Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain
Abstract
:1. Introduction
2. Results
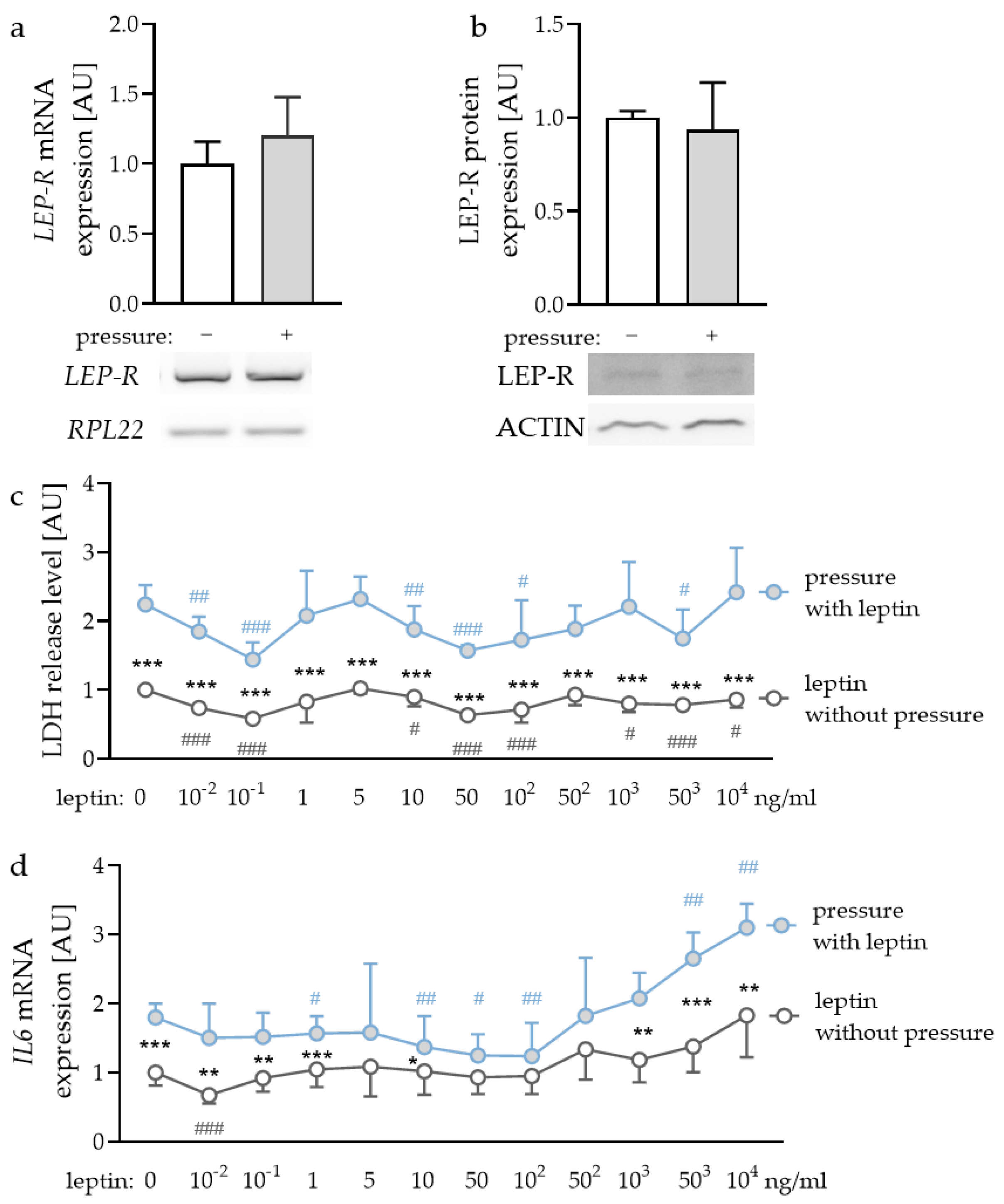
2.1. Expression of Leptin Receptors in PDLF and Effects of Different Leptin Concentrations
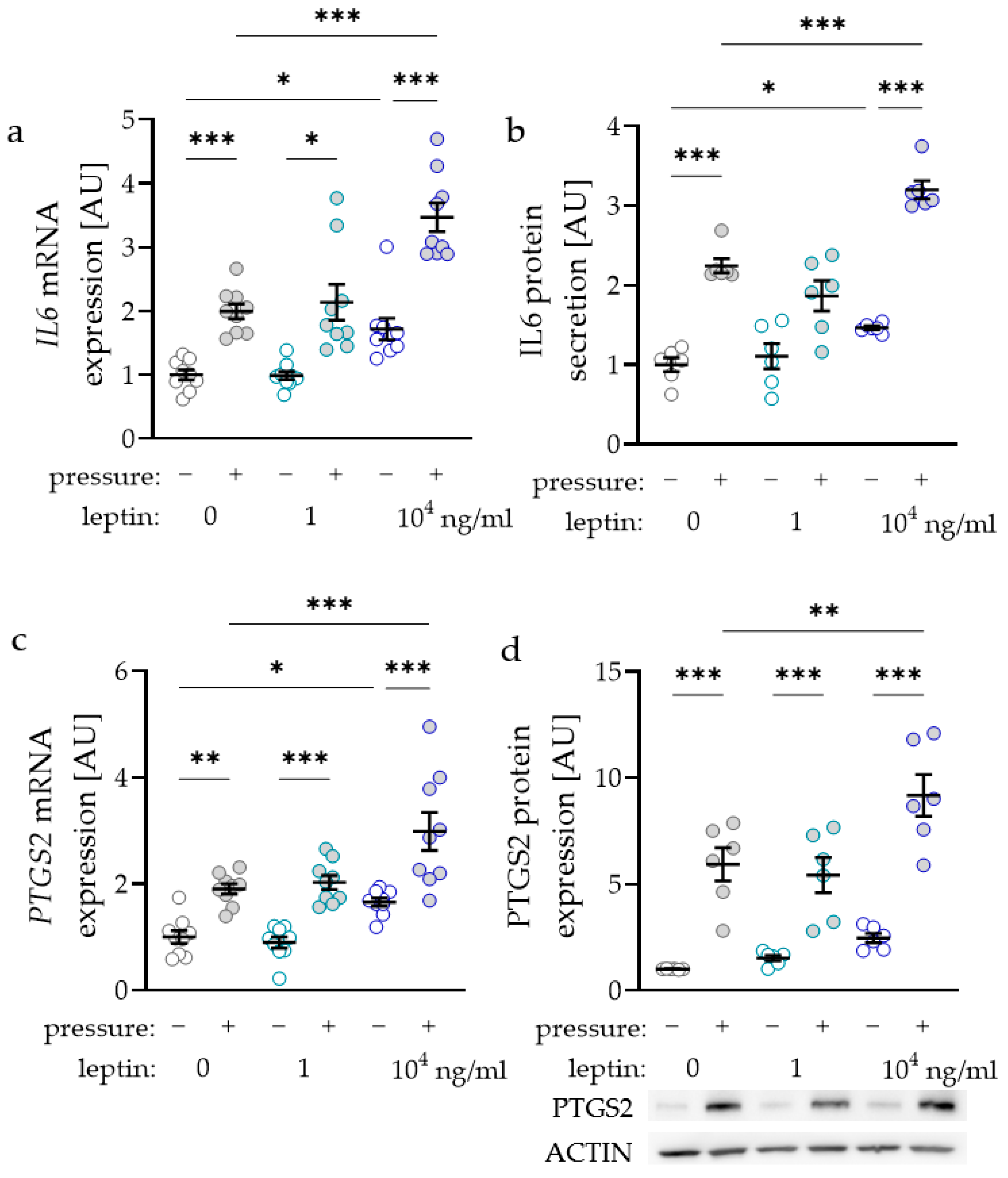
2.2. Impact of Leptin Combined with Compressive Strain on the Expression of Proinflammatory Factors
2.3. Impact of Leptin Combined with Compressive Strain on the Expression of Bone-Remodelling Factors
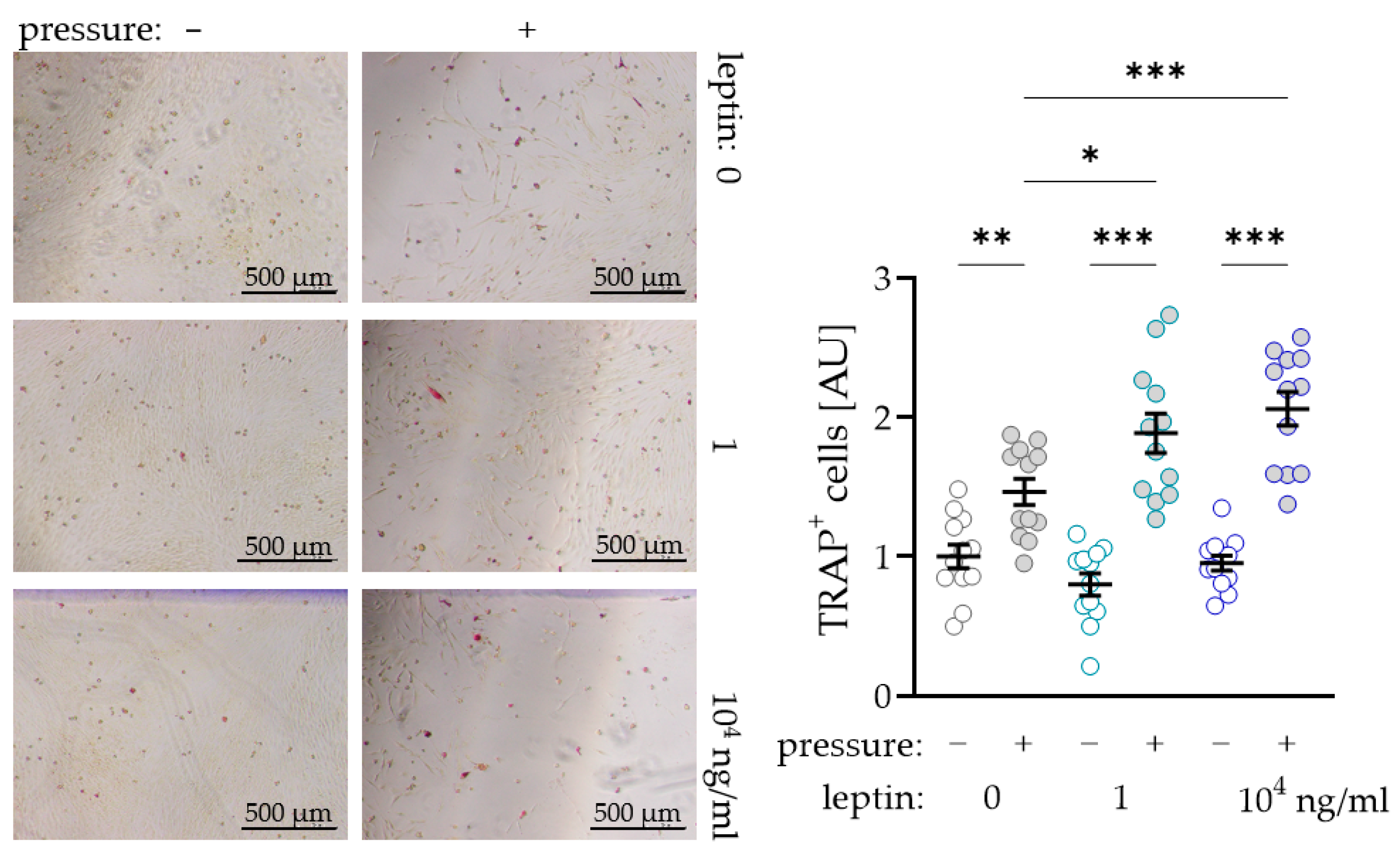
2.4. Impact of Leptin Combined with Compressive Strain on Differentiation of Osteoclasts in a Coculture Model
3. Discussion
4. Materials and Methods
4.1. Cell Culture Experiments
4.2. LDH Assay
4.3. RNA Isolation and cDNA Synthesis
4.4. Semiquantitative PCR and Agarose Gel Electrophoresis
4.5. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.6. Western Blot Analysis
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. TRAP (Tartrate-Resistant Acid Phosphatase) Staining
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Will, L.A. Orthodontic Tooth Movement: A Historic Prospective. Front. Oral Biol. 2016, 18, 46–55. [Google Scholar] [CrossRef]
- Schröder, A.; Käppler, P.; Nazet, U.; Jantsch, J.; Proff, P.; Cieplik, F.; Deschner, J.; Kirschneck, C. Effects of Compressive and Tensile Strain on Macrophages during Simulated Orthodontic Tooth Movement. Mediat. Inflamm. 2020, 2020, 2814015. [Google Scholar] [CrossRef]
- Wolf, M.; Lossdörfer, S.; Marciniak, J.; Römer, P.; Kirschneck, C.; Craveiro, R.B.; Deschner, J.; Jäger, A. CD8+ T cells mediate the regenerative PTH effect in hPDL cells via Wnt10b signaling. Innate Immun. 2016, 22, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschneck, C.; Kuchler, E.; Wolf, M.; Spanier, G.; Proff, P.; Schröder, A. Effects of the Highly COX-2-Selective Analgesic NSAID Etoricoxib on Human Periodontal Ligament Fibroblasts during Compressive Orthodontic Mechanical Strain. Mediat. Inflamm. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef]
- Kanzaki, H.; Chiba, M.; Shimizu, Y.; Mitani, H. Periodontal Ligament Cells Under Mechanical Stress Induce Osteoclastogenesis by Receptor Activator of Nuclear Factor κB Ligand Up-Regulation via Prostaglandin E2 Synthesis. J. Bone Miner. Res. 2002, 17, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment? Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Consolaro, A. Obesity and orthodontic treatment: Is there any direct relationship? Dent. Press J. Orthod. 2017, 22, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Chaffee, B.W.; Weston, S.J. Association Between Chronic Periodontal Disease and Obesity: A Systematic Review and Meta-Analysis. J. Periodontol. 2010, 81, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Rohde, J.F.; Raymond, K.; Heitmann, B.L. Association Between Periodontal Disease and Overweight and Obesity: A Systematic Review. J. Periodontol. 2015, 86, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; De Smet, S.; Yusof, M.Y.P.M.; Rajasekharan, S. Association between overweight/obesity and periodontal disease in children and adolescents: A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2017, 18, 69–82. [Google Scholar] [CrossRef]
- Nishida, N.; Tanaka, M.; Hayashi, N.; Nagata, H.; Takeshita, T.; Nakayama, K.; Morimoto, K.; Shizukuishi, S. Determination of Smoking and Obesity as Periodontitis Risks Using the Classification and Regression Tree Method. J. Periodontol. 2005, 76, 923–928. [Google Scholar] [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Issa, R.I.; Griffin, T.M. Pathobiology of obesity and osteoarthritis: Integrating biomechanics and inflammation. Pathobiol. Aging Age Relat. Dis. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and Surgical Wound Healing: A Current Review. ISRN Obes. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Seven, A.; Guzel, S.; Aslan, M.; Hamuryudan, V. Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol. Int. 2008, 29, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Margalet, V.; Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Yanes, C.G. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Procaccini, C.; De Rosa, V.; Horvath, T.L.; La Cava, A. Regulatory T cells in obesity: The leptin connection. Trends Mol. Med. 2010, 16, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Callon, K.E.; Bava, U.; Lin, C.; Naot, D.; Hill, B.L.; Grey, A.B.; Broom, N.; Myers, D.E.; Nicholson, G.; et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol. 2002, 175, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Gordeladze, J.O.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J. Cell. Biochem. 2002, 85, 825–836. [Google Scholar] [CrossRef]
- Holloway, W.R.; Collier, F.M.; Aitken, C.J.; Myers, D.E.; Hodge, J.M.; Malakellis, M.; Gough, T.J.; Collier, G.R.; Nicholson, G. Leptin Inhibits Osteoclast Generation. J. Bone Miner. Res. 2002, 17, 200–209. [Google Scholar] [CrossRef]
- Reid, I.R.; Comish, J. Direct actions of leptin on bone remodeling. Calcif. Tissue Int. 2003, 74, 313–316. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Dilsiz, A.; Kiliç, N.; Aydin, T.; Ates, F.N.; Zihni, M.; Bulut, C. Leptin Levels in Gingival Crevicular Fluid During Orthodontic Tooth Movement. Angle Orthod. 2010, 80, 504–508. [Google Scholar] [CrossRef]
- Srinivasan, B.; Chitharanjan, A.; Kailasam, V.; Lavu, V.; Ganapathy, V. Evaluation of leptin concentration in Gingival Crevicular Fluid (GCF) during orthodontic tooth movement and its correlation to the rate of tooth movement. J. Orthod. Sci. 2019, 8, 6. [Google Scholar] [CrossRef]
- Saloom, H.; Papageorgiou, S.; Carpenter, G.; Cobourne, M. Impact of Obesity on Orthodontic Tooth Movement in Adolescents: A Prospective Clinical Cohort Study. J. Dent. Res. 2017, 96, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, B.V.; Pradeep, A.R. Leptin levels in gingival crevicular fluid in periodontal health and disease. J. Periodontal Res. 2007, 42, 300–304. [Google Scholar] [CrossRef]
- Park, H.-G.; Kim, J.-H.; Cha, J.-H.; Bak, E.-J.; Yoo, Y.-J. Induction of IL-6 and IL-8 Expression by Leptin Treatment in Periodontal Ligament Cells and Gingival Fibroblasts. Int. J. Oral Biol. 2013, 38, 73–80. [Google Scholar] [CrossRef]
- Lago, F.; Diéguez, C.; Gómez-Reino, J.; Gualillo, O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pr. Rheumatol. 2007, 3, 716–724. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; Gómez, R.; López, V.; Gómez-Reino, J.J.; Lago, F.; Gualillo, O. Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. BioFactors 2011, 37, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Nazet, U.; Neubert, P.; Jantsch, J.; Spanier, G.; Proff, P.; Kirschneck, C. Sodium-chloride-induced effects on the expression profile of human periodontal ligament fibroblasts with focus on simulated orthodontic tooth movement. Eur. J. Oral Sci. 2019, 127, 386–395. [Google Scholar] [CrossRef]
- Grimm, S.; Wolff, E.; Walter, C.; Pabst, A.M.; Mundethu, A.; Jacobs, C.; Wehrbein, H.; Jacobs, C. Influence of clodronate and compressive force on IL-1ß-stimulated human periodontal ligament fibroblasts. Clin. Oral Investig. 2019, 24, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Bauer, K.; Spanier, G.; Proff, P.; Wolf, M.; Kirschneck, C. Expression kinetics of human periodontal ligament fibroblasts in the early phases of orthodontic tooth movement. J. Orofac. Orthop. 2018, 79, 337–351. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.; Nie, Y.; Chen, Z.; Wang, H.; Liao, H.; Yuelin, C. Leptin upregulates COX-2 and its downstream products in aortic endothelial cells. Exp. Ther. Med. 2017, 14, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.; Timms, E.; Tan, H.-L.; Kelley, M.; Dunstan, C.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sarosi, I.; Yan, X.-Q.; Morony, S.; Capparelli, C.; Tan, H.-L.; McCabe, S.; Elliott, R.; Scully, S.; Van, G.; et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 1566–1571. [Google Scholar] [CrossRef] [Green Version]
- Klein, D.C.; Raisz, L.G. Prostaglandins: Stimulation of Bone Resorption in Tissue Culture. Endocrinology 1970, 86, 1436–1440. [Google Scholar] [CrossRef]
- Kirschneck, C.; Küchler, E.C.; Wahlmann, U.; Proff, P.; Schröder, A. Effects of the highly COX-2-selective analgesic NSAID etoricoxib on the rate of orthodontic tooth movement and cranial growth. Ann. Anat. 2018, 220, 21–28. [Google Scholar] [CrossRef]
- Nazet, U.; Schröder, A.; Spanier, G.; Wolf, M.; Proff, P.; Kirschneck, C. Simplified method for applying static isotropic tensile strain in cell culture experiments with identification of valid RT-qPCR reference genes for PDL fibroblasts. Eur. J. Orthod. 2019, 42, 359–370. [Google Scholar] [CrossRef]
- Groeger, M.; Spanier, G.; Wolf, M.; Deschner, J.; Proff, P.; Schröder, A.; Kirschneck, C. Effects of histamine on human periodontal ligament fibroblasts under simulated orthodontic pressure. PLoS ONE 2020, 15, e0237040. [Google Scholar] [CrossRef]
- Nettelhoff, L.; Grimm, S.; Jacobs, C.; Walter, C.; Pabst, A.M.; Goldschmitt, J.; Wehrbein, H. Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin. Oral Investig. 2015, 20, 621–629. [Google Scholar] [CrossRef]
- Van Dielen, F.; Veer, C.V.; Schols, A.; Soeters, P.; Buurman, W.; Greve, J. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int. J. Obes. 2001, 25, 1759–1766. [Google Scholar] [CrossRef] [Green Version]
- Elefteriou, F.; Takeda, S.; Ebihara, K.; Magre, J.; Patano, N.; Kim, C.; Ogawa, Y.; Liu, X.; Ware, S.M.; Craigen, W.J.; et al. Serum leptin level is a regulator of bone mass. Proc. Natl. Acad. Sci. USA 2004, 101, 3258–3263. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kirschneck, C.; Batschkus, S.; Proff, P.; Köstler, J.; Spanier, G.; Schröder, A. Valid gene expression normalization by RT-qPCR in studies on hPDL fibroblasts with focus on orthodontic tooth movement and periodontitis. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Symbol | Gene Name | 5′-Forward Primer-3′ | 5′-Reverse Primer-3′ |
|---|---|---|---|
| IL-6 | interleukin-6 | TGGCAGAAAACAACCTGAACC | CCTCAAACTCCAAAAGACCAGTG |
| LEP-R | leptin receptor | CAGAAGCCAGAAACGTTTGAG | AGCCCTTGTTCTTCACCAGT |
| OPG | osteoprotegerin | TGTCTTTGGTCTCCTGCTAACTC | ACGCTCCAGGACTTATACCG |
| PPIB | peptidylprolyl isomeraseA | TTCCATCGTGTAATCAAGGACTTC | GCTCACCGTAGATGCTCTTTC |
| PTGS2 | prostaglandin-endoperoxide synthase-2 | GAGCAGGCAGATGAAATACCAGTC | TGTCACCATAGAGTGCTTCCAAC |
| RANKL | receptor activator of NFkB ligand | ATACCCTGATGAAAGGAGGA | GGGGCTCAATCTATATCTCG |
| RPL22 | ribosomal protein L22 | TGATTGCACCCACCCTGTAG | GGTTCCCAGCTTTTCCGTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, A.; Meyer, A.; Spanier, G.; Damanaki, A.; Paddenberg, E.; Proff, P.; Kirschneck, C. Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain. Int. J. Mol. Sci. 2021, 22, 6847. https://doi.org/10.3390/ijms22136847
Schröder A, Meyer A, Spanier G, Damanaki A, Paddenberg E, Proff P, Kirschneck C. Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain. International Journal of Molecular Sciences. 2021; 22(13):6847. https://doi.org/10.3390/ijms22136847
Chicago/Turabian StyleSchröder, Agnes, Andrea Meyer, Gerrit Spanier, Anna Damanaki, Eva Paddenberg, Peter Proff, and Christian Kirschneck. 2021. "Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain" International Journal of Molecular Sciences 22, no. 13: 6847. https://doi.org/10.3390/ijms22136847
APA StyleSchröder, A., Meyer, A., Spanier, G., Damanaki, A., Paddenberg, E., Proff, P., & Kirschneck, C. (2021). Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain. International Journal of Molecular Sciences, 22(13), 6847. https://doi.org/10.3390/ijms22136847







