Inhibitory Mechanisms of Lusianthridin on Human Platelet Aggregation
Abstract
:1. Introduction
2. Results
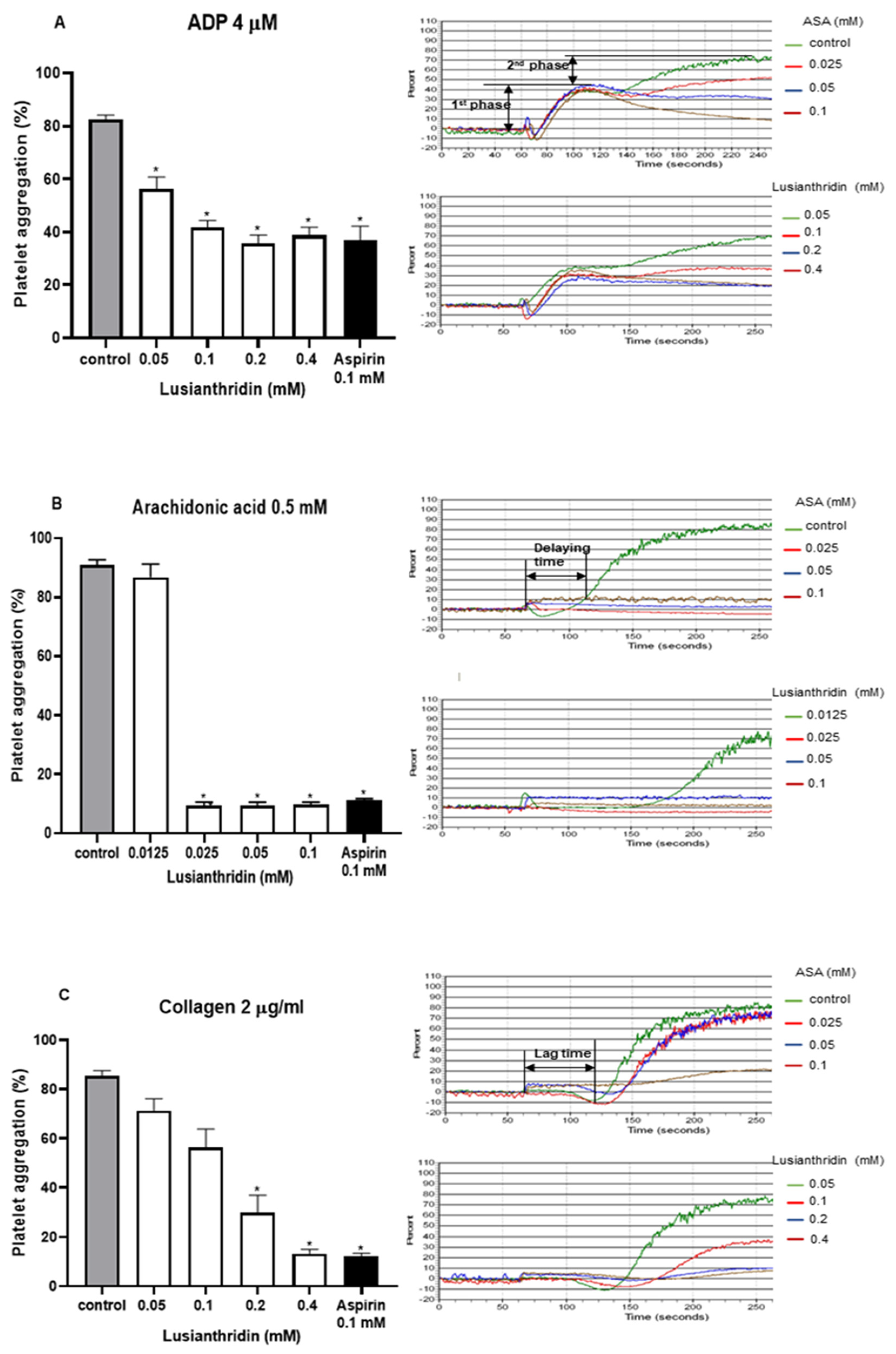
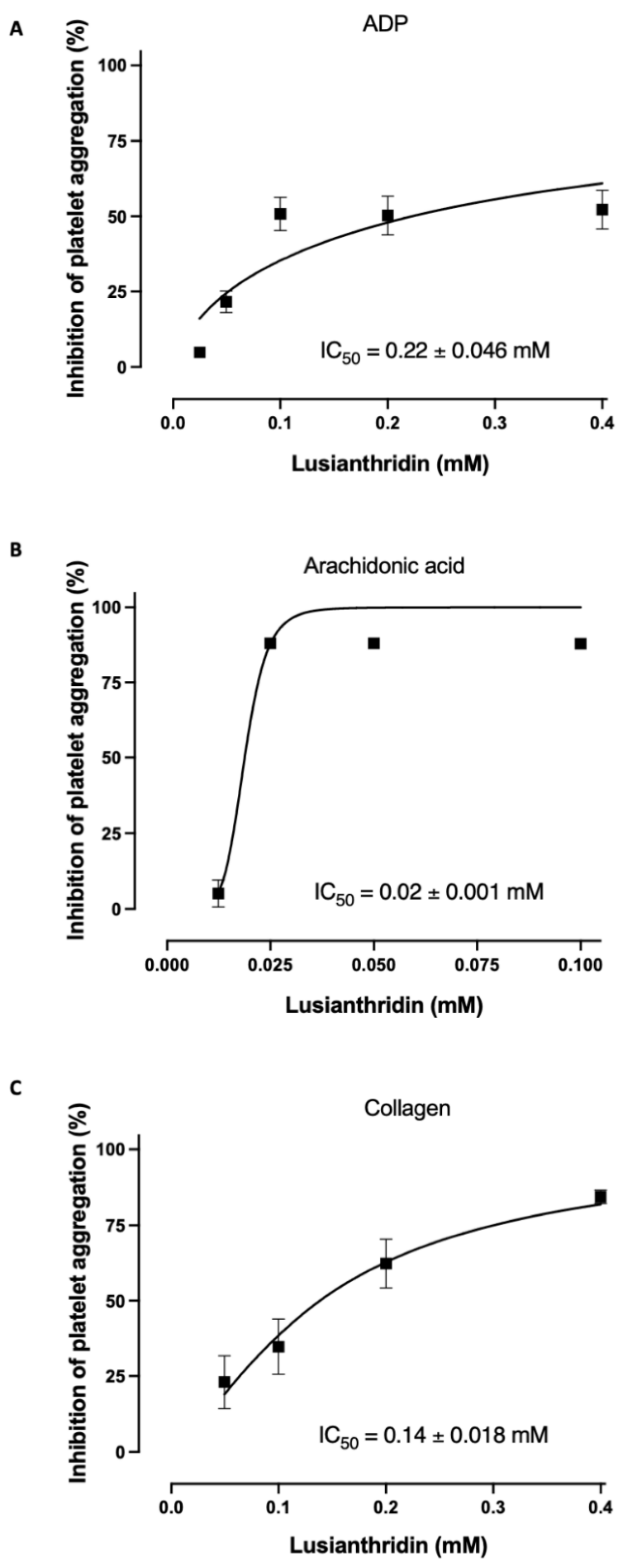
2.1. Antiplatelet Aggregation Activities of Lusianthridin
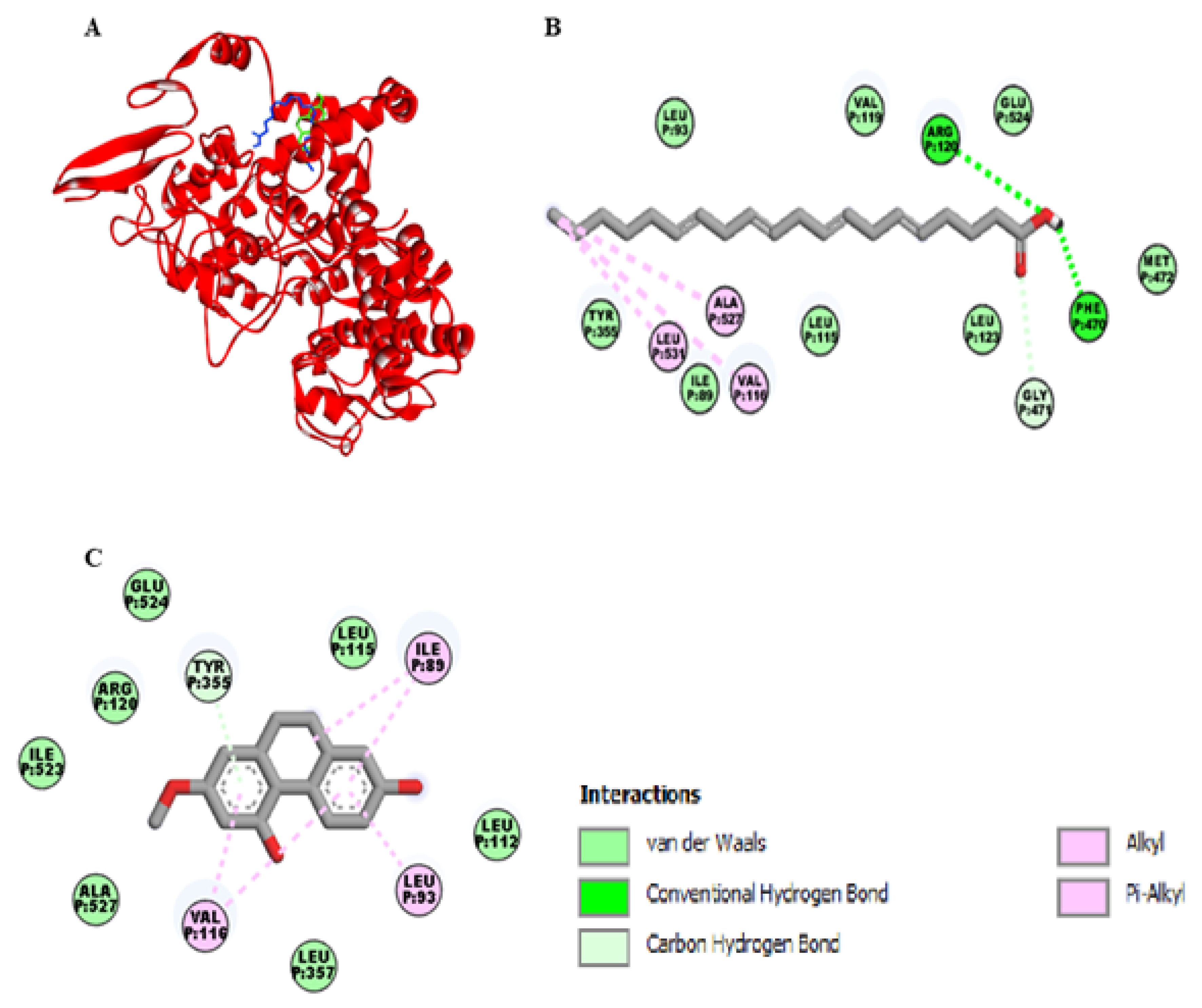
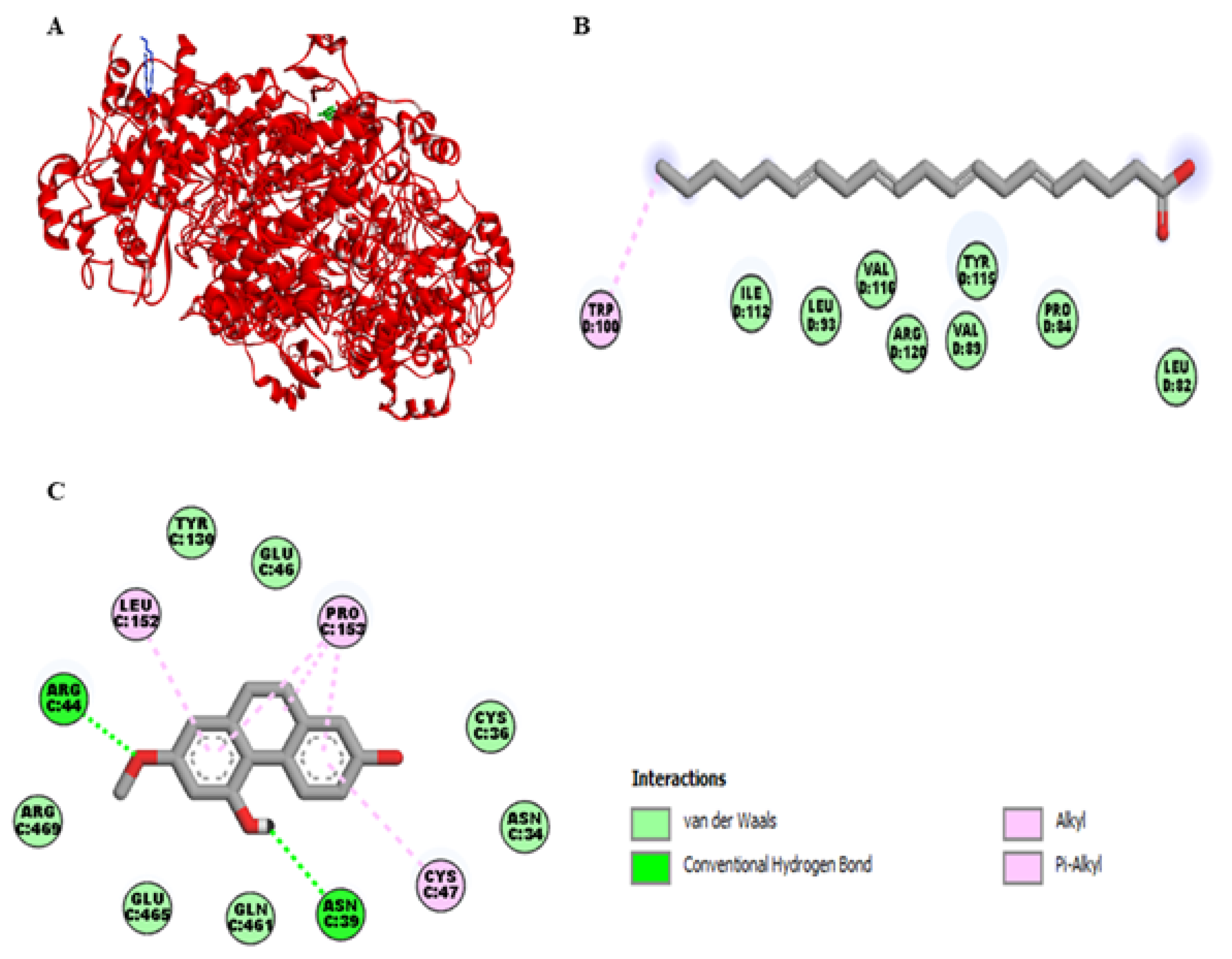
2.2. Molecular Docking Studies of Lusianthridin on COX-1 and COX-2 Enzymes
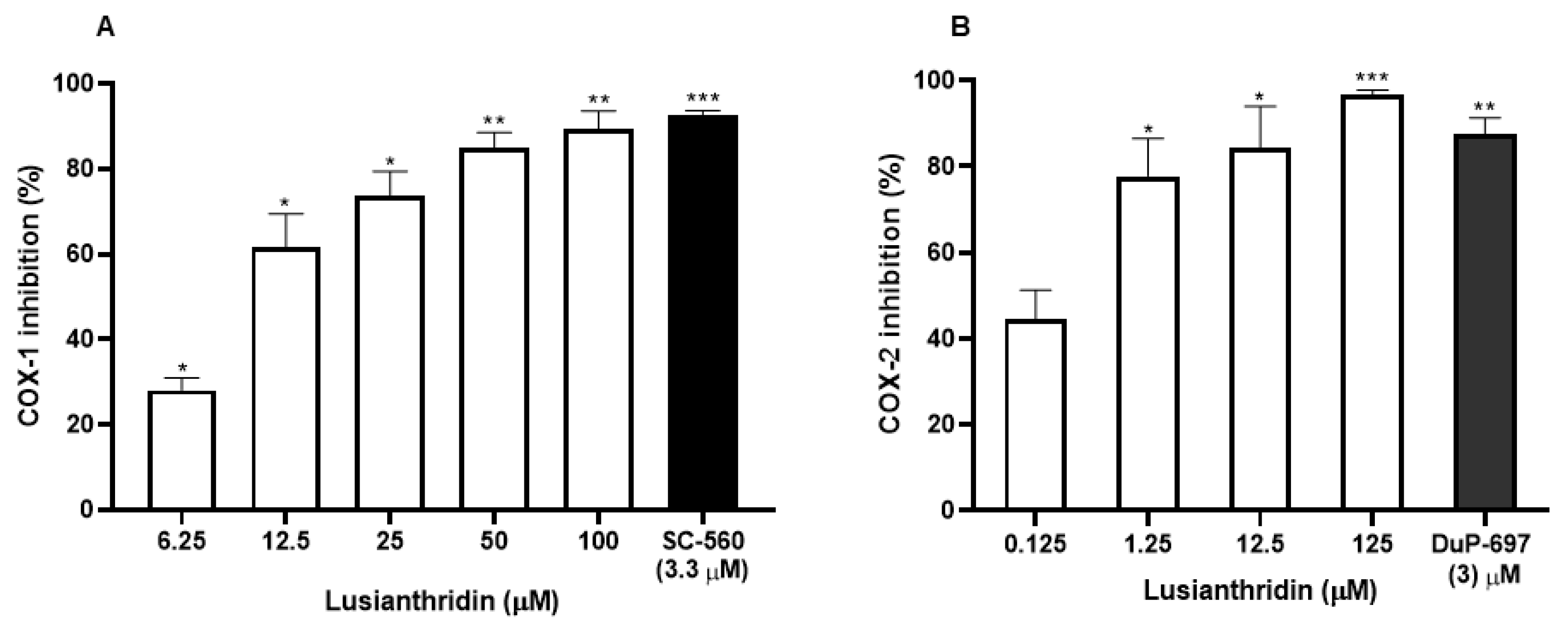
2.3. Effects of Lusianthridin on Cyclooxygenase Enzymes Activities
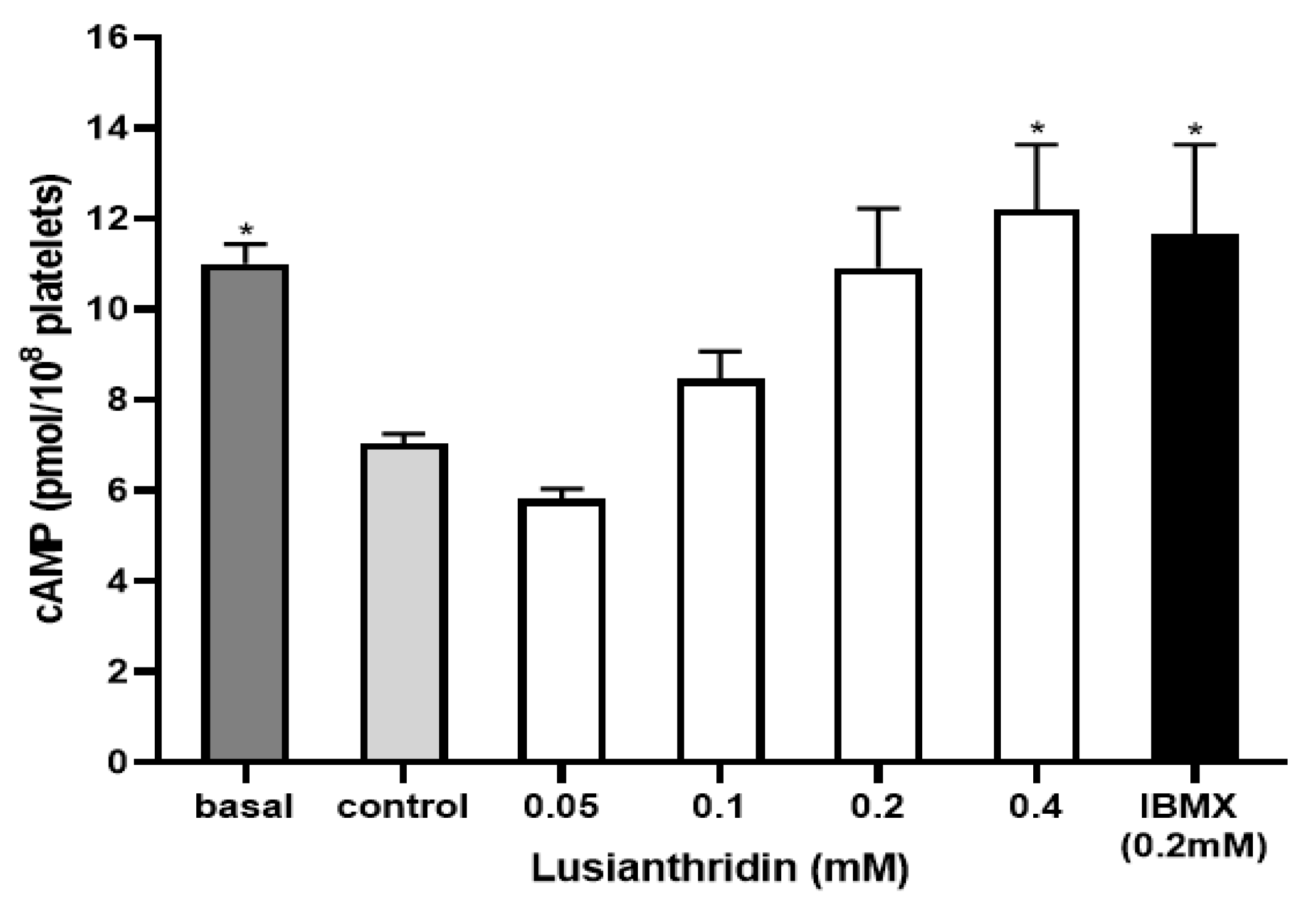
2.4. Effect of Lusianthridin on cAMP Levels
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Platelet-Rich Plasma (PRP) Preparation
4.3. Platelet Aggregation Measurement
4.4. Molecular Docking Study
4.5. Cyclooxygenase Activity Assay
4.6. Measurement of cAMP Levels
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 18 April 2020).
- Davì, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef]
- Rao, G.H. Manual of Blood Platelets: Morphology, Physiology and Pharmacology; Jaypee Brothers Medical Publishers: New Delhi, India, 2018. [Google Scholar]
- Barrett, N.; Holbrook, L.; Jones, S.; Kaiser, W.; Moraes, L.; Rana, R.; Sage, T.; Stanley, R.; Tucker, K.; Wright, B. Future innovations in antiplatelet therapies. Br. J. Pharmacol. 2008, 154, 918–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustina, W.; Kristanti, A.; Takaya, Y.; Fitriana, E.; Aminah, N. In Batatasin III a Derivative of Dihydrostilbene Compound from Yam Peel of Uwi Tuban and Its Antioxidant Activity; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK; p. 042003.
- Chen, C.-C.; Wu, L.-G.; Ko, F.-N.; Teng, C.-M. Antiplatelet aggregation principles of Dendrobium loddigesii. J. Nat. Prod. 1994, 57, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, W.; Wang, Y.; Qin, G.; Zhao, W. Chemical constituents from Dendrobium densiflorum. Phytochemistry 2001, 57, 1255–1258. [Google Scholar] [CrossRef]
- Klongkumnuankarn, P.; Busaranon, K.; Chanvorachote, P.; Sritularak, B.; Jongbunprasert, V.; Likhitwitayawuid, K. Cytotoxic and antimigratory activities of phenolic compounds from Dendrobium brymerianum. Evid.-Based Complement. Altern. Med. 2015, 2015, 350410. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-X.; Shaw, P.-C.; Sze, C.-W.; Tong, Y.; Yao, X.-S.; Ng, T.-B.; Zhang, Y.-B. Chrysotoxine, a novel bibenzyl compound, inhibits 6-hydroxydopamine induced apoptosis in SH-SY5Y cells via mitochondria protection and NF-κB modulation. Neurochem. Int. 2010, 57, 676–689. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010, 4, 592–638. [Google Scholar]
- Dressler, R.L.; Dodson, C.H. Classification and phylogeny in the Orchidacea. Ann. Mo. Bot. Gard. 1960, 47, 25–68. [Google Scholar] [CrossRef]
- Szlachetko, D.L. Genera et species Orchidalium 1. Pol. Bot. J. 2001, 46, 11–26. [Google Scholar]
- Hu, J.-M.; Chen, J.-J.; Yu, H.; Zhao, Y.-X.; Zhou, J. Five New Compounds from Dendrobium longicornu. Planta Med. 2008, 74, 535–539. [Google Scholar] [CrossRef]
- Chen, C.-C.; Huang, Y.-L.; Teng, C.-M. Antiplatelet aggregation principles from Ephemerantha lonchophylla. Planta Med. 2000, 66, 372–374. [Google Scholar] [CrossRef]
- Weber, A.A.; Reimann, S.; Schrör, K. Specific inhibition of ADP-induced platelet aggregation by clopidogrel in vitro. Br. J. Pharmacol. 1999, 126, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Brass, L.F.; Newman, D.K.; Wannemacher, K.; Zhu, L.; Stalker, T.J. Chapter 19. Signal transduction during platelet plug formation. In Platelets, 3rd ed.; Academic Press: Millbrae, CA, USA, 2013; pp. 319–346. [Google Scholar]
- Zhou, L.; Schmaier, A.H. Platelet aggregation testing in platelet-rich plasma: Description of procedures with the aim to develop standards in the field. Am. J. Clin. Pathol. 2005, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kunapuli, S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. USA 1998, 95, 8070–8074. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.C.; Liao, C.H.; Chang, Y.W.; Liou, J.T.; Day, Y.J. A new insight of antiplatelet effects of sirtinol in platelets aggregation via cyclic AMP phosphodiesterase. Biochem. Pharmacol. 2009, 77, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Giroux, V.; Dagorn, J.-C.; Iovanna, J.L. A review of kinases implicated in pancreatic cancer. Pancreatology 2009, 9, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Fitzgerald, D.; Cox, D. Platelet Function: Assessment, Diagnosis, and Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Roberts, D.E.; McNicol, A.; Bose, R. Mechanism of collagen activation in human platelets. J. Biol. Chem. 2004, 279, 19421–19430. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.; Truss, N.; Ali, F.; Dhanji, A.; Vojnovic, I.; Zain, Z.; Bishop-Bailey, D.; Paul-Clark, M.; Tucker, A.; Mitchell, J. Aspirin and the in vitro linear relationship between thromboxane A2-mediated platelet aggregation and platelet production of thromboxane A2. J. Thromb. Haemost. 2008, 6, 1933–1943. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancilla, T.; Correa-Basurto, J.; Alavés Carbajal, K.S.; Sánchez Escalante, E.T.; Trujillo Ferrara, J. Theoretical study of isoindolines to identify them as cyclooxygenase-1 and-2 inhibitors by docking simulations. J. Mex. Chem. Soc. 2007, 51, 96–102. [Google Scholar]
- Celik, S.; Albayrak, A.T.; Akyuz, S.; Ozel, A.E. Synthesis, molecular docking and ADMET study of ionic liquid as anticancer inhibitors of DNA and COX-2, TOPII enzymes. J. Biomol. Struct. Dyn. 2019, 38, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Sourirajan, A.; Khosla, P.K.; Dev, K. Docking studies in targeting proteins involved in cardiovascular disorders using phytocompounds from Terminalia arjuna. bioRxiv 2020. [Google Scholar] [CrossRef]
- Uzzaman, M.; Hoque, M.J. Physiochemical, molecular docking, and pharmacokinetic studies of Naproxen and its modified derivatives based on DFT. Int. J. Sci. Res. Manag. 2018, 6. [Google Scholar] [CrossRef]
- Madhava, G.; Ramana, K.V.; Sudhana, S.M.; Rao, D.S.; Kumar, K.H.; Lokanatha, V.; Rani, A.U.; Raju, C.N. Aryl/heteroaryl substituted celecoxib derivatives as COX-2 inhibitors: Synthesis, anti-inflammatory activity and molecular docking studies. Med. Chem. 2017, 13, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Cho, M.S.; Thiagarajan, P.; Aung, F.M.; Sood, A.K.; Afshar-Kharghan, V. A small amount of cyclooxygenase 2 (COX2) is constitutively expressed in platelets. Platelets 2017, 28, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Brenneis, C.; Maier, T.J.; Schmidt, R.; Hofacker, A.; Zulauf, L.; Jakobsson, P.-J.; Scholich, K.; Geisslinger, G. Inhibition of prostaglandin E2 synthesis by SC-560 is independent of cyclooxygenase 1 inhibition. FASEB J. 2006, 20, 1352–1360. [Google Scholar] [CrossRef]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat. Prod. Commun. 2014, 9, 825–827. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.T.; Akkawat, B.; Morales, N.P.; Rojnuckarin, P.; Luechapudiporn, R. Antiplatelet activity of deferiprone through cyclooxygenase-1 inhibition. Platelets 2019, 31, 505–512. [Google Scholar] [CrossRef]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swe, H.N.; Sritularak, B.; Rojnuckarin, P.; Luechapudiporn, R. Inhibitory Mechanisms of Lusianthridin on Human Platelet Aggregation. Int. J. Mol. Sci. 2021, 22, 6846. https://doi.org/10.3390/ijms22136846
Swe HN, Sritularak B, Rojnuckarin P, Luechapudiporn R. Inhibitory Mechanisms of Lusianthridin on Human Platelet Aggregation. International Journal of Molecular Sciences. 2021; 22(13):6846. https://doi.org/10.3390/ijms22136846
Chicago/Turabian StyleSwe, Hla Nu, Boonchoo Sritularak, Ponlapat Rojnuckarin, and Rataya Luechapudiporn. 2021. "Inhibitory Mechanisms of Lusianthridin on Human Platelet Aggregation" International Journal of Molecular Sciences 22, no. 13: 6846. https://doi.org/10.3390/ijms22136846
APA StyleSwe, H. N., Sritularak, B., Rojnuckarin, P., & Luechapudiporn, R. (2021). Inhibitory Mechanisms of Lusianthridin on Human Platelet Aggregation. International Journal of Molecular Sciences, 22(13), 6846. https://doi.org/10.3390/ijms22136846






