as a connective hub between ERLINs and APOB. E167K mutation in TM6SF2, equal to ERLIN or TM6SF2 deficiencies, leads to defective APOB stabilization, which is one of the key factors in the development of this sub-type of genetic NAFLD [150].
In total, these studies highlight the fundamental difference between metabolic and genetic NAFLD. Thus, for NAFLD patients carrying specific SNP sites, several independent molecular pathways could be involved in quicker NAFLD progression, but also accompanied by some extent of cardioprotection. In such cases, personalized, genotype-based medicine should be applied, based on presented SNP site/s, the severity of other symptoms, and the presence of other co-morbidities.
4. Pharmaceutical Strategies to Treat NAFLD and Reduce CVD Risk
The primary lifestyle interventions recommended for NAFLD patients are diet modifications and physical exercise. The most effective diet modification is a low-carbohydrate, ketogenic, low-fat and Mediterranean diet, which provides a positive effect on dyslipidemia, hepatic steatosis, and related comorbidities [76,199]. Similarly, different physical activities (high-intensity interval, aerobic, and resistance training) have been shown to reduce liver fat content and body weight, and improve plasma lipid status and IR. In addition, such exercises were followed by improvements in CVD risk factors, such as plasma levels of TG-rich VLDL1 particles and LDL-C, and reduced arterial stiffness [200]. Another type of effective NAFLD and NASH treatment, especially when accompanied with severe obesity, is bariatric surgery, which is aimed to mechanically reduce food intake [201]. Bariatric surgery also reduced the risk of CVD events among T2D and obese patients; thus, a similar effect would be also expected in the case of NASH patients [202].
Currently, there are no approved pharmacological therapies for NAFLD/NASH treatment. Existing treatments aim to reduce liver fat accumulation, stimulate metabolic pathways, and decrease liver injury. The main classes of such medications are: (1) bile acid metabolism modulators; (2) PPAR agonists; (3) thyroid hormone receptor β agonists; and (4) well-known T2DM drugs (such as GLP1-targeted drugs) [203]. Further, we focus on the FGF21 analogues, one of the most promising drugs for NAFLD/NASH treatment with documented effect on lowering CVD risk.
FGF21 (fibroblast growth factor 21) is an endocrine hormone of the FGF family, which is secreted mainly by the liver and exhibits diverse metabolic activities. The FGF21 signalling pathway begins with binding the co-receptors KLB (β-Klotho) and FGFR1 (FGF receptor 1), which forms an active FGF21/FGFR1/KLB receptor complex. Such a triple complex can phosphorylate ERK1/2 (extracellular signal-regulated kinases 1 and 2) and FRS2α (fibroblast growth factor receptor substrate 2 alpha) with multiple downstream targets [204]. The exact signalling cascade of FGF21 has not been fully resolved; it is known that in the liver, FGF21 expression is regulated by PPARα and could be also repressed by LXR [205,206]. FGF21 plays an important role in glucose and lipid metabolism, and insulin sensitivity. FGF21 is produced primarily by the liver in response to metabolic stresses, such as ketogenic diet or fasting, and is required for regulation of lipolysis, ketogenesis, and FAO [207]. Elevated levels of FGF21 (both circulation and mRNA) have been observed in cases of several metabolic disorders (NAFLD, obesity, T2DM), which suggests protective activities against those diseases [208]. In addition, FGF21 could be secreted from the brown adipose tissue and participate in thermoregulation in an ATF4-dependent way [209]. Several FGF21 analogues have been clinically tested as promising NAFLD treatments.
A long-acting FGF21 analogue, PF-05231023, had been tested on obese patients with and without T2DM. PF-05231023 significantly reduced TG levels, and increased HDL-C and adiponectin [210]. Another PEGylated FGF21 analogue, pegbelfermin, has been tested in a phase 2a study on NAFLD patients with obesity. In that study, 16 weeks of subcutaneous pegbelfermin administration resulted in a significant reduction of liver fat [211]. AKR-001 (FGF21 analogue) has a positive influence on lipoprotein profile (TG, nHDL-C, HDL-C, APOB, and APOC3) and improved insulin sensitivity [212,213]. Because FGF21 is quickly deactivated by proteolysis, some attempts have been made to prevent FGF21 cleavage by inhibiting its main protease FAP (seprase). BR103354, a FAP inhibitor, was tested in vitro, in mice and non-human primate models, where it was shown to reduce non-fasting glucose and TG levels, and improve hepatic steatosis and fibrosis. This suggests FAP inhibitors as potential anti-diabetic and anti-NASH medications [214].
The anti-atherosclerotic effect of FGF21 was studied in several clinical trials, where it was shown to significantly improve the cardiometabolic profile in obese patients with T2DM [215]. FGF21 therapy significantly improves lipid profiles, reduces vascular inflammation, and mitigates apoptosis and OS in atherosclerosis-related diseases [216].
Despite multiple positive outcomes in reports regarding safety and good tolerability of long-term FGF21 application, there are also several known drawbacks. As it was found in mice, FGF21 inhibits osteoblastogenesis via PPARγ, thus connecting bone turnover and energy metabolism [217]. Similar effects on the bone-turnover markers were also found n T2DM human patients in a trial for FGF21 analogue PF-05231023, where N-terminal propeptides and C-telopeptide cross-linking of type 1 collage were altered [210]. Further, depending on dose, up to 92% of subjects had increased titre of the anti-FGF21 antibodies, which raise the concern of immunogenicity in the case of long-term NAFLD treatment with FGF21 and its analogues [218]. However, it is necessary to note that despite the positive effect of FGF21 and its analogues on metabolic comorbidities of NAFLD and reduced liver fat, a recent systematic study suggests that better outcomes could be achieved with weight loss via diet modification and exercise [219].
In total, we could find a promising trend in developing NAFLD treatment. Currently, several medications able to treat NAFLD and provide CVD protection are in different stages of clinical trials. However, keeping in mind the defined drawbacks of the FGF21-based drugs, the efficacy of these therapies should be defined in long-term studies and in patients with severe NASH and a high risk of CVD.
5. Conclusions
The close association between NAFLD and CVD is supported by observations that CVD is the most common cause of death among NAFLD patients. Liver mitochondrial fatty-acid β-oxidation is the primary system that reacts to disbalances in nutrient flow. Subsequent alterations in the liver lipid metabolism drive NAFLD development, simultaneously creating a CVD-favouring pro-atherogenic environment via system-wide CK production, dyslipidemia, IR, and procoagulant imbalance. NAFLD development and lipid-profile alterations are regulated by a complex network of genes reacting to intracellular and environmental stresses, circadian rhythms, nutrients, and lifestyle. Currently, several promising NAFLD/NASH therapies with CVD-protecting activities are in development. However, given the extremely diverse nature of metabolic pathways involved in genetic NAFLD pathogenesis, a clear understanding of the underlying molecular mechanisms is required to provide effective care and treatment. Despite significant success in the understanding of NAFLD–CVD causality and a wide range of available pharmacological tools, great efforts should be oriented on the promotion of a healthy lifestyle, nutrition literacy, and smoking cessation, thus contributing to the prevention of the primary causes of NAFLD and CVD.
Author Contributions
S.A.D. and A.N.O. conceptualized the manuscript; S.A.D. wrote the manuscript text; M.S.B., T.V.P. and A.N.O. reviewed the text; E.E.B. and M.S.B. created the methodology; T.V.P. performed the formal analysis; E.E.B. and A.N.O. obtained funding and supervised. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant # 18-15-00254).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| ACAA2 | acetyl-CoA acyltransferase 2 |
| ACAD | acyl-CoA dehydrogenase |
| ACC | acetyl-CoA carboxylase |
| AITC | allyl isothiocyanate |
| AMPK | AMP-activated protein kinase |
| ANT2 | ADP/ATP translocase 2 |
| APOE | apolipoprotein E |
| ATF4 | activating transcription factor 4 |
| BAT | brown adipose tissue |
| CACT | acylcarnitine translocase |
| CASP1 | caspase-1 |
| CAT | catalase |
| CCl4 | carbon tetrachloride |
| CCN1 | cellular communication network factor 1 |
| CES1 | carboxylesterase 1 |
| ChO | chitosan oligosaccharide |
| CHOP | C/EBP homologous protein |
| ChREBP | carbohydrate-responsive element-binding protein |
| ClC-2 | chloride voltage-gated channel 2 |
| CLOCK | circadian locomotor output cycles kaput |
| CPT | carnitine palmitoyltransferase |
| CREBH | CAMP-responsive element-binding protein, hepatic-specific |
| CRLS1 | cardiolipin synthase 1 |
| CVD | Cardiovascular diseases |
| DAGs | diacylglycerols |
| DNL | de novo lipogenesis |
| DPP4 | dipeptidyl peptidase-4 |
| ECHS1 | enoyl-CoA hydratase, short chain 1 |
| ELAVL1 | RNA-binding protein HuR |
| ERK1/2 | extracellular signal-regulated kinases 1 and 2 |
| ETC | electron transport chain |
| FA | fatty acid |
| FABP1 | fatty-acid-binding protein 1 |
| FACS | fatty-acid synthase |
| FAT | fatty-acid translocase |
| FGF21 | fibroblast growth factor 21 |
| FGFR1 | FGF receptor 1 |
| FNDC5 | fibronectin type III domain-containing protein 5 |
| FOH | farnesol |
| FRS2α | fibroblast growth factor receptor substrate 2 alpha |
| GCN2 | general control nonderepressible 2 |
| GDF15 | growth/differentiation factor 15 |
| GGPPS | geranylgeranyl pyrophosphate synthase |
| GNMT | glycine N-methyltransferase |
| GRK2 | G protein-coupled receptor kinase 2 |
| HADH | hydroxyacyl-CoA dehydrogenase |
| HC | high cholesterol |
| HCC | hepatocellular carcinoma |
| HDL-C | high-density lipoprotein cholesterol |
| IL-6 | Interleukin 6 |
| IMP2 | insulin-like growth factor 2 mRNA binding protein 2 |
| IGFBP1 | Insulin-like growth factor-binding protein 1 |
| IR | insulin resistance |
| IRS1 | insulin receptor substrate 1 |
| JNK | c-Jun N-terminal kinase |
| KLB | β-Klotho |
| LAMP2A | lysosome-associated membrane protein 2A |
| LAP1 | lamina-associated polypeptide 1 |
| LCAD | long-chain acyl-CoA dehydrogenase |
| LCFAs | long-chain FAs |
| LCHAD | long-chain 3-hydoxyacyl-CoA dehydrogenase |
| LDH | lactate dehydrogenase |
| LDL | low-density lipoprotein |
| LKB1 | liver kinase B1 |
| LPGAT1 | lysophosphatidylglycerol acyltransferase 1 |
| LRP1 | LDL receptor-related protein-1 |
| LXR | liver X receptor |
| MCD | malonyl-CoA decarboxylase |
| MCJ | methylation-controlled J protein |
| MFN2 | mitofusin 2 |
| MetS | metabolic syndrome |
| MPC | mitochondrial pyruvate carrier 1 |
| MUPs | major urinary proteins |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NF-kB | nuclear factor kappa B |
| NLRP3 | NLR family pyrin domain-containing 3 |
| OS | oxidative stress |
| PA | palmitate |
| Plin5 | perilipin 5 |
| PCs | phosphatidylcholines |
| PDH | pyruvate dehydrogenase |
| PDK | pyruvate dehydrogenase kinase |
| PDP | pyruvate dehydrogenase phosphatase |
| PRX5 | peroxiredoxin |
| PTP1B | protein tyrosine phosphatase non-receptor type 1 |
| ROCK1 | rho-kinase 1 |
| RON | macrophage-stimulating 1 receptor |
| S100A11 | S100 calcium-binding protein A11 |
| SFA | saturated fatty acids |
| SIRT1 | sirtuin 1 |
| SLUG | snail family transcriptional repressor 2 |
| SMOC2 | secreted modular calcium-binding protein 2 |
| SOD1 | Cu/Zn-superoxide dismutase |
| SREBP1c | sterol regulatory element-binding protein-1c |
| STK25 | serine/threonine kinase 25 |
| T2DM | type 2 diabetes mellitus |
| TBK1 | TANK-binding kinase 1 |
| TC | total cholesterol |
| TCA cycle | tricarboxylic acid cycle |
| TGF-β | transforming growth factor beta |
| TFF3 | trefoil factor 3 |
| TM6SF2 | transmembrane 6 superfamily member 2 |
| TNF-α | tumor necrosis factor alpha |
| UCP2 | uncoupling protein 2 |
| VSIG4 | V-set and immunoglobulin domain-containing protein-4 |
| XBP1 | Xbp1-X-box binding protein 1 |
References
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2020. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Angelico, F.; Balla, A.; Paganini, A.M.; Cocomello, N.; Ferro, D.; Violi, F.; Sanyal, A.J.; Del Ben, M. Nonalcoholic Fatty Liver Disease and Fibrosis Associated With Increased Risk of Cardiovascular Events in a Prospective Study. Clin. Gastroenterol. Hepatol. 2020, 18, 2324–2331.e4. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, G.-C.; Tan, H.-Y.; Hao, F.-B.; Hu, J.-J. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis. Sci. Rep. 2019, 9, 11124. [Google Scholar] [CrossRef]
- Przybyszewski, E.M.; Targher, G.; Roden, M.; Corey, K.E. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease. Clin. Liver Dis. 2021, 17, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Stols-Gonçalves, D.; Hovingh, G.K.; Nieuwdorp, M.; Holleboom, A.G. NAFLD and Atherosclerosis: Two Sides of the Same Dysmetabolic Coin? Trends Endocrinol. Metab. 2019, 30, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, S.; Stanciu, C.; Girleanu, I.; Cojocariu, C.; Sfarti, C.; Singeap, A.-M.; Cuciureanu, T.; Huiban, L.; Muzica, C.M.; Zenovia, S.; et al. Nonalcoholic Fatty Liver Disease and Cardiovascular Diseases: The Heart of the Matter. Can. J. Gastroenterol. Hepatol. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primer 2019, 5, 56. [Google Scholar] [CrossRef]
- Niederseer, D.; Wernly, B.; Aigner, E.; Stickel, F.; Datz, C. NAFLD and Cardiovascular Diseases: Epidemiological, Mechanistic and Therapeutic Considerations. J. Clin. Med. 2021, 10, 467. [Google Scholar] [CrossRef]
- Oni, E.; Budoff, M.J.; Zeb, I.; Li, D.; Veledar, E.; Polak, J.F.; Blankstein, R.; Wong, N.D.; Blaha, M.J.; Agatston, A.; et al. Nonalcoholic Fatty Liver Disease Is Associated With Arterial Distensibility and Carotid Intima-Media Thickness: (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2019, 124, 534–538. [Google Scholar] [CrossRef]
- Xin, Z.; Zhu, Y.; Wang, S.; Liu, S.; Xu, M.; Wang, T.; Lu, J.; Chen, Y.; Zhao, Z.; Wang, W.; et al. Associations of subclinical atherosclerosis with nonalcoholic fatty liver disease and fibrosis assessed by non-invasive score. Liver Int. 2020, 40, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Allison, M.A.; Criqui, M.H.; Denenberg, J.O.; Wright, C.M. The association between liver fat and systemic calcified atherosclerosis. J. Vasc. Surg. 2020, 71, 204–211.e4. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, K.; Alazawi, W. Genetics of Non-Alcoholic Fatty Liver and Cardiovascular Disease: Implications for Therapy? Front. Pharmacol. 2020, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, M.A.; Ryzhkova, A.I.; Sinyov, V.V.; Galitsyna, E.V.; Melnichenko, A.A.; Demakova, N.A.; Sobenin, I.A.; Shkurat, T.P.; Orekhov, A.N. Mitochondrial Genome Mutations Associated with Myocardial Infarction. Dis. Markers 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Mitrofanov, K.Y.; Zhelankin, A.V.; Shiganova, G.M.; Sazonova, M.A.; Bobryshev, Y.V.; Postnov, A.Y.; Sobenin, I.A.; Orekhov, A.N. Analysis of mitochondrial DNA heteroplasmic mutations A1555G, C3256T, T3336C, С5178А, G12315A, G13513A, G14459A, G14846A and G15059A in CHD patients with the history of myocardial infarction. Exp. Mol. Pathol. 2016, 100, 87–91. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bezsonov, E.E.; Baig, M.S.; Popkova, T.V.; Nedosugova, L.V.; Starodubova, A.V.; Orekhov, A.N. Mitochondrial Mutations and Genetic Factors Determining NAFLD Risk. Int. J. Mol. Sci. 2021, 22, 4459. [Google Scholar] [CrossRef]
- Sliz, E.; Sebert, S.; Würtz, P.; Kangas, A.J.; Soininen, P.; Lehtimäki, T.; Kähönen, M.; Viikari, J.; Männikkö, M.; Ala-Korpela, M.; et al. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Hum. Mol. Genet. 2018, 27, 2214–2223. [Google Scholar] [CrossRef]
- Simons, N.; Isaacs, A.; Koek, G.H.; Kuč, S.; Schaper, N.C.; Brouwers, M.C.G.J. PNPLA3, TM6SF2, and MBOAT7 Genotypes and Coronary Artery Disease. Gastroenterology 2017, 152, 912–913. [Google Scholar] [CrossRef]
- Brouwers, M.C.G.J.; Simons, N.; Stehouwer, C.D.A.; Isaacs, A. Non-alcoholic fatty liver disease and cardiovascular disease: Assessing the evidence for causality. Diabetologia 2020, 63, 253–260. [Google Scholar] [CrossRef]
- Käräjämäki, A.J.; Hukkanen, J.; Kauma, H.; Kesäniemi, Y.A.; Ukkola, O. Metabolic syndrome but not genetic polymorphisms known to induce NAFLD predicts increased total mortality in subjects with NAFLD (OPERA study). Scand. J. Clin. Lab. Investig. 2020, 80, 106–113. [Google Scholar] [CrossRef]
- Grimaudo, S.; Pipitone, R.M.; Pennisi, G.; Celsa, C.; Cammà, C.; Di Marco, V.; Barcellona, M.R.; Boemi, R.; Enea, M.; Giannetti, A.; et al. Association Between PNPLA3 rs738409 C>G Variant and Liver-Related Outcomes in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 935–944.e3. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.T.; Vonghia, L.; Mogilenko, D.A.; Verrijken, A.; Molendi-Coste, O.; Fleury, S.; Deprince, A.; Nikitin, A.; Woitrain, E.; Ducrocq-Geoffroy, L.; et al. Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nat. Metab. 2019, 1, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation—A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Vos, D.Y.; van de Sluis, B. Function of the endolysosomal network in cholesterol homeostasis and metabolic-associated fatty liver disease (MAFLD). Mol. Metab. 2021, 101146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, H.S.; Cho, A.-R.; Lee, Y.-J.; Kwon, Y.-J. Non-Alcoholic Fatty Liver Disease Is an Independent Risk Factor for LDL Cholesterol Target Level. Int. J. Environ. Res. Public. Health 2021, 18, 3442. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N.; Japan Study Group of NAFLD (JSG-NAFLD). The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.D.; Armitage, A.E.; Cobbold, J.F.; Banerjee, R.; Borsani, O.; Dongiovanni, P.; Neubauer, S.; Morovat, R.; Wang, L.M.; Pasricha, S.-R.; et al. Hepatic iron is the major determinant of serum ferritin in NAFLD patients. Liver Int. 2018, 38, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Chen, J.; Luo, M.; Luo, J. Fetuin-A and Fetuin-B in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis and Meta-Regression. Int. J. Environ. Res. Public. Health 2020, 17, 2735. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, J.; Zhao, Z.; Wang, M.; Wang, Y.; Xin, Y. Systematic Review and Meta-analysis of Circulating Fetuin-A Levels in Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2020, 9, 3. [Google Scholar] [CrossRef]
- Jiménez, M.C.; Sun, Q.; Schürks, M.; Hu, F.B.; Manson, J.E.; Rexrode, K.M. Circulating Fetuin-A and Risk of Ischemic Stroke in Women. Clin. Chem. 2014, 60, 165–173. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Yilmaz, A.; Hoppe, U.C.; Schulze, P.C.; Kretzschmar, D.; Pistulli, R. Specifics of fetuin-A levels in distinct types of chronic heart failure. J. Clin. Lab. Anal. 2018, 32, e22179. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Selvaggio, F.; Sciuto, M.; Zanoli, L.; Yazdani, A.; He, F.; Marotta, F. Triglycerides to high-density lipoprotein cholesterol ratio for diagnosing nonalcoholic fatty liver disease. Minerva Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ebrahimi-mameghani, M.; Mobasseri, M.; Naghshi, S.; Ostadrahimi, A. The association of the steatosis severity, NAFLD fibrosis score and FIB-4 index with atherogenic dyslipidaemia in adult patients with NAFLD: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14131. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Leon, E.; Connelly, M.A.; Konomi, J.V.; Caltharp, S.; Cleeton, R.; Vos, M.B. Increased atherogenic lipoprotein profile in children with NON-ALCOHOLIC steatohepatitis. Pediatr. Obes. 2020, 15, e12648. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Ronca, A.; D’Erasmo, L.; Manfredini, M.; Baratta, F.; Pastori, D.; Di Martino, M.; Ceci, F.; Angelico, F.; Del Ben, M.; et al. HDL-Mediated Cholesterol Efflux and Plasma Loading Capacities Are Altered in Subjects with Metabolically- but Not Genetically Driven Non-Alcoholic Fatty Liver Disease (NAFLD). Biomedicines 2020, 8, 625. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ronda, N.; Bernini, F.; Zimetti, F. High Density Lipoprotein Cholesterol Efflux Capacity and Atherosclerosis in Cardiovascular Disease: Pathophysiological Aspects and Pharmacological Perspectives. Cells 2021, 10, 574. [Google Scholar] [CrossRef]
- Gordon, S.M.; Amar, M.J.; Jeiran, K.; Stagliano, M.; Staller, E.; Playford, M.P.; Mehta, N.N.; Vaisar, T.; Remaley, A.T. Effect of niacin monotherapy on high density lipoprotein composition and function. Lipids Health Dis. 2020, 19, 190. [Google Scholar] [CrossRef] [PubMed]
- Aissa, A.F.; Tryndyak, V.; de Conti, A.; Melnyk, S.; Gomes, T.D.U.H.; Bianchi, M.L.P.; James, S.J.; Beland, F.A.; Antunes, L.M.G.; Pogribny, I.P. Effect of methionine-deficient and methionine-supplemented diets on the hepatic one-carbon and lipid metabolism in mice. Mol. Nutr. Food Res. 2014, 58, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guan, Y.; Yang, X.; Xia, Z.; Wu, J. Association of Serum Homocysteine Levels with Histological Severity of NAFLD. J. Gastrointestin. Liver Dis. 2020, 29, 51–58. [Google Scholar] [CrossRef]
- Yuan, S.; Mason, A.M.; Carter, P.; Burgess, S.; Larsson, S.C. Homocysteine, B vitamins, and cardiovascular disease: A Mendelian randomization study. BMC Med. 2021, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Djuric, D.; Jakovljevic, V.; Zivkovic, V.; Srejovic, I. Homocysteine and homocysteine-related compounds: An overview of the roles in the pathology of the cardiovascular and nervous systems. Can. J. Physiol. Pharmacol. 2018, 96, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Fricker, Z.P.; Pedley, A.; Massaro, J.M.; Vasan, R.S.; Hoffmann, U.; Benjamin, E.J.; Long, M.T. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin. Gastroenterol. Hepatol. 2019, 17, 1157–1164.e4. [Google Scholar] [CrossRef]
- Katsarou, A.; Moustakas, I.I.; Pyrina, I.; Lembessis, P.; Koutsilieris, M.; Chatzigeorgiou, A. Metabolic inflammation as an instigator of fibrosis during non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 1993–2011. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, K.J.; eun Yoo, M.; Kim, G.; Yoon, H.; Jo, K.; Youn, J.-C.; Yun, M.; Park, J.Y.; Shim, C.Y.; et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J. Hepatol. 2018, 68, 764–772. [Google Scholar] [CrossRef]
- Chiu, L.S.; Pedley, A.; Massaro, J.M.; Benjamin, E.J.; Mitchell, G.F.; McManus, D.D.; Aragam, J.; Vasan, R.S.; Cheng, S.; Long, M.T. The association of non-alcoholic fatty liver disease and cardiac structure and function—Framingham Heart Study. Liver Int. 2020, 40, 2445–2454. [Google Scholar] [CrossRef]
- Hodson, L. Hepatic fatty acid synthesis and partitioning: The effect of metabolic and nutritional state. Proc. Nutr. Soc. 2019, 78, 126–134. [Google Scholar] [CrossRef]
- Roumans, K.H.M.; Basset Sagarminaga, J.; Peters, H.P.F.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Liver fat storage pathways: Methodologies and dietary effects. Curr. Opin. Lipidol. 2021, 32, 9–15. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Itoh, M.; Ogawa, Y.; Suganami, T. Chronic inflammation as a molecular basis of nonalcoholic steatohepatitis: Role of macrophages and fibroblasts in the liver. Nagoya J. Med. Sci. 2020, 82, 391–397. [Google Scholar] [PubMed]
- Chornyi, S.; IJlst, L.; van Roermund, C.W.T.; Wanders, R.J.A.; Waterham, H.R. Peroxisomal Metabolite and Cofactor Transport in Humans. Front. Cell Dev. Biol. 2021, 8, 613892. [Google Scholar] [CrossRef] [PubMed]
- Miotto, P.M.; Petrick, H.L.; Holloway, G.P. Acute insulin deprivation results in altered mitochondrial substrate sensitivity conducive to greater fatty acid transport. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E345–E353. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Fu, Q.; Ma, C.; Kruhlak, M.; Zhang, H.; Luo, J.; Heinrich, B.; Yu, S.J.; Zhang, Q.; Wilson, A.; et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4+ T cell apoptosis promoting HCC development. Cell Death Dis. 2018, 9, 620. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Bocco, B.M.L.C. Hepatic mediators of lipid metabolism and ketogenesis: Focus on fatty liver and diabetes. Curr. Diabetes Rev. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef]
- Colbert, C.L.; Kim, C.-W.; Moon, Y.-A.; Henry, L.; Palnitkar, M.; McKean, W.B.; Fitzgerald, K.; Deisenhofer, J.; Horton, J.D.; Kwon, H.J. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18820–18825. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, N.H.; Yun, J.S.; Cho, E.S.; Cha, Y.H.; Cho, S.B.; Lee, S.-H.; Cha, S.Y.; Kim, S.-Y.; Choi, J.; et al. Snail augments fatty acid oxidation by suppression of mitochondrial ACC2 during cancer progression. Life Sci. Alliance 2020, 3, e202000683. [Google Scholar] [CrossRef]
- Harriman, G.; Greenwood, J.; Bhat, S.; Huang, X.; Wang, R.; Paul, D.; Tong, L.; Saha, A.K.; Westlin, W.F.; Kapeller, R.; et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Natl. Acad. Sci. USA 2016, 113, E1796–E1805. [Google Scholar] [CrossRef]
- Goedeke, L.; Bates, J.; Vatner, D.F.; Perry, R.J.; Wang, T.; Ramirez, R.; Li, L.; Ellis, M.W.; Zhang, D.; Wong, K.E.; et al. Acetyl-CoA Carboxylase Inhibition Reverses NAFLD and Hepatic Insulin Resistance but Promotes Hypertriglyceridemia in Rodents: Hepatology. Hepatology 2018, 68, 2197–2211. [Google Scholar] [CrossRef]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. AMPK Re-Activation Suppresses Hepatic Steatosis but its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef]
- Ming, Y.; Yin, Y.; Sun, Z. Interaction of Nuclear Receptor Subfamily 4 Group A Member 1 (Nr4a1) and Liver Linase B1 (LKB1) Mitigates Type 2 Diabetes Mellitus by Activating Monophosphate-Activated Protein Kinase (AMPK)/Sirtuin 1 (SIRT1) Axis and Inhibiting Nuclear Factor-kappa B (NF-κB) Activation. Med. Sci. Monit. 2020, 26, e920278-1. [Google Scholar] [PubMed]
- Martínez-Jiménez, V.; Cortez-Espinosa, N.; Rodríguez-Varela, E.; Vega-Cárdenas, M.; Briones-Espinoza, M.; Ruíz-Rodríguez, V.M.; López-López, N.; Briseño-Medina, A.; Turiján-Espinoza, E.; Portales-Pérez, D.P. Altered levels of sirtuin genes (SIRT1, SIRT2, SIRT3 and SIRT6) and their target genes in adipose tissue from individual with obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, E.; Morgan, K.; French, S.W.; Morgan, T.R. Acetyl-l-carnitine and lipoic acid improve mitochondrial abnormalities and serum levels of liver enzymes in a mouse model of nonalcoholic fatty liver disease. Nutr. Res. 2013, 33, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.W.; Cho, W.K.; Jun, J.H.; Kwon, H.J.; Jang, K.-S.; Kim, H.-J.; Jeon, H.J.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; et al. Prevention of free fatty acid-induced hepatic lipotoxicity by carnitine via reversal of mitochondrial dysfunction. Liver Int. 2011, 31, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Frigini, E.N.; Barrera, E.E.; Pantano, S.; Porasso, R.D. Role of membrane curvature on the activation/deactivation of Carnitine Palmitoyltransferase 1A: A coarse grain molecular dynamic study. Biochim. Biophys. Acta BBA Biomembr. 2020, 1862, 183094. [Google Scholar] [CrossRef]
- Auguet, T.; Berlanga, A.; Guiu-Jurado, E.; Martinez, S.; Porras, J.; Aragonès, G.; Sabench, F.; Hernandez, M.; Aguilar, C.; Sirvent, J.; et al. Altered Fatty Acid Metabolism-Related Gene Expression in Liver from Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 22173–22187. [Google Scholar] [CrossRef]
- Naguib, G.; Morris, N.; Yang, S.; Fryzek, N.; Haynes-Williams, V.; Huang, W.A.; Norman-Wheeler, J.; Rotman, Y. Dietary fatty acid oxidation is decreased in non-alcoholic fatty liver disease: A palmitate breath test study. Liver Int. 2020, 40, 590–597. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Selen Alpergin, E.S.; Zhao, L.; Hartung, T.; Scafidi, S.; Riddle, R.C.; Wolfgang, M.J. Loss of Hepatic Mitochondrial Long-Chain Fatty Acid Oxidation Confers Resistance to Diet-Induced Obesity and Glucose Intolerance. Cell Rep. 2017, 20, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofano, M.; Ferramosca, A.; Di Giacomo, M.; Fusco, C.; Boscaino, F.; Luongo, D.; Vera Rotondi, A.; Maurano, F.; Cocca, E.; Mazzarella, G.; et al. Mechanisms underlying the hormetic effect of conjugated linoleic acid: Focus on Nrf2, mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2021, 167, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Selen, E.S.; Choi, J.; Wolfgang, M.J. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 2021, 6, e135626. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.-M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.A.; Deja, S.; Satapati, S.; Fu, X.; Burgess, S.C.; Browning, J.D. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 2019, 4, e127737. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Alterås, E.K.; Lindquist, C.; Svardal, A.; Skorve, J.; Berge, R.K. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr. Metab. 2018, 15, 10. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, Y.; Malik, V.; Li, X.; Peng, X.; Zhang, F.F.; Shan, Z.; Liu, L. Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages and Risk of Cardiovascular Disease: A Meta-Analysis and Systematic Review. Adv. Nutr. 2021, 12, 89–101. [Google Scholar] [CrossRef]
- Haslam, D.E.; Peloso, G.M.; Herman, M.A.; Dupuis, J.; Lichtenstein, A.H.; Smith, C.E.; McKeown, N.M. Beverage Consumption and Longitudinal Changes in Lipoprotein Concentrations and Incident Dyslipidemia in US Adults: The Framingham Heart Study. J. Am. Heart Assoc. 2020, 9, e014083. [Google Scholar] [CrossRef]
- Mamounis, K.J.; Yasrebi, A.; Roepke, T.A. Linoleic acid causes greater weight gain than saturated fat without hypothalamic inflammation in the male mouse. J. Nutr. Biochem. 2017, 40, 122–131. [Google Scholar] [CrossRef]
- Ghosh, S.; O’Connell, J.F.; Carlson, O.D.; González-Mariscal, I.; Kim, Y.; Moaddel, R.; Ghosh, P.; Egan, J.M. Linoleic acid in diets of mice increases total endocannabinoid levels in bowel and liver: Modification by dietary glucose. Obes. Sci. Pract. 2019, 5, 383–394. [Google Scholar] [CrossRef]
- Chandra, A.; Røsjø, H.; Svensson, M.; Vigen, T.; Ihle-Hansen, H.; Orstad, E.B.; Rønning, O.M.; Lyngbakken, M.N.; Nygård, S.; Berge, T.; et al. Plasma linoleic acid levels and cardiovascular risk factors: Results from the Norwegian ACE 1950 Study. Eur. J. Clin. Nutr. 2020, 74, 1707–1717. [Google Scholar] [CrossRef]
- Softic, S.; Gupta, M.K.; Wang, G.-X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K. Fructose-mediated effects on gene expression and epigenetic mechanisms associated with NAFLD pathogenesis. Cell. Mol. Life Sci. 2020, 77, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.-J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Shin, H.-S.; Choi, H.S.; Park, J.-W.; Jo, I.; Oh, E.-S.; Lee, K.-Y.; Lee, B.-H.; Johnson, R.J.; Kang, D.-H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef]
- Vavrova, E.; Lenoir, V.; Alves-Guerra, M.-C.; Denis, R.G.; Castel, J.; Esnous, C.; Dyck, J.R.B.; Luquet, S.; Metzger, D.; Bouillaud, F.; et al. Muscle expression of a malonyl-CoA-insensitive carnitine palmitoyltransferase-1 protects mice against high-fat/high-sucrose diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E649–E660. [Google Scholar] [CrossRef] [PubMed]
- Van Weeghel, M.; Abdurrachim, D.; Nederlof, R.; Argmann, C.A.; Houtkooper, R.H.; Hagen, J.; Nabben, M.; Denis, S.; Ciapaite, J.; Kolwicz, S.C.; et al. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-CoA sensitivity of CPT1B. Cardiovasc. Res. 2018, 114, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Meyer, J.G.; Wang, G.-X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.P.M.M.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab. 2019, 30, 735–753.e4. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-L.; Qiu, G.; Pian, L.; Guo, L.; Cao, H.; Liu, J.; Zhao, Y.; Li, X.; Xu, Z.; et al. Hepatic neddylation targets and stabilizes electron transfer flavoproteins to facilitate fatty acid β-oxidation. Proc. Natl. Acad. Sci. USA 2020, 117, 2473–2483. [Google Scholar] [CrossRef]
- Montagna, C.; Cirotti, C.; Rizza, S.; Filomeni, G. When S -Nitrosylation Gets to Mitochondria: From Signaling to Age-Related Diseases. Antioxid. Redox Signal. 2020, 32, 884–905. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Gao, X.; Li, L.; Yuan, Y.; Liu, F.; Zhang, L.; Wu, J.; Hu, P.; Zhang, X.; et al. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology 2015, 61, 870–882. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Chen, L.; Liu, Y.; Ren, Z. Reactive Oxygen Species Induces Lipid Droplet Accumulation in HepG2 Cells by Increasing Perilipin 2 Expression. Int. J. Mol. Sci. 2018, 19, 3445. [Google Scholar] [CrossRef] [PubMed]
- Trevino, M.B.; Mazur-Hart, D.; Machida, Y.; King, T.; Nadler, J.; Galkina, E.V.; Poddar, A.; Dutta, S.; Imai, Y. Liver Perilipin 5 Expression Worsens Hepatosteatosis But Not Insulin Resistance in High Fat-Fed Mice. Mol. Endocrinol. 2015, 29, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zheng, S.; Attie, A.D.; Keller, M.P.; Bernlohr, D.A.; Blaner, W.S.; Newberry, E.P.; Davidson, N.O.; Chen, A. Perilipin 5 and liver fatty acid binding protein function to restore quiescence in mouse hepatic stellate cells. J. Lipid Res. 2018, 59, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, A. Perilipin 5 restores the formation of lipid droplets in activated hepatic stellate cells and inhibits their activation. Lab. Investig. 2016, 96, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.N.; Meex, R.C.; Lo, J.C.Y.; Ryan, A.; Nie, S.; Montgomery, M.K.; Watt, M.J. Perilipin 5 Deletion in Hepatocytes Remodels Lipid Metabolism and Causes Hepatic Insulin Resistance in Mice. Diabetes 2019, 68, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sreenivasan, U.; Hu, H.; Saladino, A.; Polster, B.M.; Lund, L.M.; Gong, D.; Stanley, W.C.; Sztalryd, C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res. 2011, 52, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Loh, K.; Song, Z.; Yang, H.; Zhang, Y.; Lin, S. Update on glycerol-3-phosphate acyltransferases: The roles in the development of insulin resistance. Nutr. Diabetes 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Benador, I.Y.; Veliova, M.; Mahdaviani, K.; Petcherski, A.; Wikstrom, J.D.; Assali, E.A.; Acín-Pérez, R.; Shum, M.; Oliveira, M.F.; Cinti, S.; et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018, 27, 869–885.e6. [Google Scholar] [CrossRef]
- Tan, Y.; Jin, Y.; Wang, Q.; Huang, J.; Wu, X.; Ren, Z. Ren Perilipin 5 Protects against Cellular Oxidative Stress by Enhancing Mitochondrial Function in HepG2 Cells. Cells 2019, 8, 1241. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Engel, K.M.; Gassler, N.; Bracht, T.; Sitek, B.; Buhl, E.M.; Kalampoka, S.; Pinoé-Schmidt, M.; van Helden, J.; Schiller, J.; et al. Deletion of Perilipin 5 Protects against Hepatic Injury in Nonalcoholic Fatty Liver Disease via Missing Inflammasome Activation. Cells 2020, 9, 1346. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Vucur, M.; Luedde, T.; Schneiders, S.; Kalampoka, S.; Weiss, T.; Weiskirchen, R. Perilipin 5 and Lipocalin 2 Expression in Hepatocellular Carcinoma. Cancers 2019, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, M.; Han, X.; Bi, Y.; Zhang, W.; Wu, Z.; Wu, G. Perilipin 5 deficiency promotes atherosclerosis progression through accelerating inflammation, apoptosis, and oxidative stress. J. Cell. Biochem. 2019, 120, 19107–19123. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Z.; Liu, P.; Zhang, S. Comparative proteomic study of liver lipid droplets and mitochondria in mice housed at different temperatures. FEBS Lett. 2019, 593, 2118–2138. [Google Scholar] [CrossRef] [PubMed]
- Badenes, M.; Amin, A.; González-García, I.; Félix, I.; Burbridge, E.; Cavadas, M.; Ortega, F.J.; de Carvalho, É.; Faísca, P.; Carobbio, S.; et al. Deletion of iRhom2 protects against diet-induced obesity by increasing thermogenesis. Mol. Metab. 2020, 31, 67–84. [Google Scholar] [CrossRef]
- Giles, D.A.; Moreno-Fernandez, M.E.; Stankiewicz, T.E.; Graspeuntner, S.; Cappelletti, M.; Wu, D.; Mukherjee, R.; Chan, C.C.; Lawson, M.J.; Klarquist, J.; et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017, 23, 829–838. [Google Scholar] [CrossRef]
- Mass Sanchez, P.B.; Krizanac, M.; Weiskirchen, R.; Asimakopoulos, A. Understanding the Role of Perilipin 5 in Non-Alcoholic Fatty Liver Disease and Its Role in Hepatocellular Carcinoma: A Review of Novel Insights. Int. J. Mol. Sci. 2021, 22, 5284. [Google Scholar] [CrossRef] [PubMed]
- Herker, E.; Vieyres, G.; Beller, M.; Krahmer, N.; Bohnert, M. Lipid Droplet Contact Sites in Health and Disease. Trends Cell Biol. 2021, 31, 345–358. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, H.; Qiu, S.; Chen, G.; Chen, Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: A retrospective cohort research. Lipids Health Dis. 2019, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Shi, D.; Ding, J.; Cheng, Z.Y.; Li, H.Y.; Li, J.S.; Pu, H.Q.; Yang, A.M.; He, C.L.; Zhang, J.P.; et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. 2019, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Zaccardi, F.; Karppi, J.; Kurl, S.; Laukkanen, J.A. Is High Serum LDL/HDL Cholesterol Ratio an Emerging Risk Factor for Sudden Cardiac Death? Findings from the KIHD Study. J. Atheroscler. Thromb. 2017, 24, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef]
- Huang, D.; Liu, B.; Huang, K.; Huang, K. Enoyl coenzyme A hydratase 1 protects against high-fat-diet-induced hepatic steatosis and insulin resistance. Biochem. Biophys. Res. Commun. 2018, 499, 403–409. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Sun, H.; Liu, X.; Zheng, Y.; Xu, D.; Wang, J.; Jia, D.; Han, X.; Liu, F.; et al. Defective Phosphatidylglycerol Remodeling Causes Hepatopathy, Linking Mitochondrial Dysfunction to Hepatosteatosis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Xiong, H.; Hu, Y.; Wang, W.; Mei, G.; Wang, H.; Li, Y.; Zhou, Z.; Meng, F.; Zhang, P.; et al. Cardiolipin Synthase 1 Ameliorates NASH Through Activating Transcription Factor 3 Transcriptional Inactivation. Hepatology 2020, 72, 1949–1967. [Google Scholar] [CrossRef]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernández, V.; Vasconcelos, N.; et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tussy, P.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; Simón, J.; Barbier-Torres, L.; Gomez-Santos, B.; Nuñez-Garcia, M.; Azkargorta, M.; Gutiérrez-de Juan, V.; Serrano-Macia, M.; et al. miR-873-5p targets mitochondrial GNMT-Complex II interface contributing to non-alcoholic fatty liver disease. Mol. Metab. 2019, 29, 40–54. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Wang, Y.; Zhang, G.; Cheng, Y.; Feng, Z.; Bai, X.; Liu, J. High ratio of ω-3/ω-6 polyunsaturated fatty acids targets mTORC1 to prevent high-fat diet-induced metabolic syndrome and mitochondrial dysfunction in mice. J. Nutr. Biochem. 2020, 79, 108330. [Google Scholar] [CrossRef]
- Kang, S.G.; Choi, M.J.; Jung, S.-B.; Chung, H.K.; Chang, J.Y.; Kim, J.T.; Kang, Y.E.; Lee, J.H.; Hong, H.J.; Jun, S.M.; et al. Differential roles of GDF15 and FGF21 in systemic metabolic adaptation to the mitochondrial integrated stress response. iScience 2021, 24, 102181. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Kwon, Y.; Hartleben, G.; Jülg, J.; Sekar, R.; Krahmer, N.; Najafi, B.; Loft, A.; Gancheva, S.; Stemmer, K.; et al. Hepatic Rab24 controls blood glucose homeostasis via improving mitochondrial plasticity. Nat. Metab. 2019, 1, 1009–1026. [Google Scholar] [CrossRef]
- Barbier-Torres, L.; Fortner, K.A.; Iruzubieta, P.; Delgado, T.C.; Giddings, E.; Chen, Y.; Champagne, D.; Fernández-Ramos, D.; Mestre, D.; Gomez-Santos, B.; et al. Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat. Commun. 2020, 11, 3360. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Zhang, Y.; Park, S.-Y.; Joseph, A.-M.; Han, C.; Park, H.-J.; Kalavalapalli, S.; Chun, S.-K.; Morgan, D.; Kim, J.-S.; et al. Mitochondrial ATP transporter depletion protects mice against liver steatosis and insulin resistance. Nat. Commun. 2017, 8, 14477. [Google Scholar] [CrossRef]
- Cruces-Sande, M.; Vila-Bedmar, R.; Arcones, A.C.; González-Rodríguez, Á.; Rada, P.; Gutiérrez-de-Juan, V.; Vargas-Castrillón, J.; Iruzubieta, P.; Sánchez-González, C.; Formentini, L.; et al. Involvement of G protein-coupled receptor kinase 2 (GRK2) in the development of non-alcoholic steatosis and steatohepatitis in mice and humans. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Ruddy, M.; Chakravarthy, M.; et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab. 2017, 26, 394–406.e6. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Ikehara, T.; Kashiwagi, Y.; Hashimoto, K.; Nanchi, I.; Shimazaki, A.; Nambu, H.; Yukioka, H. ACC2 Deletion Enhances IMCL Reduction Along With Acetyl-CoA Metabolism and Improves Insulin Sensitivity in Male Mice. Endocrinology 2018, 159, 3007–3019. [Google Scholar] [CrossRef]
- Huang, H.; Lee, S.-H.; Sousa-Lima, I.; Kim, S.S.; Hwang, W.M.; Dagon, Y.; Yang, W.-M.; Cho, S.; Kang, M.-C.; Seo, J.A.; et al. Rho-kinase/AMPK axis regulates hepatic lipogenesis during overnutrition. J. Clin. Investig. 2018, 128, 5335–5350. [Google Scholar] [CrossRef]
- Li, C.-X.; Gao, J.-G.; Wan, X.-Y.; Chen, Y.; Xu, C.-F.; Feng, Z.-M.; Zeng, H.; Lin, Y.-M.; Ma, H.; Xu, P.; et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J. Gastroenterol. 2019, 25, 5120–5133. [Google Scholar] [CrossRef]
- Goetzman, E.S.; Bharathi, S.S.; Zhang, Y.; Zhao, X.-J.; Dobrowolski, S.F.; Peasley, K.; Sims-Lucas, S.; Monga, S.P. Impaired mitochondrial medium-chain fatty acid oxidation drives periportal macrovesicular steatosis in sirtuin-5 knockout mice. Sci. Rep. 2020, 10, 18367. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, Q.; Song, N.; Yan, Z.; Lin, R.; Wu, S.; Jiang, L.; Hong, S.; Xie, J.; Zhou, H.; et al. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020, 11, 5807. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Zhao, Y.; Sun, Q.; Zhang, J.; Shen, D.; Wu, J.; Shen, N.; Fu, X.; Sun, X.; et al. Geranylgeranyl diphosphate synthase (GGPPS) regulates non-alcoholic fatty liver disease (NAFLD)-fibrosis progression by determining hepatic glucose/fatty acid preference under high-fat diet conditions: GGPPS regulates NAFLD-fibrosis by glycolysis. J. Pathol. 2018, 246, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Zhang, J.; Liu, B.; Deng, X.; Xin, S.; Xu, K. N-glycosylation of CREBH improves lipid metabolism and attenuates lipotoxicity in NAFLD by modulating PPARα and SCD-1. FASEB J. 2020, 34, 15338–15363. [Google Scholar] [CrossRef]
- Regué, L.; Minichiello, L.; Avruch, J.; Dai, N. Liver-specific deletion of IGF2 mRNA binding protein-2/IMP2 reduces hepatic fatty acid oxidation and increases hepatic triglyceride accumulation. J. Biol. Chem. 2019, 294, 11944–11951. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Rondini, E.A.; Kocarek, T.A. Farnesol induces fatty acid oxidation and decreases triglyceride accumulation in steatotic HepaRG cells. Toxicol. Appl. Pharmacol. 2019, 365, 61–70. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, H.; Yang, R.; Luan, X.; Zhang, L.; Jin, Q.; Jin, Y.; Xue, J. Mouse trefoil factor 3 ameliorated high-fat-diet-induced hepatic steatosis via increasing peroxisome proliferator-activated receptor-α-mediated fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E436–E445. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Mera, P.; Casas, J.; Salvador, J.; Rodríguez, A.; Alonso, S.; Sebastián, D.; Soler-Vázquez, M.C.; Montironi, C.; Recalde, S.; et al. Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD. FASEB J. 2020, 34, 11816–11837. [Google Scholar] [CrossRef]
- Mohammed, S.; Nicklas, E.H.; Thadathil, N.; Selvarani, R.; Royce, G.H.; Kinter, M.; Richardson, A.; Deepa, S.S. Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radic. Biol. Med. 2021, 164, 315–328. [Google Scholar] [CrossRef]
- Kim, M.H.; Seong, J.B.; Huh, J.-W.; Bae, Y.C.; Lee, H.-S.; Lee, D.-S. Peroxiredoxin 5 ameliorates obesity-induced non-alcoholic fatty liver disease through the regulation of oxidative stress and AMP-activated protein kinase signaling. Redox Biol. 2020, 28, 101315. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, H.-W.; Song, S.-E.; Bae, J.-H.; Im, S.-S.; Hwang, I.; Ha, H.; Song, D.-K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflüg. Arch. Eur. J. Physiol. 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Yue, W.; Bi, Y.; Shen, X.; Gao, J.; Xu, X.; Lu, Z. GCN2 deficiency protects against high fat diet induced hepatic steatosis and insulin resistance in mice. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 3257–3267. [Google Scholar] [CrossRef]
- Luo, X.; Shi, X.; Sun, Z.; Xiao, J.; Song, H.; Lu, G.; Xu, X. GCN2 Deficiency Enhances Protective Effects of Exercise on Hepatic Steatosis. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar]
- Feng, W.; Lei, T.; Wang, Y.; Feng, R.; Yuan, J.; Shen, X.; Wu, Y.; Gao, J.; Ding, W.; Lu, Z. GCN2 deficiency ameliorates cardiac dysfunction in diabetic mice by reducing lipotoxicity and oxidative stress. Free Radic. Biol. Med. 2019, 130, 128–139. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, T.; Yuan, J.; Wu, Y.; Shen, X.; Gao, J.; Feng, W.; Lu, Z. GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol. 2018, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Liu, M.; Xi, Y.; Chen, M.; Tian, L.; Xie, J.; Chen, M.; Wang, Z.; Yang, M.; Yu, Z.; et al. Long-term exercise prevents hepatic steatosis: A novel role of FABP1 in regulation of autophagy-lysosomal machinery. FASEB J. 2019, 33, 11870–11883. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.N.; Dey, A.; Cai, J.; Zhang, J.; Tian, Y.; Kennett, M.; Ma, Y.; Liang, T.J.; Patterson, A.D.; Hankey-Giblin, P.A. Metabolic Profiling Reveals Aggravated Non-Alcoholic Steatohepatitis in High-Fat High-Cholesterol Diet-Fed Apolipoprotein E-Deficient Mice Lacking Ron Receptor Signaling. Metabolites 2020, 10, 326. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; An, W.; Song, J.; Zhang, Y.; Zhao, X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2018, 129, 546–555. [Google Scholar] [CrossRef]
- Nardo, A.D.; Grün, N.G.; Zeyda, M.; Dumanic, M.; Oberhuber, G.; Rivelles, E.; Helbich, T.H.; Markgraf, D.F.; Roden, M.; Claudel, T.; et al. Impact of osteopontin on the development of non-alcoholic liver disease and related hepatocellular carcinoma. Liver Int. 2020, 40, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, A.; Haller, A.; Cash, J.G.; Nam, C.; Igel, E.; Roebroek, A.J.M.; Hui, D.Y. Mutation in the distal NPxY motif of LRP1 alleviates dietary cholesterol-induced dyslipidemia and tissue inflammation. J. Lipid Res. 2021, 62, 100012. [Google Scholar] [CrossRef]
- Li, B.-T.; Sun, M.; Li, Y.-F.; Wang, J.-Q.; Zhou, Z.-M.; Song, B.-L.; Luo, J. Disruption of the ERLIN–TM6SF2–APOB complex destabilizes APOB and contributes to non-alcoholic fatty liver disease. PLoS Genet. 2020, 16, e1008955. [Google Scholar] [CrossRef]
- Ma, S.Y.; Sun, K.S.; Zhang, M.; Zhou, X.; Zheng, X.H.; Tian, S.Y.; Liu, Y.S.; Chen, L.; Gao, X.; Ye, J.; et al. Disruption of Plin5 degradation by CMA causes lipid homeostasis imbalance in NAFLD. Liver Int. 2020, 40, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Khare, S.; Angdisen, J.J.; Zhang, Q.; Stuckel, A.; Mooney, B.P.; Ridenhour, S.E.; Gitan, R.S.; Hammoud, G.M.; Ibdah, J.A. Defects in long-chain 3-hydroxy acyl-CoA dehydrogenase lead to hepatocellular carcinoma: A novel etiology of hepatocellular carcinoma. Int. J. Cancer 2020, 147, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Queiroz, J.; Hussain, M.M. Nonalcoholic fatty liver disease in CLOCK mutant mice. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, H.; Jiang, L.; Shang, Q.; Yin, L.; Lin, J.D.; Wu, W.-S.; Rui, L. Hepatic Slug epigenetically promotes liver lipogenesis, fatty liver disease, and type 2 diabetes. J. Clin. Investig. 2020, 130, 2992–3004. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Hernandez-Ono, A.; Fedotova, T.; Östlund, C.; Lee, M.J.; Gibeley, S.B.; Liang, C.-C.; Dauer, W.T.; Ginsberg, H.N.; Worman, H.J. Nuclear envelope–localized torsinA-LAP1 complex regulates hepatic VLDL secretion and steatosis. J. Clin. Investig. 2019, 129, 4885–4900. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Gonzales, N.M.; Lonard, D.M.; Putluri, N.; Zhu, B.; Dacso, C.C.; York, B.; O’Malley, B.W. XBP1 links the 12-hour clock to NAFLD and regulation of membrane fluidity and lipid homeostasis. Nat. Commun. 2020, 11, 6215. [Google Scholar] [CrossRef]
- Huh, J.Y.; Reilly, S.M.; Abu-Odeh, M.; Murphy, A.N.; Mahata, S.K.; Zhang, J.; Cho, Y.; Seo, J.B.; Hung, C.-W.; Green, C.R.; et al. TANK-Binding Kinase 1 Regulates the Localization of Acyl-CoA Synthetase ACSL1 to Control Hepatic Fatty Acid Oxidation. Cell Metab. 2020, 32, 1012–1027.e7. [Google Scholar] [CrossRef]
- Liou, C.-J.; Wei, C.-H.; Chen, Y.-L.; Cheng, C.-Y.; Wang, C.-L.; Huang, W.-C. Fisetin Protects Against Hepatic Steatosis Through Regulation of the Sirt1/AMPK and Fatty Acid β-Oxidation Signaling Pathway in High-Fat Diet-Induced Obese Mice. Cell. Physiol. Biochem. 2018, 49, 1870–1884. [Google Scholar] [CrossRef]
- Liou, C.-J.; Wu, S.-J.; Shen, S.-C.; Chen, L.-C.; Chen, Y.-L.; Huang, W.-C. Phloretin ameliorates hepatic steatosis through regulation of lipogenesis and Sirt1/AMPK signaling in obese mice. Cell Biosci. 2020, 10, 114. [Google Scholar] [CrossRef]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef]
- Domínguez-Pérez, M.; Simoni-Nieves, A.; Rosales, P.; Nuño-Lámbarri, N.; Rosas-Lemus, M.; Souza, V.; Miranda, R.U.; Bucio, L.; Uribe Carvajal, S.; Marquardt, J.U.; et al. Cholesterol burden in the liver induces mitochondrial dynamic changes and resistance to apoptosi. J. Cell. Physiol. 2019, 234, 7213–7223. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, C.; Bjørndal, B.; Bakke, H.G.; Slettom, G.; Karoliussen, M.; Rustan, A.C.; Thoresen, G.H.; Skorve, J.; Nygård, O.K.; Berge, R.K. A mitochondria-targeted fatty acid analogue influences hepatic glucose metabolism and reduces the plasma insulin/glucose ratio in male Wistar rats. PLoS ONE 2019, 14, e0222558. [Google Scholar] [CrossRef] [PubMed]
- Muyyarikkandy, M.S.; McLeod, M.; Maguire, M.; Mahar, R.; Kattapuram, N.; Zhang, C.; Surugihalli, C.; Muralidaran, V.; Vavilikolanu, K.; Mathews, C.E.; et al. Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD. FASEB J. 2020, 34, 14832–14849. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Hsieh, Y.-C.; Chan, C.-C.; Sun, H.-J.; Huang, Y.-H.; Hou, M.-C.; Lin, H.-C. Human relaxin-2 attenuates hepatic steatosis and fibrosis in mice with non-alcoholic fatty liver disease. Lab. Investig. 2019, 99, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, B.; Fan, M.; Candas-Green, D.; Jiang, J.X.; Wei, R.; Wang, Y.; Chen, H.-W.; Hu, Y.; Li, J.J. Low-Level Saturated Fatty Acid Palmitate Benefits Liver Cells by Boosting Mitochondrial Metabolism via CDK1-SIRT3-CPT2 Cascade. Dev. Cell 2020, 52, 196–209.e9. [Google Scholar] [CrossRef]
- Lian, J.; Watts, R.; Quiroga, A.D.; Beggs, M.R.; Alexander, R.T.; Lehner, R. Ces1d deficiency protects against high-sucrose diet-induced hepatic triacylglycerol accumulation. J. Lipid Res. 2019, 60, 880–891. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.-P.; Wang, J.; Lu, W.-H.; Xie, L.-Y.; Lv, J.; Li, H.-X.; Yang, S.-F. Expression of Vsig4 attenuates macrophage-mediated hepatic inflammation and fibrosis in high fat diet (HFD)-induced mice. Biochem. Biophys. Res. Commun. 2019, 516, 858–865. [Google Scholar] [CrossRef]
- Ju, L.; Sun, Y.; Xue, H.; Chen, L.; Gu, C.; Shao, J.; Lu, R.; Luo, X.; Wei, J.; Ma, X.; et al. CCN1 promotes hepatic steatosis and inflammation in non-alcoholic steatohepatitis. Sci. Rep. 2020, 10, 3201. [Google Scholar] [CrossRef]
- Lee, M.; Shin, E.; Bae, J.; Cho, Y.; Lee, J.-Y.; Lee, Y.; Lee, B.-W.; Kang, E.S.; Cha, B.-S. Dipeptidyl peptidase-4 inhibitor protects against non-alcoholic steatohepatitis in mice by targeting TRAIL receptor-mediated lipoapoptosis via modulating hepatic dipeptidyl peptidase-4 expression. Sci. Rep. 2020, 10, 19429. [Google Scholar] [CrossRef]
- Cansby, E.; Nuñez-Durán, E.; Magnusson, E.; Amrutkar, M.; Booten, S.L.; Kulkarni, N.M.; Svensson, L.T.; Borén, J.; Marschall, H.-U.; Aghajan, M.; et al. Targeted Delivery of Stk25 Antisense Oligonucleotides to Hepatocytes Protects Mice Against Nonalcoholic Fatty Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 597–618. [Google Scholar] [CrossRef]
- Yuting, Y.; Lifeng, F.; Qiwei, H. Secreted modular calcium-binding protein 2 promotes high fat diet (HFD)-induced hepatic steatosis through enhancing lipid deposition, fibrosis and inflammation via targeting TGF-β1. Biochem. Biophys. Res. Commun. 2019, 509, 48–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Zong, C.; Jiang, M.; Hu, H.; Cheng, X.; Ni, J.; Yi, X.; Jiang, B.; Tian, F.; Chang, M.-W.; et al. Hepatic HuR modulates lipid homeostasis in response to high-fat diet. Nat. Commun. 2020, 11, 3067. [Google Scholar] [CrossRef] [PubMed]
- Canivet, C.M.; Bonnafous, S.; Rousseau, D.; Leclere, P.S.; Lacas-Gervais, S.; Patouraux, S.; Sans, A.; Luci, C.; Bailly-Maitre, B.; Iannelli, A.; et al. Hepatic FNDC5 is a potential local protective factor against Non-Alcoholic Fatty Liver. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Li, C.; Zhu, T.; Gao, J.; Zhou, H.; Zheng, Y.; Chang, Q.; Wang, M.; Wu, J.; et al. S100A11 Promotes Liver Steatosis via FOXO1-Mediated Autophagy and Lipogenesis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 697–724. [Google Scholar] [CrossRef] [PubMed]
- Mirea, A.-M.; Stienstra, R.; Kanneganti, T.-D.; Tack, C.J.; Chavakis, T.; Toonen, E.J.M.; Joosten, L.A.B. Mice Deficient in the IL-1β Activation Genes Prtn3, Elane, and Casp1 Are Protected Against the Development of Obesity-Induced NAFLD. Inflammation 2020, 43, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Cui, H.; Zhang, Y. Lack of ClC-2 Alleviates High Fat Diet-Induced Insulin Resistance and Non-Alcoholic Fatty Liver Disease. Cell. Physiol. Biochem. 2018, 45, 2187–2198. [Google Scholar] [CrossRef]
- Shum, M.; Shintre, C.A.; Althoff, T.; Gutierrez, V.; Segawa, M.; Saxberg, A.D.; Martinez, M.; Adamson, R.; Young, M.R.; Faust, B.; et al. ABCB10 exports mitochondrial biliverdin, driving metabolic maladaptation in obesity. Sci. Transl. Med. 2021, 13, eabd1869. [Google Scholar] [CrossRef]
- McGlaughon, J.L.; Pasquali, M.; Wallace, K.; Ross, J.; Senol-Cosar, O.; Shen, W.; Weaver, M.A.; Feigenbaum, A.; Lyon, E.; Enns, G.M.; et al. Assessing the strength of evidence for genes implicated in fatty acid oxidation disorders using the ClinGen clinical validity framework. Mol. Genet. Metab. 2019, 128, 122–128. [Google Scholar] [CrossRef]
- Li, Q.; Tomcik, K.; Zhang, S.; Puchowicz, M.A.; Zhang, G.-F. Dietary regulation of catabolic disposal of 4-hydroxynonenal analogs in rat liver. Free Radic. Biol. Med. 2012, 52, 1043–1053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wanders, R.J.A.; Vaz, F.M.; Waterham, H.R.; Ferdinandusse, S. Fatty Acid Oxidation in Peroxisomes: Enzymology, Metabolic Crosstalk with Other Organelles and Peroxisomal Disorders. In Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases; Lizard, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1299, pp. 55–70. ISBN 978-3-030-60203-1. [Google Scholar]
- Dahan, N.; Francisco, T.; Falter, C.; Rodrigues, T.; Kalel, V.; Kunze, M.; Hansen, T.; Schliebs, W.; Erdmann, R. Current advances in the function and biogenesis of peroxisomes and their roles in health and disease. Histochem. Cell Biol. 2021, 155, 513–524. [Google Scholar] [CrossRef]
- Bharathi, S.S.; Zhang, Y.; Gong, Z.; Muzumdar, R.; Goetzman, E.S. Role of mitochondrial acyl-CoA dehydrogenases in the metabolism of dicarboxylic fatty acids. Biochem. Biophys. Res. Commun. 2020, 527, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Grünig, D.; Duthaler, U.; Krähenbühl, S. Effect of Toxicants on Fatty Acid Metabolism in HepG2 Cells. Front. Pharmacol. 2018, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.; Aja, S.; Scafidi, S.; Wolfgang, M.J. Loss of Adipose Fatty Acid Oxidation Does Not Potentiate Obesity at Thermoneutrality. Cell Rep. 2016, 14, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef]
- Ménégaut, L.; Thomas, C.; Lagrost, L.; Masson, D. Fatty acid metabolism in macrophages: A target in cardio-metabolic diseases. Curr. Opin. Lipidol. 2016, 1. [Google Scholar] [CrossRef]
- Nomura, M.; Liu, J.; Yu, Z.-X.; Yamazaki, T.; Yan, Y.; Kawagishi, H.; Rovira, I.I.; Liu, C.; Wolfgang, M.J.; Mukouyama, Y.; et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J. Mol. Cell. Cardiol. 2019, 127, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Wek, R.C. Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef]
- Masson, G.R. Towards a model of GCN2 activation. Biochem. Soc. Trans. 2019, 47, 1481–1488. [Google Scholar] [CrossRef]
- Brouwers, M.C.G.J.; Simons, N.; Stehouwer, C.D.A.; Koek, G.H.; Schaper, N.C.; Isaacs, A. Relationship Between Nonalcoholic Fatty Liver Disease Susceptibility Genes and Coronary Artery Disease: Hepatology Communications. Hepatol. Commun. 2019, 3, 587–596. [Google Scholar] [CrossRef]
- Krawczyk, M.; Portincasa, P.; Lammert, F. PNPLA3-associated steatohepatitis: Toward a gene-based classification of fatty liver disease. Semin. Liver Dis. 2013, 33, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Smagris, E.; BasuRay, S.; Li, J.; Huang, Y.; Lai, K.V.; Gromada, J.; Cohen, J.C.; Hobbs, H.H. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 2015, 61, 108–118. [Google Scholar] [CrossRef] [PubMed]
- BasuRay, S.; Smagris, E.; Cohen, J.C.; Hobbs, H.H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 2017, 66, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Nick, A.; Hölttä-Vuori, M.; Thiele, C.; Isokuortti, E.; Lallukka-Brück, S.; Zhou, Y.; Hakkarainen, A.; Lundbom, N.; Peltonen, M.; et al. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Saki, S.; Saki, N.; Poustchi, H.; Malekzadeh, R. Assessment of Genetic Aspects of Non-alcoholic Fatty Liver and Premature Cardiovascular Events. Middle East J. Dig. Dis. 2020, 12, 65–88. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.; Guo, Y.; Zhu, T.; Garcia-Barrio, M.T.; Jiang, Z.; Willer, C.J.; Zhang, J.; Chen, Y.E. Hepatic Transmembrane 6 Superfamily Member 2 Regulates Cholesterol Metabolism in Mice. Gastroenterology 2016, 150, 1208–1218. [Google Scholar] [CrossRef]
- Wright, F.A.; Bonzerato, C.G.; Sliter, D.A.; Wojcikiewicz, R.J.H. The erlin2 T65I mutation inhibits erlin1/2 complex–mediated inositol 1,4,5-trisphosphate receptor ubiquitination and phosphatidylinositol 3-phosphate binding. J. Biol. Chem. 2018, 293, 15706–15714. [Google Scholar] [CrossRef]
- Saeed, N.; Nadeau, B.; Shannon, C.; Tincopa, M. Evaluation of Dietary Approaches for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients 2019, 11, 3064. [Google Scholar] [CrossRef]
- Shojaee-Moradie, F.; Cuthbertson, D.J.; Barrett, M.; Jackson, N.C.; Herring, R.; Thomas, E.L.; Bell, J.; Kemp, G.J.; Wright, J.; Umpleby, A.M. Exercise Training Reduces Liver Fat and Increases Rates of VLDL Clearance But Not VLDL Production in NAFLD. J. Clin. Endocrinol. Metab. 2016, 101, 4219–4228. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.-C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e5. [Google Scholar] [CrossRef]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. JAMA 2019, 322, 1271. [Google Scholar] [CrossRef] [PubMed]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef]
- Smati, S.; Régnier, M.; Fougeray, T.; Polizzi, A.; Fougerat, A.; Lasserre, F.; Lukowicz, C.; Tramunt, B.; Guillaume, M.; Burnol, A.-F.; et al. Regulation of hepatokine gene expression in response to fasting and feeding: Influence of PPAR-α and insulin-dependent signalling in hepatocytes. Diabetes Metab. 2020, 46, 129–136. [Google Scholar] [CrossRef]
- Uebanso, T.; Taketani, Y.; Yamamoto, H.; Amo, K.; Tanaka, S.; Arai, H.; Takei, Y.; Masuda, M.; Yamanaka-Okumura, H.; Takeda, E. Liver X receptor negatively regulates fibroblast growth factor 21 in the fatty liver induced by cholesterol-enriched diet. J. Nutr. Biochem. 2012, 23, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Barb, D.; Bril, F.; Kalavalapalli, S.; Cusi, K. Plasma Fibroblast Growth Factor 21 Is Associated With Severity of Nonalcoholic Steatohepatitis in Patients With Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3327–3336. [Google Scholar] [CrossRef]
- Pereira, R.O.; Marti, A.; Olvera, A.C.; Tadinada, S.M.; Bjorkman, S.H.; Weatherford, E.T.; Morgan, D.A.; Westphal, M.; Patel, P.H.; Kirby, A.K.; et al. OPA1 deletion in brown adipose tissue improves thermoregulation and systemic metabolism via FGF21. eLife 2021, 10, e66519. [Google Scholar] [CrossRef]
- Kim, A.M.; Somayaji, V.R.; Dong, J.Q.; Rolph, T.P.; Weng, Y.; Chabot, J.R.; Gropp, K.E.; Talukdar, S.; Calle, R.A. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab. 2017, 19, 1762–1772. [Google Scholar] [CrossRef]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2018, 392, 2705–2717. [Google Scholar] [CrossRef]
- Kaufman, A.; Abuqayyas, L.; Denney, W.S.; Tillman, E.J.; Rolph, T. AKR-001, an Fc-FGF21 Analog, Showed Sustained Pharmacodynamic Effects on Insulin Sensitivity and Lipid Metabolism in Type 2 Diabetes Patients. Cell Rep. Med. 2020, 1, 100057. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.; Hanouneh, I.A.; Noureddin, M.; Rolph, T.; Alkhouri, N. Fibroblast growth factor (FGF)-21 based therapies: A magic bullet for nonalcoholic fatty liver disease (NAFLD)? Expert Opin. Investig. Drugs 2020, 29, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Yang, E.H.; Quan, W.; Nam, E.H.; Cheon, H.G. Discovery of a novel fibroblast activation protein (FAP) inhibitor, BR103354, with anti-diabetic and anti-steatotic effects. Sci. Rep. 2020, 10, 21280. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, J.; Tang, S.; Rye, K.-A.; Ong, K.L. The role of fibroblast growth factor 21 in atherosclerosis. Atherosclerosis 2017, 257, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tabari, F.S.; Karimian, A.; Parsian, H.; Rameshknia, V.; Mahmoodpour, A.; Majidinia, M.; Maniati, M.; Yousefi, B. The roles of FGF21 in atherosclerosis pathogenesis. Rev. Endocr. Metab. Disord. 2019. [Google Scholar] [CrossRef]
- Wei, W.; Dutchak, P.A.; Wang, X.; Ding, X.; Wang, X.; Bookout, A.L.; Goetz, R.; Mohammadi, M.; Gerard, R.D.; Dechow, P.C.; et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 3143–3148. [Google Scholar] [CrossRef]
- Charles, E.D.; Neuschwander-Tetri, B.A.; Pablo Frias, J.; Kundu, S.; Luo, Y.; Tirucherai, G.S.; Christian, R. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity 2019, 27, 41–49. [Google Scholar] [CrossRef]
- Kenneally, S.; Sier, J.H.; Moore, J.B. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: A systematic review. BMJ Open Gastroenterol. 2017, 4, e000139. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).