n-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation
Abstract
1. Introduction
2. Results
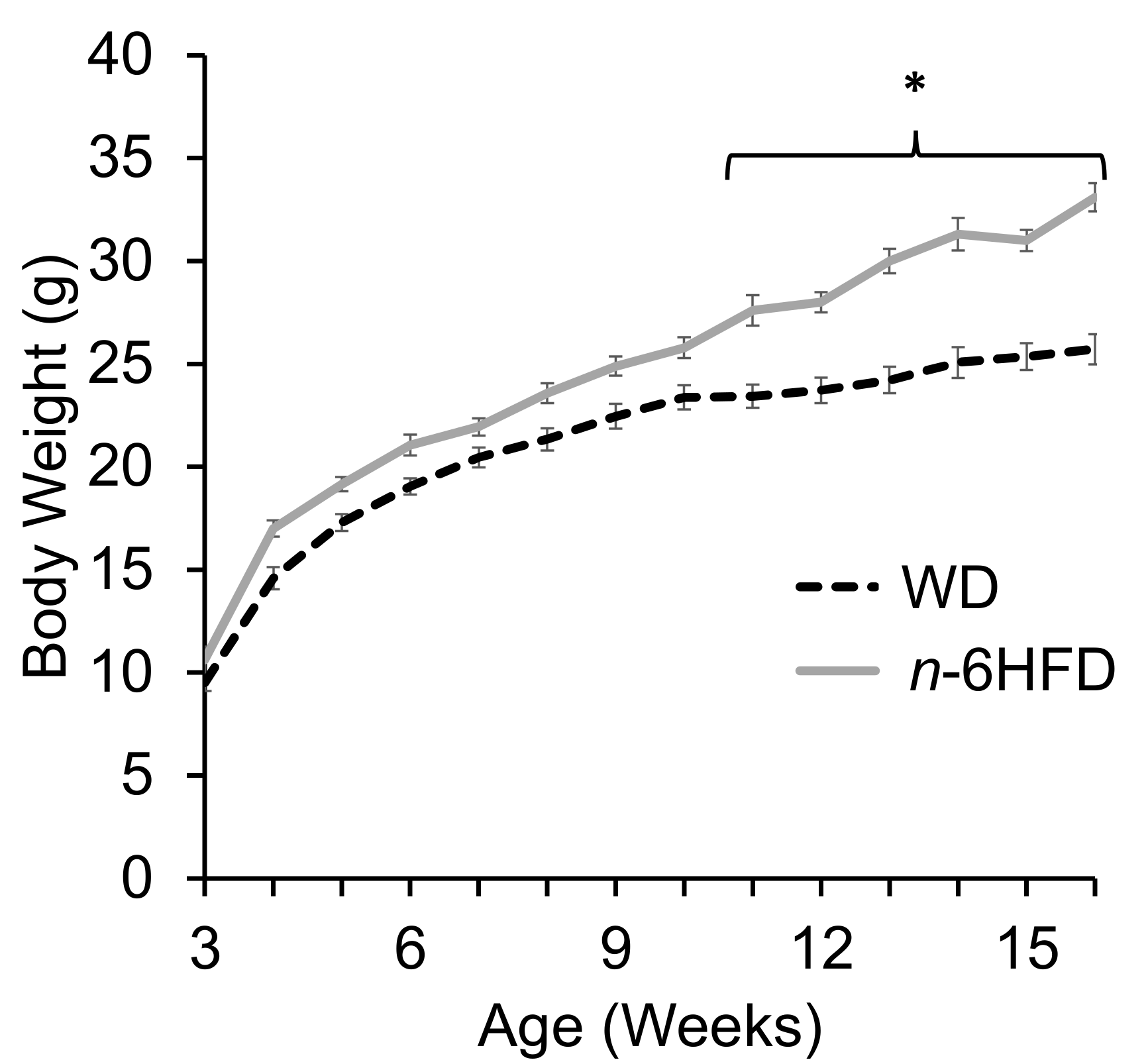
2.1. Body Weight and Plasma Fatty Acid Profile
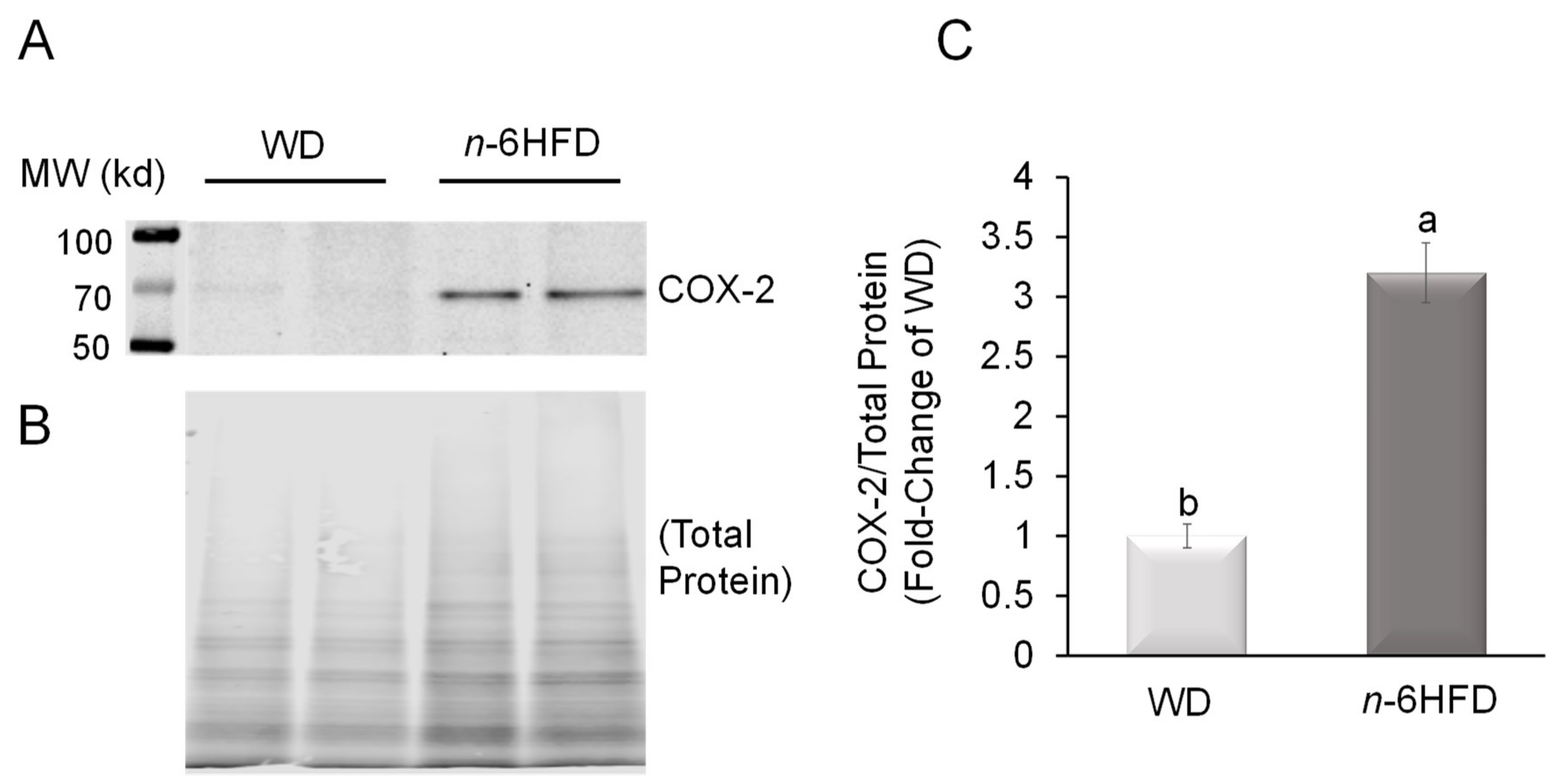
2.2. Histopathological Analysis and COX-2 Expression
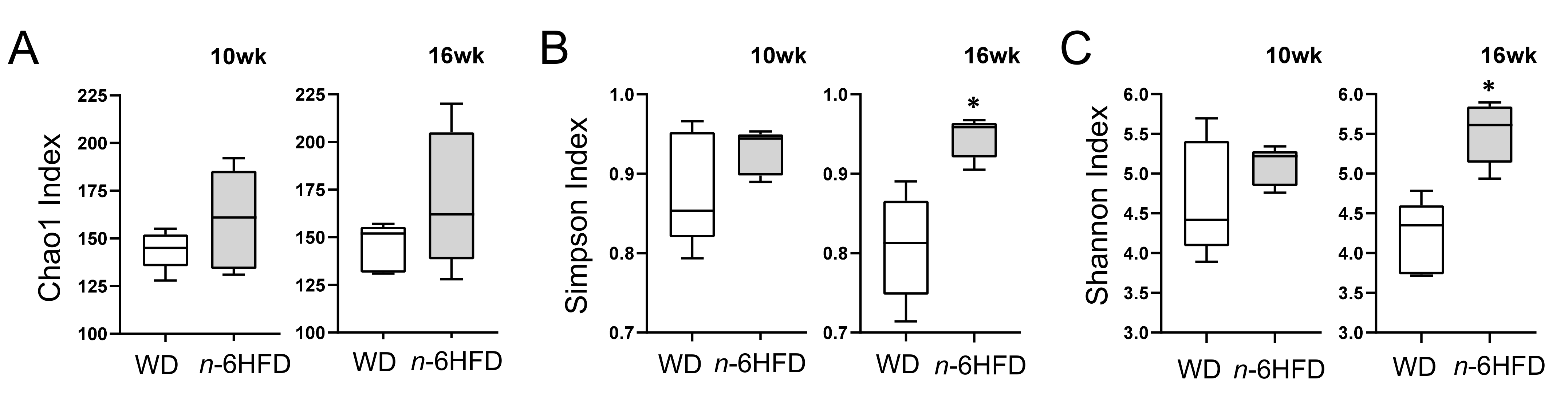
2.3. Diversity of the Cecal Microbiota
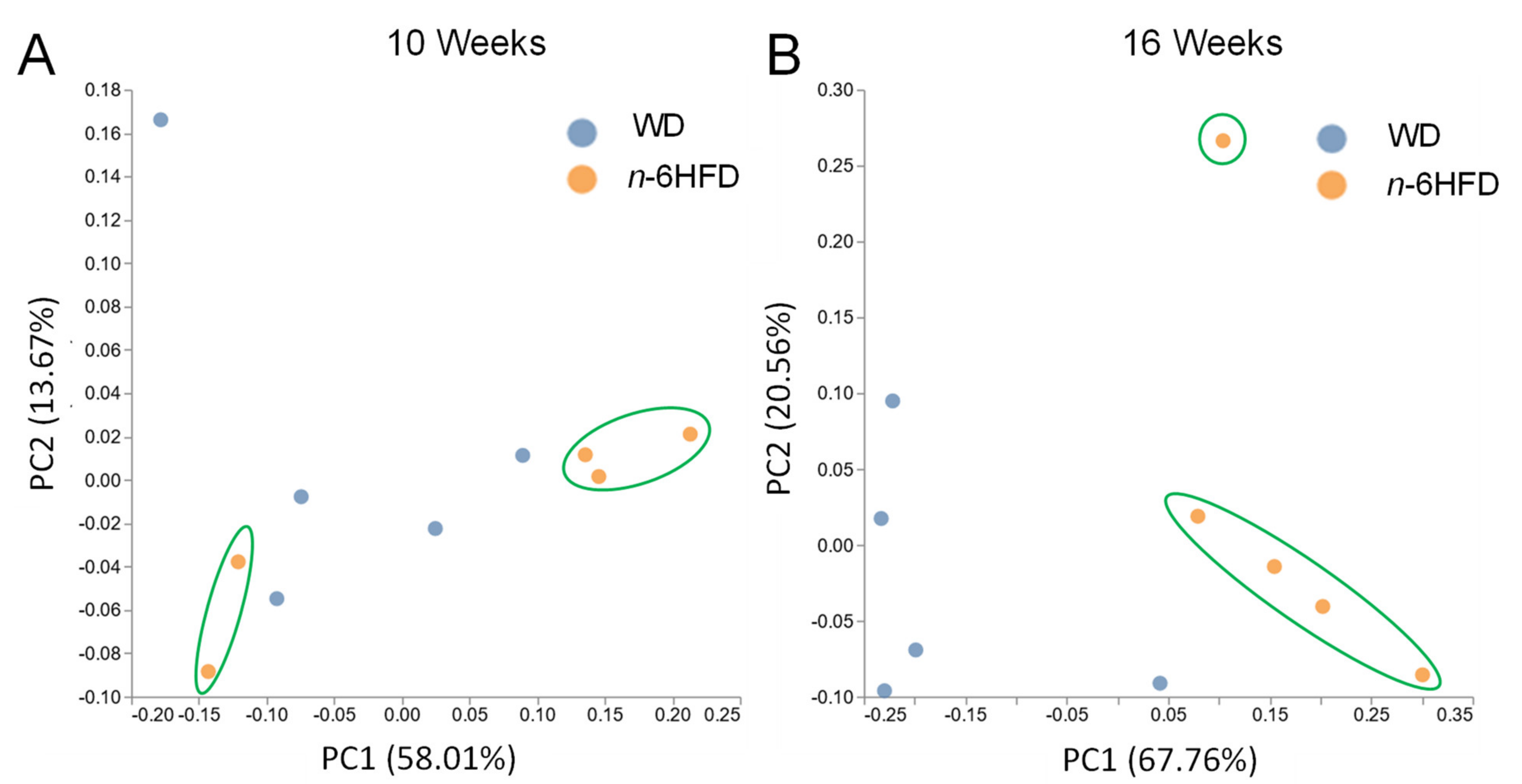
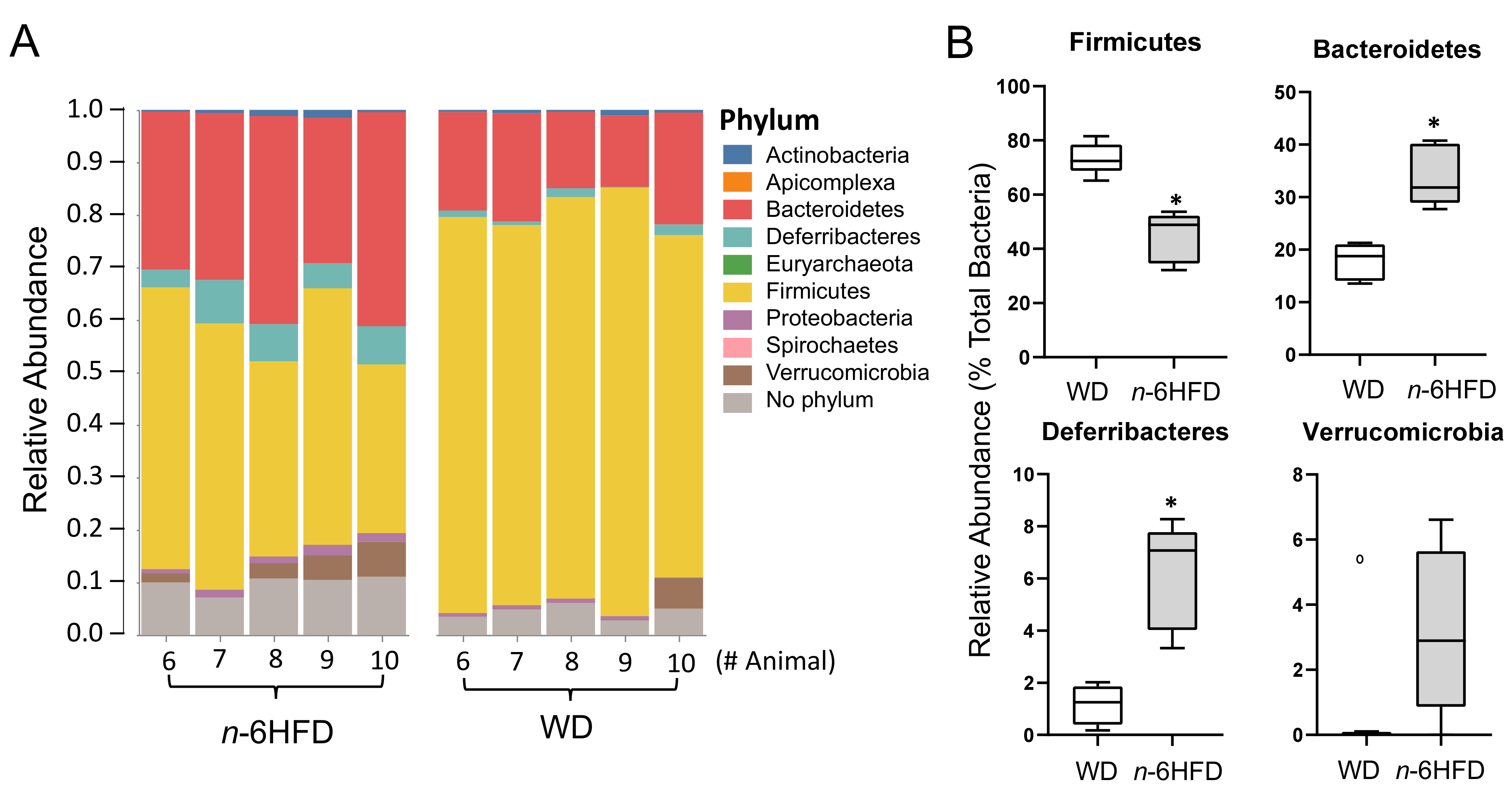
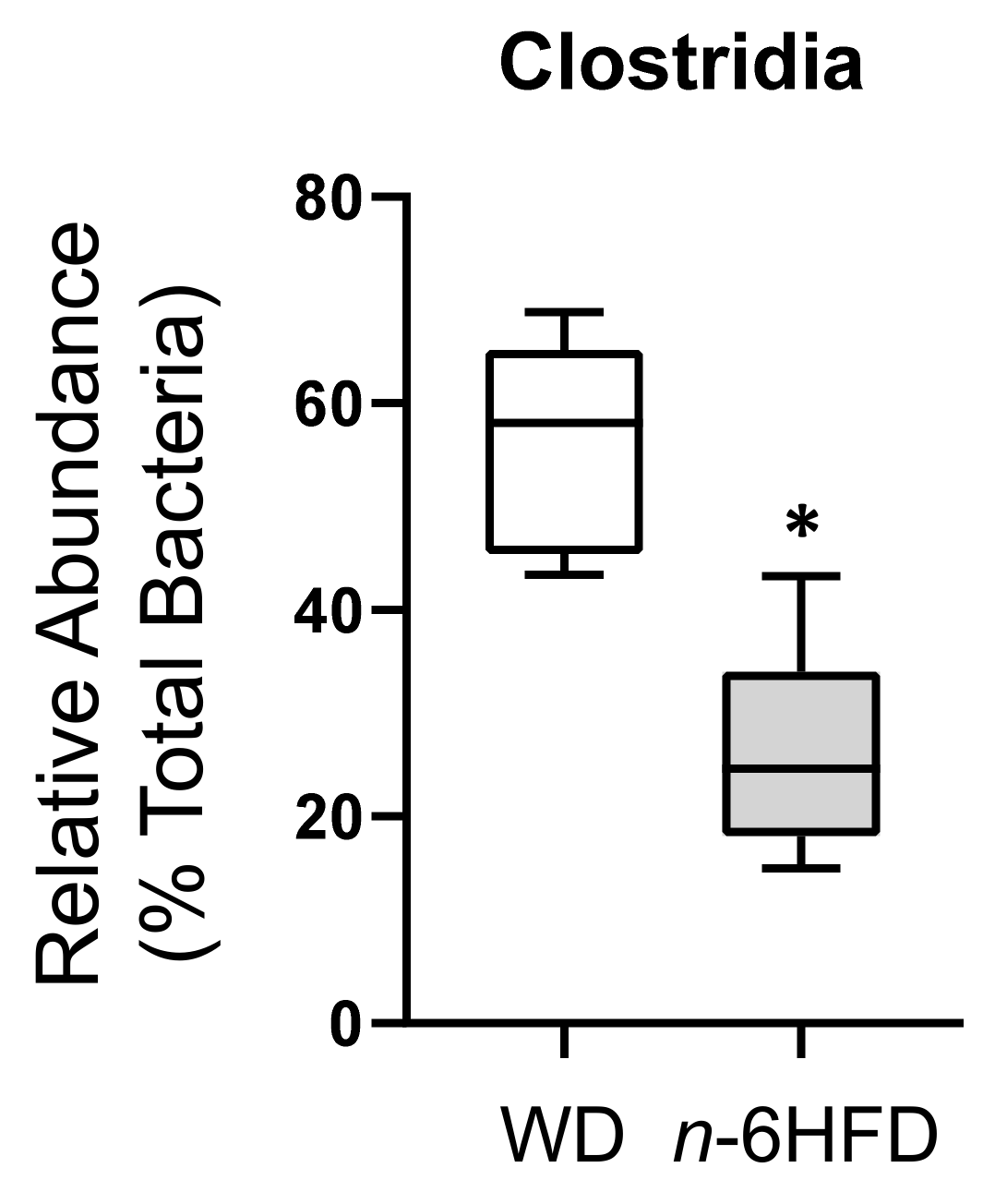
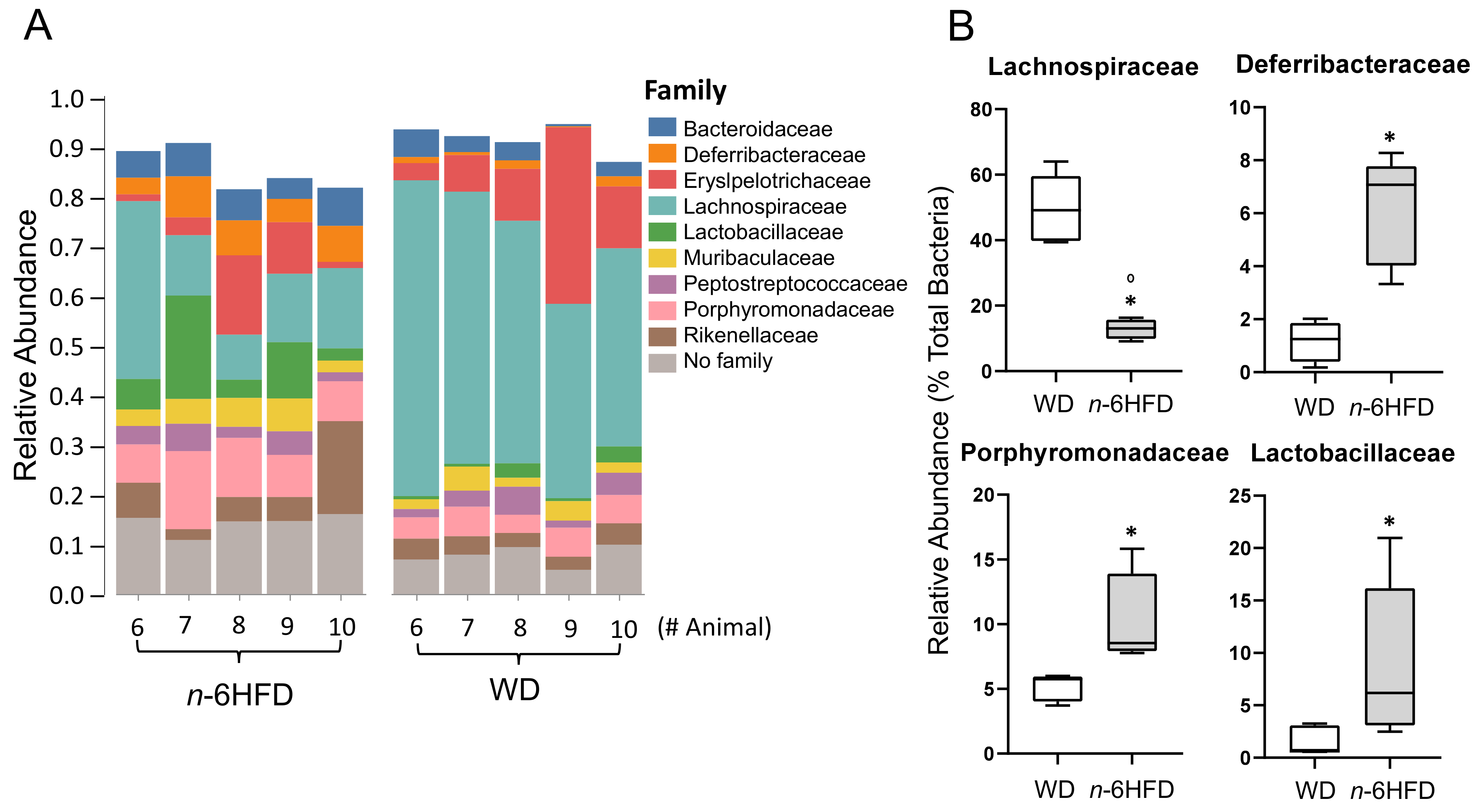
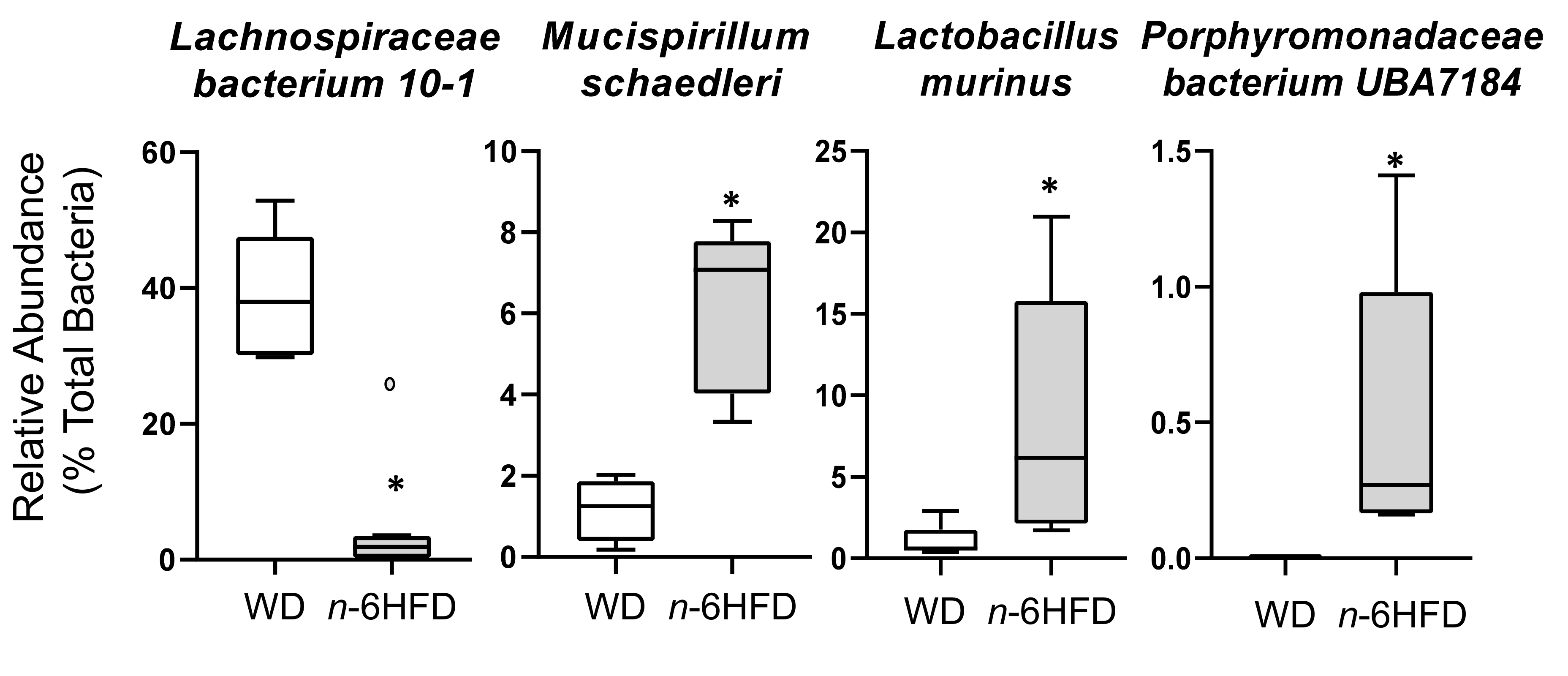
2.4. Microbiota Community Composition
3. Discussion
4. Materials and Methods
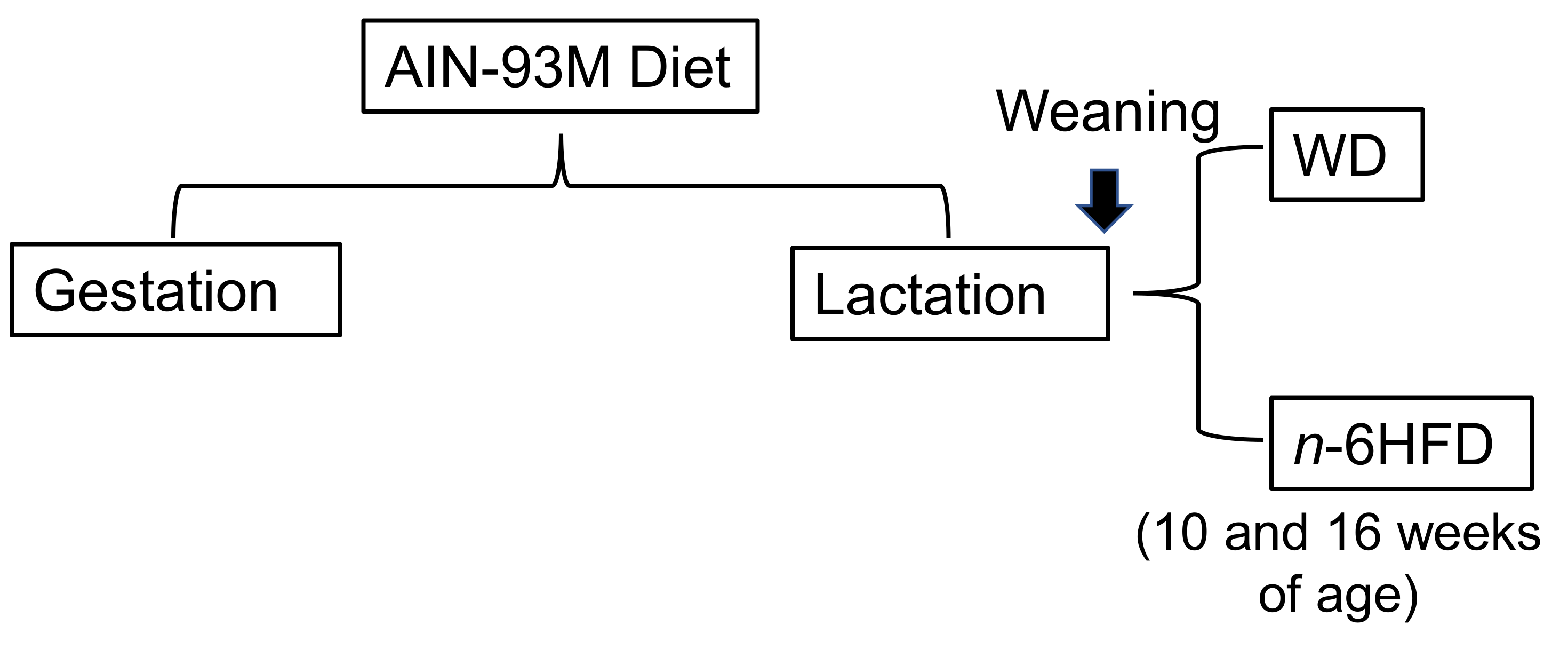
4.1. Animals, Diets, and Samples Collection
4.2. Western Blot Analysis
4.3. Sequencing of Microbiota in Cecal Pellets
4.4. Plasma Fatty Acid Profile
4.5. Colon Histopathology
4.6. Microbiome and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | cholic acid |
| CD | Chron’s disease |
| COX-2 | cyclooxygenase-2 |
| FXR | farnesoid X receptor |
| HFD | high fat diet |
| IBD | inflammatory bowel disease |
| LFD | low fat diet |
| LA | linoleic acid |
| MUFA | monounsaturated fatty acids |
| NGS | next generation sequencing |
| n-6HFD | n-6 high fat diet |
| NSP | non-starch polysaccharide |
| PC | principal component |
| %E | percent energy |
| PUFA | polyunsaturated fatty acids |
| SFA | saturated fatty acids |
| TβMCA | taurine-conjugated β-muricholic acid |
| UC | ulcerative colitis |
| WD | Western diet |
References
- Ruel, J.; Ruane, D.; Mehandru, S.; Gower-Rousseau, C.; Colombel, J.F. IBD across the age spectrum: Is it the same disease? Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 88–98. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Ventham, N.T.; Kennedy, N.A.; Nimmo, E.R.; Satsangi, J. Beyond gene discovery in inflammatory bowel disease: The emerging role of epigenetics. Gastroenterology 2013, 145, 293–308. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- IBD in EPIC Study Investigators; Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar]
- Pickens, C.A.; Albuquerque Pereira, M.F.; Fenton, J.I. Long-chain ω-6 plasma phospholipid polyunsaturated fatty acids and association with colon adenomas in adult men: A cross-sectional study. Eur. J. Cancer Prev. 2017, 26, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castillo, N.; Silva-Gómez, J.A.; Campos-Perez, W.; Barron-Cabrera, E.; Hernandez-Cañaveral, I.; Garcia-Cazarin, M.; Marquez-Sandoval, Y.; Gonzalez-Becerra, K.; Barron-Gallardo, C.; Martinez-Lopez, E. High Dietary ω-6:ω-3 PUFA Ratio Is Positively Associated with Excessive Adiposity and Waist Circumference. Obes. Facts 2018, 11, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef]
- Cariello, M.; Contursi, A.; Gadaleta, R.M.; Piccinin, E.; De Santis, S.; Piglionica, M.; Spaziante, A.F.; Sabbà, C.; Villani, G.; Moschetta, A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 2020, 12, 1084. [Google Scholar] [CrossRef]
- Bamia, C.; Lagiou, P.; Buckland, G.; Grioni, S.; Agnoli, C.; Taylor, A.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; et al. Mediterranean diet and colorectal cancer risk: Results from a European cohort. Eur. J. Epidemiol. 2013, 28, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Mediterranean Diet: Prevention of Colorectal Cancer. Front. Nutr. 2017, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Patrone, V.; Minuti, A.; Lizier, M.; Miragoli, F.; Lucchini, F.; Trevisi, E.; Rossi, F.; Callegari, M.L. Differential effects of coconut versus soy oil on gut microbiota composition and predicted metabolic function in adult mice. BMC Genom. 2018, 19, 808. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Ferrari, S.; Lizier, M.; Lucchini, F.; Minuti, A.; Tondelli, B.; Trevisi, E.; Rossi, F.; Callegari, M.L. Short-term modifications in the distal gut microbiota of weaning mice induced by a high-fat diet. Microbiology 2012, 158, 983–992. [Google Scholar] [CrossRef] [PubMed]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef]
- Kübeck, R.; Bonet-Ripoll, C.; Hoffmann, C.; Walker, A.; Müller, V.M.; Schüppel, V.L.; Lagkouvardos, I.; Scholz, B.; Engel, K.H.; Daniel, H.; et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol. Metab. 2016, 5, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Molcan, E.; DeCoffe, D.; Dai, C.; Gibson, D.L. Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br. J. Nutr. 2013, 110, 515–523. [Google Scholar] [CrossRef]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef]
- USDA, Agricultural Research Service. Nutrient Intakes from Food and Beverages: Mean Amounts Consumed Per Individual, by Gender and Age, What We Eat in America, NHANES 2013–2014; United States Department of Agriculture, Agriculture Research Service: Beltsville, MD, USA, 2016.
- Luo, T.; Goldfinger, T.; Shay, N. Metabolic Syndrome Is Reduced in C57BL/6J Mice Fed High-Fat Diets Supplemented with Oak Tannins. Curr. Dev. Nutr. 2020, 4, nzaa033. [Google Scholar] [CrossRef]
- Meriwether, D.; Sulaiman, D.; Volpe, C.; Dorfman, A.; Grijalva, V.; Dorreh, N.; Solorzano-Vargas, R.S.; Wang, J.; O’Connor, E.; Papesh, J.; et al. Apolipoprotein A-I mimetics mitigate intestinal inflammation in COX2-dependent inflammatory bowel disease model. J. Clin. Investig. 2019, 129, 3670–3685. [Google Scholar] [CrossRef]
- Just, S.; Mondot, S.; Ecker, J.; Wegner, K.; Rath, E.; Gau, L.; Streidl, T.; Hery-Arnaud, G.; Schmidt, S.; Lesker, T.R.; et al. The gut microbiota drives the impact of bile acids and fat source in diet on mouse metabolism. Microbiome 2018, 6, 134. [Google Scholar] [CrossRef]
- Djuric, Z.; Turgeon, D.K.; Ren, J.; Neilson, A.; Plegue, M.; Waters, I.G.; Chan, A.; Askew, L.M.; Ruffin, M.T., 4th; Sen, A.; et al. Effects of a Mediterranean Diet Intervention on Anti- and Pro-Inflammatory Eicosanoids, Epithelial Proliferation, and Nuclear Morphology in Biopsies of Normal Colon Tissue. Nutr. Cancer 2015, 67, 721–729. [Google Scholar] [CrossRef][Green Version]
- Romagnolo, D.F.; Donovan, M.G.; Doetschman, T.C.; Selmin, O.I. n-6 Linoleic Acid Induces Epigenetics Alterations Associated with Colonic Inflammation and Cancer. Nutrients 2019, 11, 171. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Wang, C.Z.; Huang, W.H.; Zhang, C.F.; Wan, J.Y.; Wang, Y.; Yu, C.; Williams, S.; He, T.C.; Du, W.; Much, M.W.; et al. Role of intestinal microbiome in American ginseng-mediated colon cancer prevention in high fat diet-fed AOM/DSS mice [corrected]. Clin. Transl. Oncol. 2018, 20, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.; Marschall, H.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Selmin, O.I.; Fang, C.; Lyon, A.M.; Doetschman, T.C.; Thompson, P.A.; Martinez, J.D.; Smith, J.W.; Lance, P.M.; Romagnolo, D.F. Inactivation of adenomatous polyposis Coli reduces bile acid/farnesoid X receptor expression through Fxr gene CpG methylation in mouse colon tumors and human colon cancer cells. J. Nutr. 2016, 146, 236–242. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wren, S.N.; Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. A Villin-driven Fxr transgene modulates enterohepatic bile acid homeostasis and response to an n-6-enriched high-fat diet. Int. J. Mol. Sci. 2020, 21, 7829. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S.; et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems 2018, 3, e00188–17. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Song, G.; Tang, X.; Song, H.; Deng, A.; Wang, W.; Wu, L.; Qin, H. Development of an XBP1 agonist, HLJ2, as a potential therapeutic agent for ulcerative colitis. Eur. J. Pharm. Sci. 2017, 109, 56–64. [Google Scholar] [CrossRef]
- Caruso, R.; Mathes, T.; Martens, E.C.; Kamada, N.; Nusrat, A.; Inohara, N.; Núñez, G. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci. Immunol. 2019, 4, eaaw4341. [Google Scholar] [CrossRef]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Gomes Neto, J.C.; Boekschoten, M.V.; Schwab, C.; Urich, T.; et al. Lifestyle and horizontal gene transfer-mediated evolution of mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems 2017, 2, e00171–16. [Google Scholar] [CrossRef]
- Daniel, S.G.; Ball, C.L.; Besselsen, D.G.; Doetschman, T.; Hurwitz, B.L. Functional changes in the gut microbiome contribute to transforming growth factor beta-deficient colon cancer. mSystems 2017, 2, e00065–17. [Google Scholar] [CrossRef] [PubMed]
- Chen. L.L.; Zou, Y.Y.; Lu, F.G.; Li, F.J.; Lian, G.H. Efficacy profiles for different concentrations of Lactobacillus acidophilus in experimental colitis. World J. Gastroenterol. 2013, 19, 5347–5356. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, J.; Jackson, M.I.; Zhao, F.Q.; Yan, L.; Combs, G.F. Fatty liver accompanies an increase in lactobacillus species in the hind gut of C57BL/6 mice fed a high-fat diet. J. Nutr. 2013, 143, 627–631. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, D.; Peng, C.; Tan, C.; Wang, Q.; Gong, J. Effects of theabrownin on serum metabolites and gut microbiome in rats with a high-sugar diet. Food Funct. 2019, 10, 7063–7080. [Google Scholar] [CrossRef]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Konson, A.; Mahajna, J.A.; Danon, A.; Rimon, G.; Agbaria, R. The involvement of nuclear factor-kappa B in cyclooxygenase-2 overexpression in murine colon cancer cells transduced with herpes simplex virus thymidine kinase gene. Cancer Gene Ther. 2006, 13, 1093–1104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.O.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.B.; Bernalier-Donadille, A.; Duncan, S.H.; O’Toole, P.W.; Scott, P.K.; Flint, J.H. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb. Genom. 2016, 2, e000043. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Ren, D.; Yan, W.; Wang, Y.; Liu, H.; Shen, M. Linoleic acid inhibits Lactobacillus activity by destroying cell membrane and affecting normal metabolism. J. Sci. Food Agric. 2020, 100, 2057–2064. [Google Scholar] [CrossRef]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. mBio 2013, 4, e00692–13. [Google Scholar] [CrossRef]
- Suzuki, R.; Miyamoto, S.; Yasui, Y.; Sugie, S.; Tanaka, T. Global gene expression analysis of the mouse colonic mucosa treated with azoxymethane and dextran sodium sulfate. BMC Cancer 2007, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Gilda, J.E.; Gomes, A.V. The necessity of and strategies for improving confidence in the accuracy of Western blots. Expert Rev. Proteom. 2014, 11, 549–560. [Google Scholar]
- Weaver, K.L.; Ivester, P.; Seeds, M.; Case, L.D.; Arm, J.P.; Chilton, F.H. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J. Biol. Chem. 2009, 284, 15400–15407. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y. Rapid Separation of Fatty Acid Methyl Esters. Agilent Application Note. Food Testing & Agriculture. 2018, pp. 1–7. Available online: https://www.agilent.com/cs/library/applications/application-fatty-acid-methyl-esters-db-fastfame-5994-0116-en-us-agilent.pdf (accessed on 24 June 2021).
- Mann, P.C.; Vahle, J.; Keenan, C.M.; Baker, J.F.; Bradley, A.E.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; et al. International harmonization of toxicologic pathology nomenclature: An overview and review of basic principles. Toxicol. Pathol. 2012, 40 (Suppl. 4), 7S–13S. [Google Scholar] [CrossRef]
- Ames, S.K.; Hysom, D.A.; Gardner, S.N.; Lloyd, G.S.; Gokhale, M.B.; Allen, J.E. Scalable metagenomic taxonomy classification using a reference genome database. Bioinformatics 2013, 29, 2253–2260. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Minot, S.S.; Krumm, N.; Greenfield, N.B. OneCodex: A sensitive and accurate data platform for genomic microbial identification. bioRxiv 2015. [Google Scholar] [CrossRef]
- Millen, B.E.; Abrams, S.; Adams-Campbell, L.; Anderson, C.A.; Brenna, J.T.; Campbell, W.W.; Clinton, S.; Hu, F.; Nelson, M.; Neuhouser, M.L.; et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv. Nutr. 2016, 7, 438–444. [Google Scholar] [CrossRef]



| Experimental Diets | WD | n-6HFD | ||
|---|---|---|---|---|
| Experimental Periods | 10 Weeks (n = 5) | 16 Weeks (n = 5) | 10 Weeks (n = 5) | 16 Weeks (n = 5) |
| Number of animals with normal histopathology | 5 | 4 | 4 | 2 |
| Number of animals with colonic events: Mucosal fibrosis: | ||||
| Minimal | 0 | 0 | 0 | 0 |
| Mild | 0 | 0 | 0 | 1 |
| Moderate | 0 | 0 | 0 | 0 |
| Marked | 0 | 0 | 0 | 0 |
| Mucosal hyperplasia: | ||||
| Minimal | 0 | 0 | 0 | 0 |
| Mild | 0 | 0 | 0 | 0 |
| Moderate | 0 | 1 | 0 | 0 |
| Marked | 0 | 0 | 0 | 1 |
| Inflammation, chronic-active: | ||||
| Minimal | 0 | 1 | 1 | 2 |
| Mild | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 |
| Marked | 0 | 0 | 0 | 0 |
| Inflammation, chronic: | ||||
| Minimal | 0 | 1 | 0 | 0 |
| Mild | 0 | 0 | 0 | 1 |
| Moderate | 0 | 0 | 0 | 0 |
| Marked | 0 | 0 | 0 | 0 |
| Diet Formula | AIN-93M (g/Kg) | WD (g/Kg) | n-6HFD (g/Kg) | |||
|---|---|---|---|---|---|---|
| Casein | 140.0 | 140.0 | 140.0 | |||
| L-Cystine | 1.8 | 1.8 | 1.8 | |||
| Corn Starch | 465.7 | 267.5 | 87.5 | |||
| Maltodextrin | 155.0 | 155.0 | 155.0 | |||
| Sucrose | 100.0 | 100.0 | 100.0 | |||
| Soybean Oil | 40.0 | 200.0 | ||||
| Palm Oil | 110.0 | |||||
| Cellulose | 50.0 | 155.0 | 290.0 | |||
| Mineral Mix, AIN-93M-MX (94049) | 35.0 | 35.0 | 35.0 | |||
| Mineral Mix, AIN-93-VX (94047) | 10.0 | 10.0 | 10.0 | |||
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | |||
| TBHQ, Antioxidant | 0.01 | 0.02 | 0.04 | |||
| Nutrient Composition | % Weight | % Kcal | % Weight | % Kcal | % Weight | % Kcal |
| Protein | 12.4 | 13.7 | 12.4 | 13.7 | 12.4 | 13.7 |
| Carbohydrate | 68.3 | 75.9 | 52.8 | 58.4 | 32.3 | 35.9 |
| Fat | 4.1 | 10.3 | 11.1 | 27.8 | 20.1 | 50.3 |
| Energy (Kcal/g) | 3.6 | 3.6 | 3.6 | |||
| Diet Formula | AIN-93M (g/Kg) % | WD (g/Kg) % | n-6HFD (g/Kg) % |
|---|---|---|---|
| Total fat (g/Kg) | 41.4 | 111.4 | 201.4 |
| SFA (g/Kg) | 6.0 15.1 | 56.2 51.3 | 30.0 15.1 |
| MUFA (g/Kg) | 9.4 23.5 | 42.7 39.0 | 48.8 23.5 |
| PUFA (g/Kg) | 24.5 61.4 | 10.7 9.7 | 122.4 61.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmin, O.I.; Papoutsis, A.J.; Hazan, S.; Smith, C.; Greenfield, N.; Donovan, M.G.; Wren, S.N.; Doetschman, T.C.; Snider, J.M.; Snider, A.J.; et al. n-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation. Int. J. Mol. Sci. 2021, 22, 6919. https://doi.org/10.3390/ijms22136919
Selmin OI, Papoutsis AJ, Hazan S, Smith C, Greenfield N, Donovan MG, Wren SN, Doetschman TC, Snider JM, Snider AJ, et al. n-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation. International Journal of Molecular Sciences. 2021; 22(13):6919. https://doi.org/10.3390/ijms22136919
Chicago/Turabian StyleSelmin, Ornella I., Andreas J. Papoutsis, Sabine Hazan, Christopher Smith, Nick Greenfield, Micah G. Donovan, Spencer N. Wren, Thomas C. Doetschman, Justin M. Snider, Ashley J. Snider, and et al. 2021. "n-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation" International Journal of Molecular Sciences 22, no. 13: 6919. https://doi.org/10.3390/ijms22136919
APA StyleSelmin, O. I., Papoutsis, A. J., Hazan, S., Smith, C., Greenfield, N., Donovan, M. G., Wren, S. N., Doetschman, T. C., Snider, J. M., Snider, A. J., Chow, S. H.-H., & Romagnolo, D. F. (2021). n-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation. International Journal of Molecular Sciences, 22(13), 6919. https://doi.org/10.3390/ijms22136919







