Efficacy of a Chitin-Based Water-Soluble Derivative in Inducing Purpureocillium lilacinum against Nematode Disease (Meloidogyne incognita)
Abstract
1. Introduction
2. Results
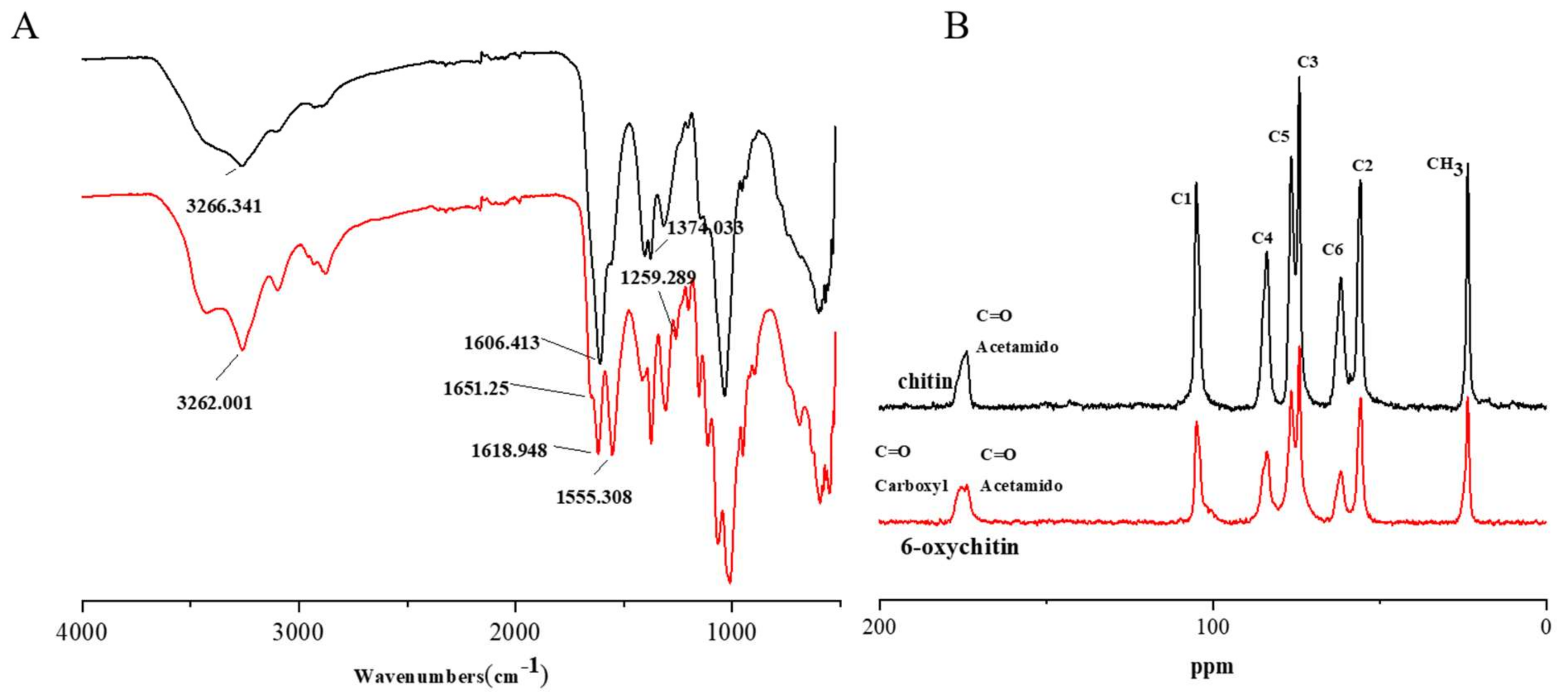
2.1. Characterization
2.2. Screening for Nematicidal Microorganisms Utilizing Chitin
2.3. Screening of the Optimum Water-Soluble Chitin Derivatives
2.4. Screening the Factors Affecting Nematicidal Activity
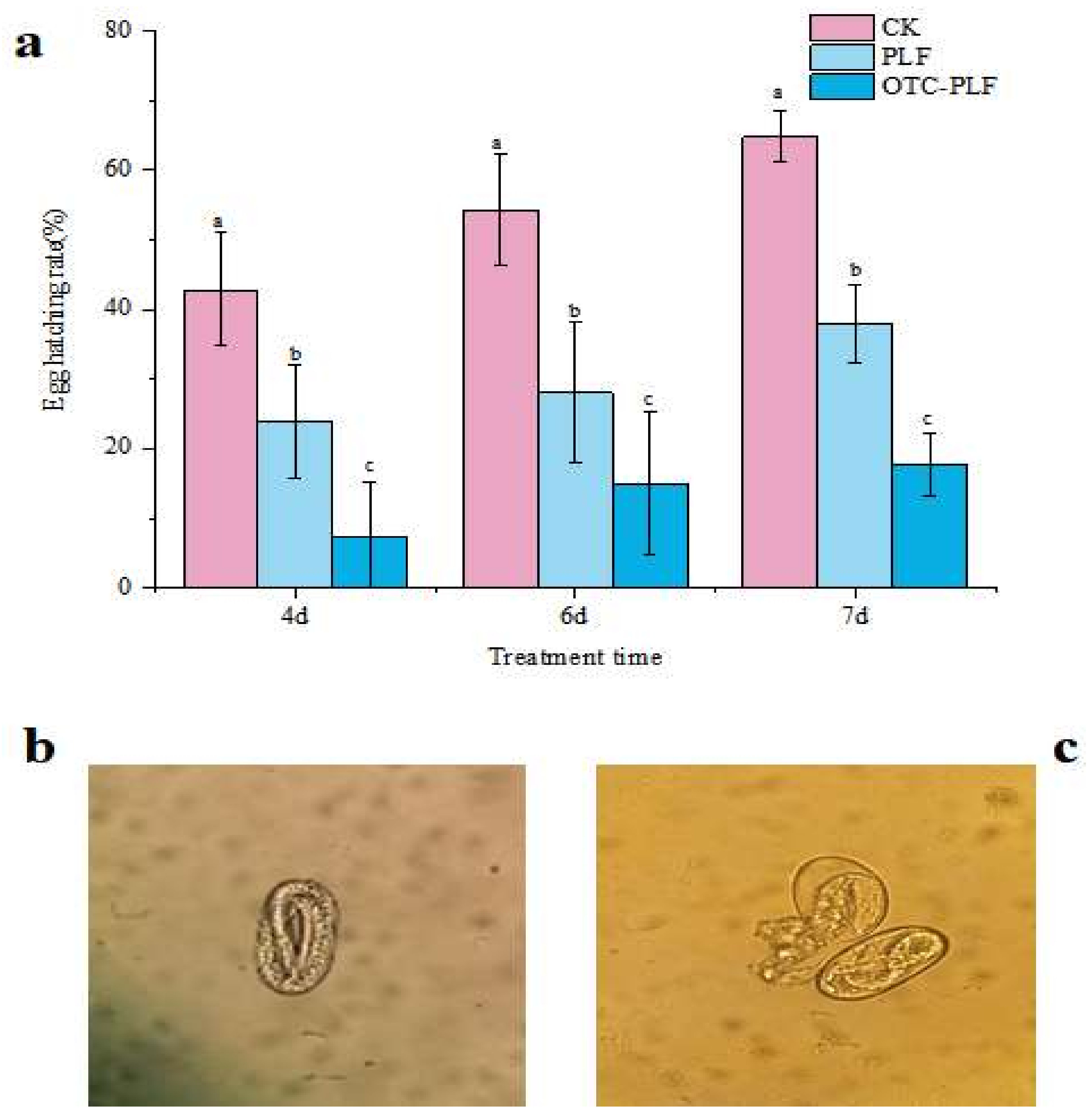
2.5. Anti-Nematode Assay
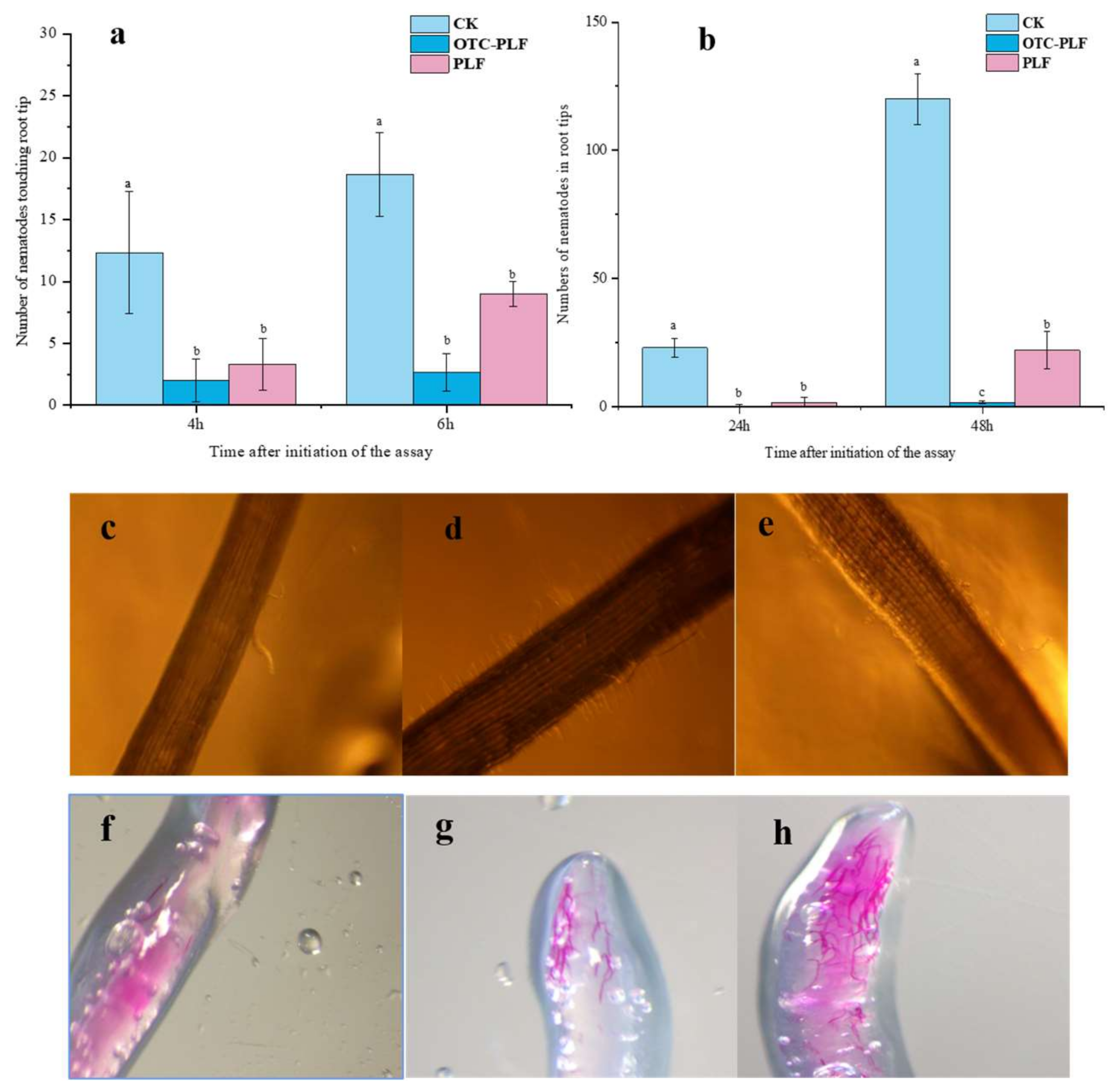
2.6. Effect of OTC-PLF on M. Incognita in Cucumber
2.6.1. Effect of OTC-PLF on Nematode Infecting Cucumber
2.6.2. Effect on the Invasion of Root-Knot Nematode
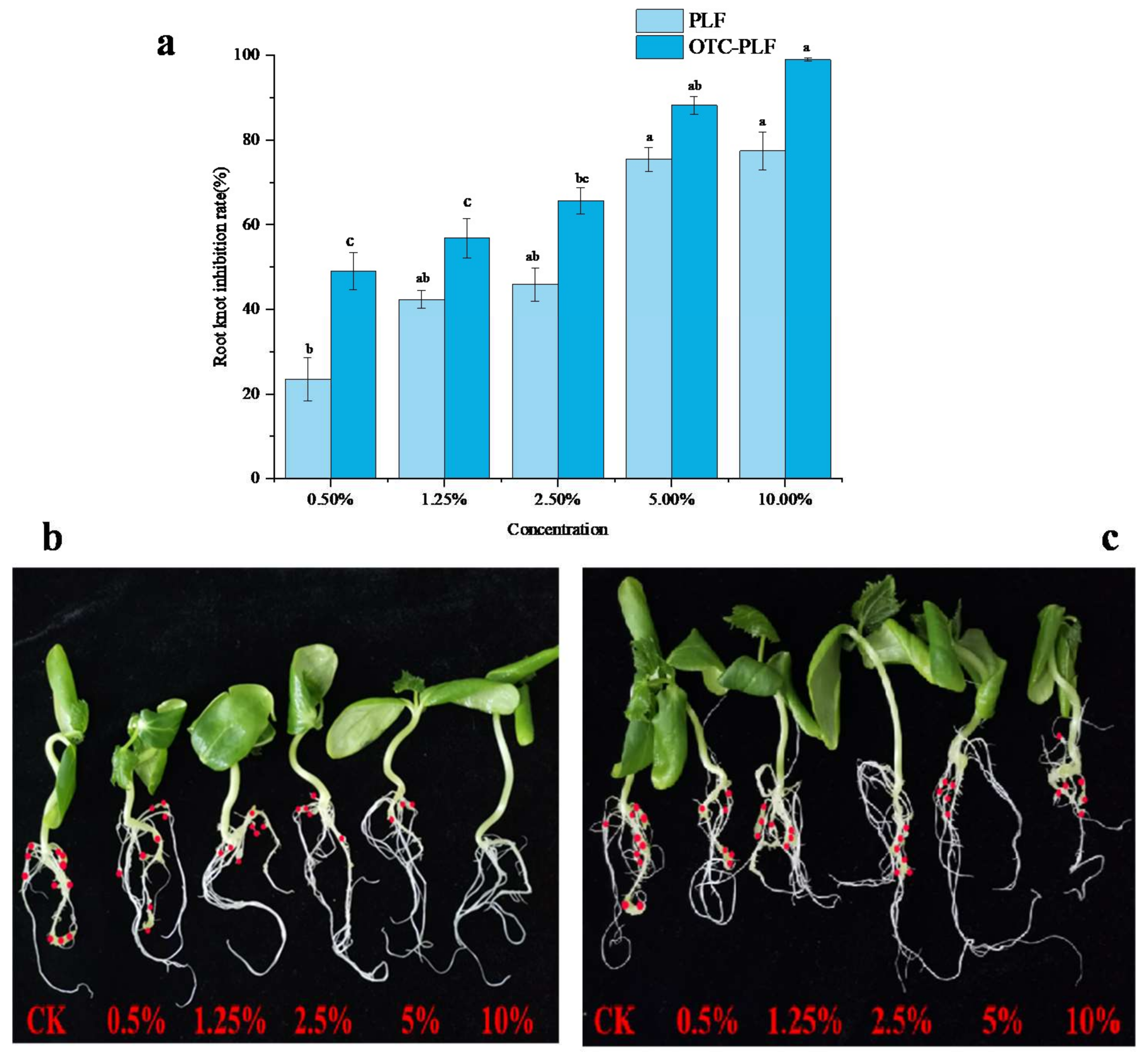
2.7. Phytotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of C-6 Oxidized Chitin (6-Oxychitin)
4.2.1. Preparation of the C-6 Oxidized chitin (6-Oxychitin)
4.2.2. Characterization and Analytical Method
4.3. Culturing and Collecting of Nematode
4.4. Screening for Nematicidal Microorganisms Utilizing Chitin
4.5. Screening of the Optimum Water-Soluble Chitin Derivatives
4.6. Screening the Factors Affecting Nematicidal Activity
4.7. Anti-Nematode Assay
4.7.1. Effect on J2s Locomotion Behaviour
4.7.2. Egg Hatching Assay
4.7.3. Effect on Nematode Morphology
4.8. Effect of OTC-PLF on Nematode Infecting Cucumber
4.8.1. Plant Materials and Pluronic Gel Preparation
4.8.2. Effect on Root Infection in Pluronic Gel
4.8.3. Effect on the Invasion of Root-Knot Nematodes, M. Incognita
4.9. Phytotoxicity Assay
- RLS: root length of the experimental group;
- RLC: root length of the control group;
- GSS: Germination number of seeds in the experimental group;
- GSC: Germination number of seeds in the experimental group;
- when GI < 80%, this value indicates phytotoxicity, while GI > 100%, the value may indicate growth promotion. At the same time, when RGI < 0.8, root elongation was inhibited, while when RGI > 1.2, root elongation was stimulated.
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Wincker, P. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Suarez, N.; Boada, L.D.; Henriquez-Hernandez, L.A.; Gonzalez-Moreo, F.; Suarez-Perez, A.; Camacho, M.; Zumbado, M.; Almeida-Gonzalez, M.; del Mar Travieso-Aja, M.; Luzardo, O.P. Continued implication of the banned pesticides carbofuran and aldicarb in the poisoning of domestic and wild animals of the Canary Islands (Spain). Sci. Total Environ. 2015, 505, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Ajwa, H.A.; Trout, T.; Mueller, J.; Wilhelm, S.; Nelson, S.D.; Soppe, R. Application of alternative fumigants through drip irrigation systems. Phytopathology 2002, 92, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Liang, W.; Xie, Z.; Cheng, J.; Du, Y.; Zhao, J. A temperature-responsive release cellulose-based microcapsule loaded with chlorpyrifos for sustainable pest control. J. Hazard. Mater. 2021, 403, 123654. [Google Scholar] [CrossRef]
- Handelsman, J. Biocontrol of Soilborne Plant Pathogens. Plant Cell Online 1996, 8, 1855–1869. [Google Scholar] [CrossRef]
- Chao, W.L. Colonization of the rhizosphere by biological control agents applied to seeds. Phytopathology 1986, 76, 60–65. [Google Scholar] [CrossRef]
- Khalil, M.S.; Badawy, M.E.I. Nematicidal Activity of a Biopolymer Chitosan atDifferent Molecular Weights against Root-Knot Nematode, Meloidogyne incognita. Plant Prot. Ence 2012, 48, 170–178. [Google Scholar]
- Radwan, M.A.; Farrag, S.A.A.; Abu-Elamayem, M.M.; Ahmed, N.S. Extraction, characterization, and nematicidal activity of chitin and chitosan derived from shrimp shell wastes. Biol. Fertil. Soils 2012, 48, 463–468. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ezziyyani, M.; Sánchez, C.P.; Candela, M.E. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur. J. Plant Pathol. 2003, 109, 633–637. [Google Scholar] [CrossRef]
- Gao, K.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, Characterization, and Anti-Phytopathogen Evaluation of 6-Oxychitosan Derivatives Containing N-Quaternized Moieties in Its Backbone. Int. J. Polym. Sci. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Li, B.; Liu, B.; Shan, C.; Ibrahim, M.; Lou, Y.; Wang, Y.; Xie, G.; Li, H.-y.; Sun, G. Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag. Sci. 2013, 69, 312–320. [Google Scholar] [CrossRef]
- Zhao, X.; She, X.; Du, Y.; Liang, X. Induction of antiviral resistance and stimulary effect by oligochitosan in tobacco. Pestic. Biochem. Physiol. 2007, 87, 78–84. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, P. Chitosan Oligosaccharide Fluorinated Derivative Control Root-Knot Nematode (Meloidogyne incognita) Disease Based on the Multi-Efficacy Strategy. Mar. Drugs 2020, 18, 273. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Rodriguezkabana, R.; Morganjones, G.; Gintis, B.O. Effects of chitin amendments to soil on Heterodera glycines, microbial population, and colonization of cysts by fungi. Nematropica 1984, 14, 10–25. [Google Scholar]
- Culbreath, A.K.; Rodriguezkabana, R.; Morganjones, G. The Use of Hemicellulosic Waste Matter for Reduction of the Phytotoxic Effects of Chitin and Control of Root-Knot Nematodes. Nematropica 1985, 15, 49–75. [Google Scholar]
- Mwaheb, M.A.; Hussain, M.; Tian, J.; Zhang, X.; Hamid, M.I.; El-Kassim, N.A.; Hassan, G.M.; Xiang, M.; Liu, X. Synergetic suppression of soybean cyst nematodes by chitosan and Hirsutella minnesotensis via the assembly of the soybean rhizosphere microbial communities. Biol. Control 2017, 115, 85–94. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hur, J.Y.; Park, S.K. Biocontrol of Botrytis cinerea by chitin-based cultures of Paenibacillus elgii HOA73. Eur. J. Plant Pathol. 2019, 155, 253–263. [Google Scholar] [CrossRef]
- Suarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Env. Microbiol. 2021. [Google Scholar] [CrossRef]
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibanez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan Increases Tomato Root Colonization by Pochonia chlamydosporia and Their Combination Reduces Root-Knot Nematode Damage. Front. Plant Sci. 2017, 8, 1415. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sorlie, M.; Varum, K.M.; Eijsink, V.G.H. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Jang, J.; Park, C.R.; Ko, S.W. Preparation and solubility in acid and water of partially deacetylated chitins. Biomacromolecules 2000, 1, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shi, X.; Li, X.; Cai, J.; Duan, B.; Du, Y. Homogeneous synthesis and characterization of quaternized chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2012, 87, 422–426. [Google Scholar] [CrossRef]
- Tokura, S.; Tamura, H. O-Carboxymethyl−Chitin Concentration in Granulocytes during Bone Repair. Biomacromolecules 2001, 2, 417–421. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Cosani, A.; Terbojevich, M. 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins. Carbohydr. Polym. 1999, 39, 361–367. [Google Scholar] [CrossRef]
- Yang, J.; Cai, J.; Wu, K.; Li, D.; Hu, Y.; Li, G.; Du, Y. Preparation, characterization and anticoagulant activity in vitro of heparin-like 6-carboxylchitin derivative. Int. J. Biol. Macromol. 2012, 50, 1158–1164. [Google Scholar] [CrossRef]
- Sun, L.; Du, Y.; Yang, J.; Shi, X.; Li, J.; Wang, X.; Kennedy, J.F. Conversion of crystal structure of the chitin to facilitate preparation of a 6-carboxychitin with moisture absorption–retention abilities. Carbohydr. Polym. 2006, 66, 168–175. [Google Scholar] [CrossRef]
- Vasyukova, N.I.; Zinovieva, S.V.; Udalova, Z.V.; Gerasimova, N.G.; Ozeretskovskaya, O.L.; Sonin, M.D. Jasmonic acid and tomato resistance to the root-knot nematode Meloidogyne incognita. Dokl. Biol. Sci. 2009, 428, 448–450. [Google Scholar] [CrossRef]
- Branch, C.; Hwang, C.F.; Navarre, D.A.; Williamson, V.M. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol. Plant Microbe Interact. 2004, 17, 351–356. [Google Scholar] [CrossRef]
- Xue, Z.; He, X.; Zuo, Y. Community composition and catabolic functional diversity of soil microbes affected by Hedysarum scoparium in arid desert regions of northwest China. Arid Land Res. Manag. 2019, 34, 1–19. [Google Scholar] [CrossRef]
- Tang, H.; Li, C.; Wen, L.; Li, W.; Shi, L.; Cheng, K.; Xiao, X. Microbial carbon source utilization in rice rhizosphere and non-rhizosphere soils in a 34-year fertilized paddy field. J. Basic Microbiol. 2020, 60, 1004–1013. [Google Scholar] [CrossRef]
- Mota, L.C.B.M.; dos Santos, M.A. Chitin and chitosan on Meloidogyne javanica management and on chitinase activity in tomato plants. Trop. Plant Pathol. 2016, 41, 84–90. [Google Scholar] [CrossRef]
- Anusha, B.G.; Kulkarni, S.; Harlapur, S.I. Morphological and Biological Variability of Different Isolates of Paecilomyces lilacinus Against Root Knot Nematode in Tomato. Int. J. Curr. Microbiol. Appl. Ences 2017, 6, 1243–1257. [Google Scholar] [CrossRef]
- Shemshura, O.N.; Bekmakhanova, N.E.; Mazunina, M.N.; Meyer, S.L.F.; Rice, C.P.; Masler, E.P. Isolation and identification of nematode-antagonistic compounds from the fungus Aspergillus candidus. Fems Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef][Green Version]
- Oka, Y.; Chet, I.; Spiegel, Y. Control of rootknot nematode Meloidogyne javanica by Bacillus cereus. Biocontrol Sci. Technol. 1993, 3, 115–126. [Google Scholar] [CrossRef]
- Nimnoi, P.; Pongsilp, N.; Ruanpanun, P. Monitoring the efficiency of Streptomyces galilaeus strain KPS-C004 against root knot disease and the promotion of plant growth in the plant-parasitic nematode infested soils. Biol. Control 2017, 114, 158–166. [Google Scholar] [CrossRef]
- Culbreath, A.K.; Rodriguez-Kabana, R.; Morgan-Jones, G. Chitin and Paecilomyces Lilacinus for Control of Meloidogyne arenaria. Nematropica 1986, 16, 153–166. [Google Scholar]
- Ladner, D.C.; Tchounwou, P.B.; Lawrence, G.W. Evaluation of the Effect of Ecologic on Root Knot Nematode, Meloidogyne incognita, and Tomato Plant, Lycopersicon esculenum. Int. J. Environ. Res. Public Health 2008, 5, 104–110. [Google Scholar] [CrossRef]
- Gao, K.; Zhan, J.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Synthesis and effects of the selective oxidation of chitosan in induced disease resistance against Botrytis cinerea. Carbohydr. Polym. 2021, 265, 118073. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Mittal, A.; Naik, S.N. Statistical optimization of growth media for Paecilomyces lilacinus 6029 using non-edible oil cakes. Ann. Microbiol. 2014, 64, 515–520. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.B.; Shivakumar, S.B.; Devappa, S. Solid-state fermentation of Jatropha seed cake for optimization of lipase, protease and detoxification of anti-nutrients in Jatropha seed cake using Aspergillus versicolor CJS-98. J. Biosci. Bioeng. 2013, 117, 208–214. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Dalela, M. Nematicidal activity of Paecilomyces lilacinus 6029 cultured on Karanja cake medium. Microb. Pathog. 2014, 75, 16–20. [Google Scholar] [CrossRef]
- Djian, C.; Arpin, N.; Favre-Bonvin, J.; Pijarowski, L.; Ponchet, M. Acetic Acid: A Selective Nematicidal Metabolite From Culture Filtrates of Paecilomyces Lilacinus (Thom) Samson and Trichoderma Longibrachiatum Rifai. Nematologica 1991, 37, 101–112. [Google Scholar]
- Khan, A.; Williams, K.L.; Nevalainen, H.K.M. Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol. Control 2004, 31, 346–352. [Google Scholar] [CrossRef]
- Chivangkul, T.; Pengprecha, S.; Padungros, P.; Siraleartmukul, K.; Prasongsuk, S.; Muangsin, N. Enhanced water-solubility and mucoadhesion of N,N,N-trimethyl-N-gluconate-N-homocysteine thiolactone chitosan. Carbohydr. Polym. 2014, 108, 224–231. [Google Scholar] [CrossRef]
- Pierre, G.; Salah, R.; Gardarin, C.; Traikia, M.; Petit, E.; Delort, A.M.; Mameri, N.; Moulti-Mati, F.; Michaud, P. Enzymatic degradation and bioactivity evaluation of C-6 oxidized chitosan. Int. J. Biol. Macromol. 2013, 60, 383–392. [Google Scholar] [CrossRef]
- Atamian, H.S.; Roberts, P.A.; Kaloshian, I. High and Low Throughput Screens with Root-knot Nematodes Meloidogyne spp. Jove J. Vis. Exp. 2012, 61, 3629. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, R.; Wang, X.; Li, P. The bioactivity of new chitin oligosaccharide dithiocarbamate derivatives evaluated against nematode disease (Meloidogyne incognita). Carbohydr. Polym. 2019, 224, 115155. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Yan, P.S.; Chen, H.; He, J. Optimization of production conditions for mushroom polysaccharides with high yield and antitumor activity. Carbohydr. Polym. 2012, 87, 2569–2575. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Hobert, O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J. Neurobiol. 2003, 56, 178–197. [Google Scholar] [CrossRef]
- Bian, T.; Zhu, X.; Guo, J.; Zhuang, Z.; Cai, Z.; Zhao, X. Cai, Toxic effect of the novel chiral insecticide IPP and its biodegradation intermediate in nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 164, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Meyer, S.L.F.; Massoud, S.; Chitwood, D. Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology 2000, 2, 540–544. [Google Scholar]
- Abolafia, J. A low-cost technique to manufacture a container to process meiofauna for scanning electron microscopy. Microsc. Res. Tech. 2015, 78, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.; Dutta, T.K.; Banakar, P.; Rao, U. Comparing the defence-related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci. Rep. 2016, 6, 22846. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Chunyan, C.; Kaijing, Z.; Zhen, T.; Jian, X.; Shuqiong, Y.; Qunfeng, L.; Ji, L.; Jin-Feng, C. Comparative transcriptomics reveals suppressed expression of genes related to auxin and the cell cycle contributes to the resistance of cucumber against Meloidogyne incognita. BMC Genom. 2018, 19, 583–597. [Google Scholar]
- Bybd, D.W., Jr.; Kirkpatrick, T.; Barker, K.R. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar] [PubMed]
- Magdaleno, A.; Peralta Gavensky, M.; Fassiano, A.V.; Rios de Molina, M.C.; Santos, M.; March, H.; Moretton, J.; Juarez, A.B. Phytotoxicity and genotoxicity assessment of imazethapyr herbicide using a battery of bioassays. Environ. Sci. Pollut. Res. 2015, 22, 19194–19202. [Google Scholar] [CrossRef]




| Sample | Corrected Mortality (%) | |
|---|---|---|
| 24 h | 48 h | |
| Purpureocillium lilacinum | 20.27 ± 0.90 ab | 56.08 ± 4.16 bc |
| Chitin+ Purpureocillium lilacinum | 20.68 ± 5.26 ab | 74.17 ± 6.12 a |
| Aspergillus sp. | 27.19 ± 14.60 a | 61.25 ± 2.57 ab |
| Chitin+ Aspergillus sp. | 17.07 ± 2.66 ab | 43.68 ± 14.30 cd |
| B. licheniformis | 13.18 ± 3.29 b | 55.89 ± 9.14 bc |
| Chitin+ B. licheniformis | 16.43 ± 4.65 ab | 49.20 ± 14.33 bcd |
| B. subtilis | 22.30 ± 4.33 ab | 37.66 ± 4.55 d |
| Chitin+ B. subtilis | 21.97 ± 7.47 ab | 50.31 ± 1.48 bcd |
| St. roseofulvus | 11.97 ± 3.26 b | 60.17 ± 11.13 ab |
| Chitin+ St. roseofulvus | 17.26 ± 5.44 ab | 56.92 ± 7.23 bc |
| St. cuspidosporus | 14.09 ± 2.23 b | 49.92 ± 6.63 bcd |
| Chitin+ St. cuspidosporus | 18.20 ± 4.42 ab | 42.44 ± 6.80 cd |
| Samples | Corrected Mortality (%) | |
|---|---|---|
| 24 h | 48 h | |
| No treated | 43.89 ± 9.18 d | 53.49 ± 13.92 d |
| Chitin | 41.73 ± 5.35 d | 68.12 ± 21.88 cd |
| Chitosan | 47.50 ± 3.13 d | 78.07 ± 6.99 ab |
| 6-oxychitin | 94.07 ± 1.96 a | 95.94 ± 0.62 a |
| CSC-3000 Da | 50.94 ± 3.23 cd | 79.79 ± 4.69 ab |
| CSC-10000 Da | 79.20 ± 12.42 b | 50.27 ± 8.54 d |
| Chitin oligosaccharides | 62.62 ± 10.98 c | 78.16 ± 12.74 ab |
| Concentration | Percentage Germination (%) | Germination Index (GI %) | Relative Growth Index (RGI) | |
|---|---|---|---|---|
| OTC-PLF | 20.00% | 89.20 ± 3.22 b | 106.37 ± 3.27 bc | 1.04 ± 0.02 a |
| 10.00% | 90.00 ± 2.00 b | 110.69 ± 6.06 b | 1.08 ± 0.07 ab | |
| 5.00% | 79.59 ± 2.04 de | 100.56 ± 1.88 cd | 1.11 ± 0.04 ab | |
| 2.50% | 93.75 ± 2.09 a | 120.63 ± 0.6 a | 1.13 ± 0.02 ab | |
| 1.25% | 84.00 ± 2.00 c | 91.69 ± 4.11 f | 0.96 ± 0.02 a | |
| PLF | 20.00% | 60.78 ± 1.98 f | 69.8 ± 1.78 g | 1.01 ± 0.01 a |
| 10.00% | 80.00 ± 2.00 de | 95.08 ± 3.99 df | 1.04 ± 0.02 a | |
| 5.00% | 78.00 ± 1.53 e | 89.72 ± 3.03 f | 1.01 ± 0.03 a | |
| 2.50% | 78.43 ± 1.96 de | 93.13 ± 4.05 df | 1.04 ± 0.03 b | |
| 1.25% | 77.67 ± 1.53 e | 89.18 ± 4.87 f | 1.01 ± 0.05 a | |
| PC | 88.00 ± 2.00 b | 95.39 ± 1.92 df | 0.95 ± 0.01 a | |
| NC | 82.00 ± 2.00 b | 100 cd | 1 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Qin, Y.; Gao, K.; Fan, Z.; Wang, L.; Xing, R.; Liu, S.; Li, P. Efficacy of a Chitin-Based Water-Soluble Derivative in Inducing Purpureocillium lilacinum against Nematode Disease (Meloidogyne incognita). Int. J. Mol. Sci. 2021, 22, 6870. https://doi.org/10.3390/ijms22136870
Zhan J, Qin Y, Gao K, Fan Z, Wang L, Xing R, Liu S, Li P. Efficacy of a Chitin-Based Water-Soluble Derivative in Inducing Purpureocillium lilacinum against Nematode Disease (Meloidogyne incognita). International Journal of Molecular Sciences. 2021; 22(13):6870. https://doi.org/10.3390/ijms22136870
Chicago/Turabian StyleZhan, Jiang, Yukun Qin, Kun Gao, Zhaoqian Fan, Linsong Wang, Ronge Xing, Song Liu, and Pengcheng Li. 2021. "Efficacy of a Chitin-Based Water-Soluble Derivative in Inducing Purpureocillium lilacinum against Nematode Disease (Meloidogyne incognita)" International Journal of Molecular Sciences 22, no. 13: 6870. https://doi.org/10.3390/ijms22136870
APA StyleZhan, J., Qin, Y., Gao, K., Fan, Z., Wang, L., Xing, R., Liu, S., & Li, P. (2021). Efficacy of a Chitin-Based Water-Soluble Derivative in Inducing Purpureocillium lilacinum against Nematode Disease (Meloidogyne incognita). International Journal of Molecular Sciences, 22(13), 6870. https://doi.org/10.3390/ijms22136870





