Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights
Abstract
:1. Introduction
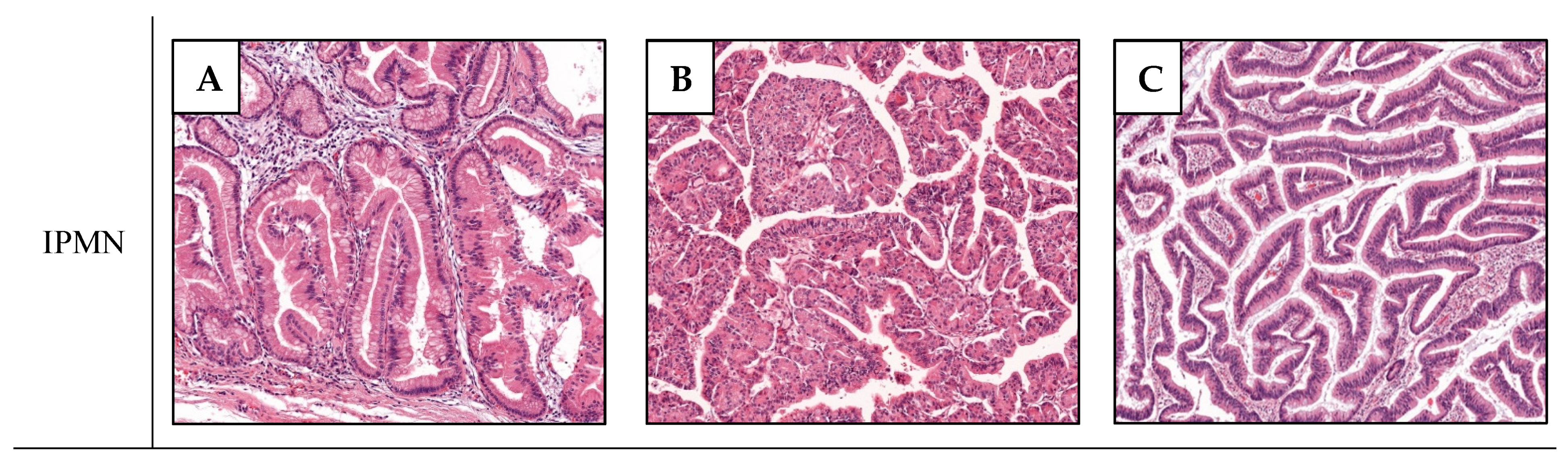
2. Non-Invasive IPMNs and Associated Carcinomas
3. Clinical Presentation of IPMCs
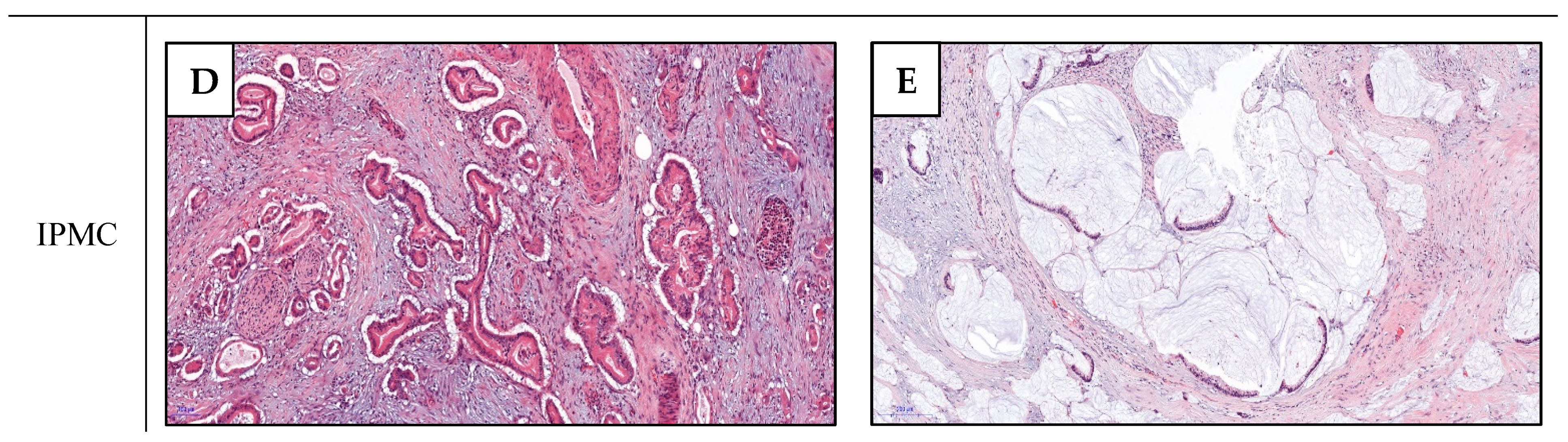
4. Pathological Features and Survival of IPMCs
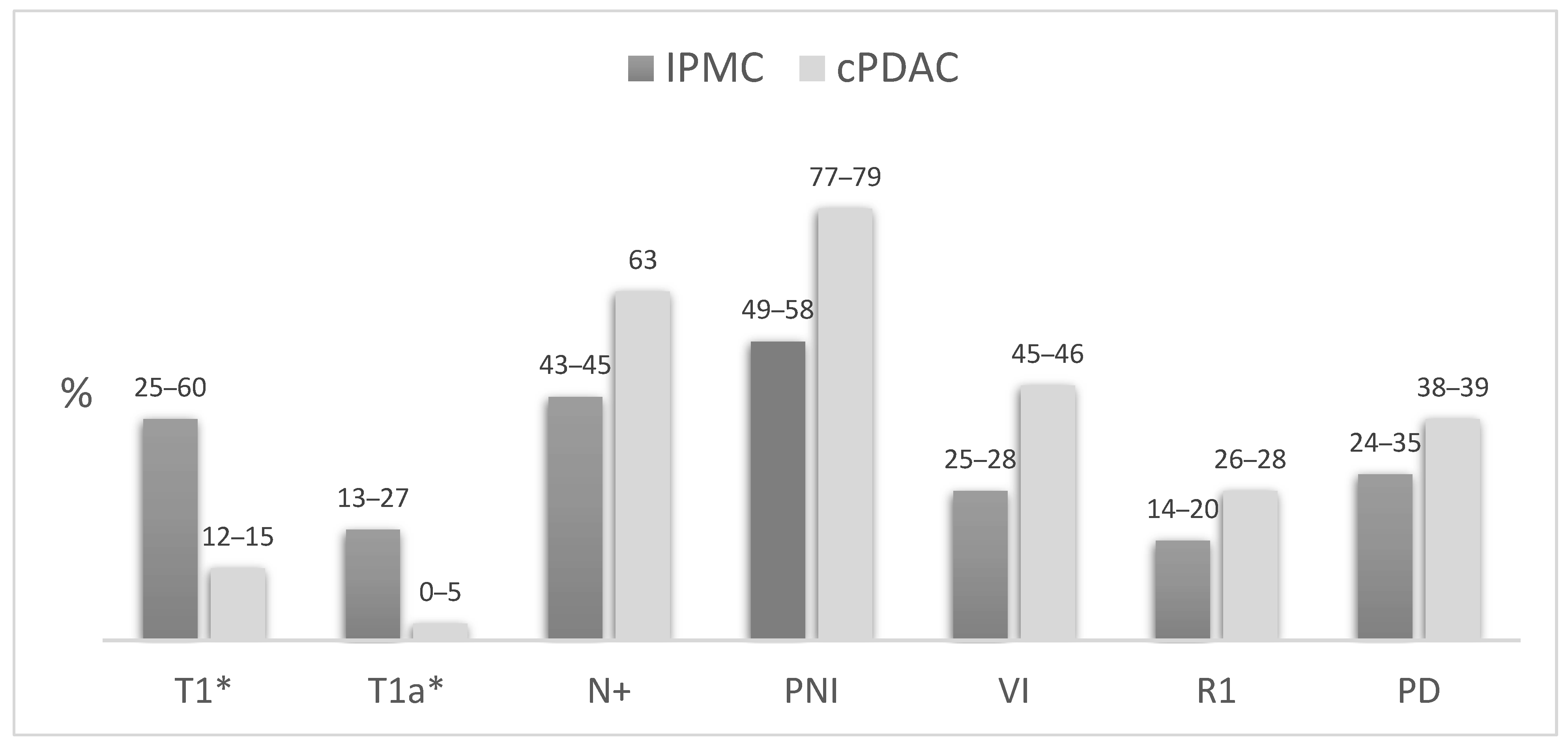
4.1. IPMC Versus cPDAC
4.2. Colloid Versus Tubular IPMC
5. Molecular Features
5.1. Mutationnal Landscape
5.1.1. GNAS and KRAS
5.1.2. Other Mutations
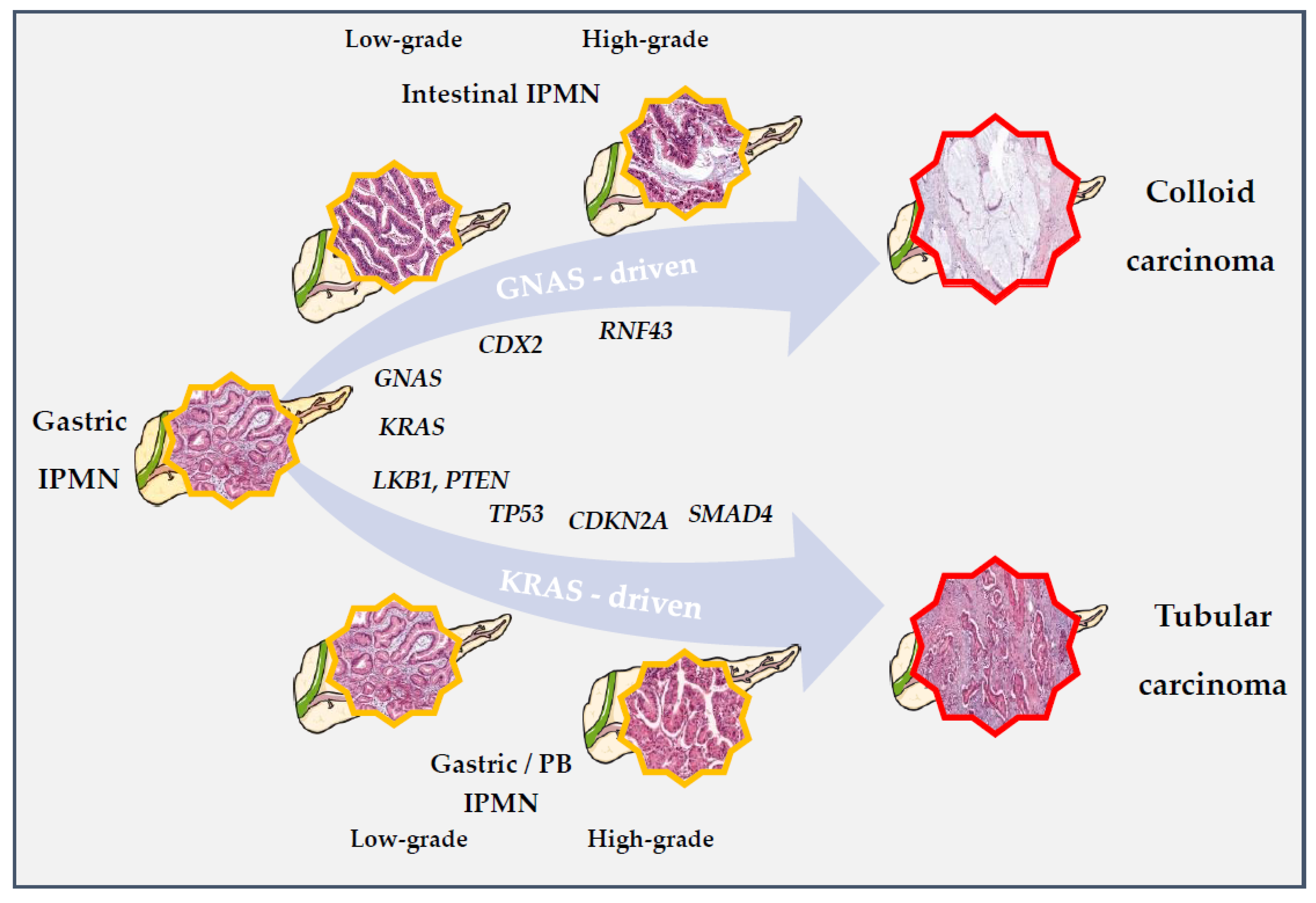
5.2. Carcinogenesis
5.2.1. Murine Models of IPMN Initiation
5.2.2. IPMN to IPMC Sequence
PDAC Derived from and Concomitant with an IPMN
Pathways of Progression
5.2.3. Tumor Heterogeneity
6. Therapeutic Implications
6.1. Adjuvant Therapy
6.2. Future Therapeutic Strategies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Partensky, C.; Bray, F. More Deaths from Pancreatic Cancer than Breast Cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, A.; Andersson, R.; Ansari, D. The Actual 5-Year Survivors of Pancreatic Ductal Adenocarcinoma Based on Real-World Data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef] [PubMed]
- Histological Typing of Tumours of the Exocrine Pancreas, 2nd ed.; Klöppel, G.; Weltgesundheitsorganisation (Eds.) International Histological Classification of Tumours; Corr. Printing; Springer: Berlin, Germany, 1998; ISBN 978-3-540-60280-4. [Google Scholar]
- Ricci, C.; Migliori, M.; Imbrogno, A.; Mazzotta, E.; Felicani, C.; Serra, C.; Bergonzoni, B.; Calculli, L.; Casadei, R. Prevalence of Asymptomatic Intraductal Papillary Mucinous Neoplasms in Healthy and Ill Populations Detected by Ultrasonography: A Single-Center Study of 6353 Outpatients. Pancreas 2019, 48, 113–120. [Google Scholar] [CrossRef]
- Laurent, L.; Vullierme, M.; Rebours, V.; Maire, F.; Hentic, O.; Francoz, C.; Durand, F.; Ruszniewski, P.; Lévy, P. Estimation of the Prevalence of Intraductal Papillary Mucinous Neoplasm of the Pancreas in the French Population through Patients Waiting for Liver Transplantation. United Eur. Gastroenterol. J. 2017, 5, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiocchi, G.L.; Portolani, N.; Grazioli, L.; Mazza, G.; Gheza, F.; Bartoli, M.; Vanzetti, E.; Giulini, S.M. Management of Pancreatic Intraductal Papillary Mucinous Neoplasm in an Academic Hospital (2005Y2010). Pancreas 2013, 42, 5. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Brennan, M.F.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.; Fong, Y.; Schattner, M.; DiMaio, C.; Janakos, M.; Jarnagin, W.R.; et al. Cystic Lesions of the Pancreas: Changes in the Presentation and Management of 1424 Patients at a Single Institution over a 15-Year Time Period. J. Am. Coll. Surg. 2011, 212, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Valsangkar, N.P.; Morales-Oyarvide, V.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Warshaw, A.L.; Fernández-del Castillo, C. 851 Resected Cystic Tumors of the Pancreas: A 33-Year Experience at the Massachusetts General Hospital. Surgery 2012, 152, S4–S12. [Google Scholar] [CrossRef] [Green Version]
- Lokuhetty, D.; Organisation Mondiale de la Santé; Centre International de Recherche sur le Cancer. WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-832-4499-8. [Google Scholar]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Keane, M.G.; Afghani, E. A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. J. Clin. Med. 2021, 10, 1284. [Google Scholar] [CrossRef]
- Lupinacci, R.M.; Goloudina, A.; Buhard, O.; Bachet, J.-B.; Maréchal, R.; Demetter, P.; Cros, J.; Bardier-Dupas, A.; Collura, A.; Cervera, P.; et al. Prevalence of Microsatellite Instability in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology 2018, 154, 1061–1065. [Google Scholar] [CrossRef]
- Lewis, R.; Drebin, J.A.; Callery, M.P.; Fraker, D.; Kent, T.S.; Gates, J.; Vollmer, C.M. A Contemporary Analysis of Survival for Resected Pancreatic Ductal Adenocarcinoma. HPB 2013, 15, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Waters, J.A.; Schnelldorfer, T.; Aguilar-Saavedra, J.R.; Chen, J.-H.; Yiannoutsos, C.T.; Lillemoe, K.D.; Farnell, M.B.; Sarr, M.G.; Schmidt, C.M. Survival after Resection for Invasive Intraductal Papillary Mucinous Neoplasm and for Pancreatic Adenocarcinoma: A Multi-Institutional Comparison According to American Joint Committee on Cancer Stage. J. Am. Coll. Surg. 2011, 213, 275–283. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hayashidani, Y.; Hashimoto, Y.; Nakashima, A.; Sueda, T. Invasive Intraductal Papillary-Mucinous Neoplasm of the Pancreas: Comparison with Pancreatic Ductal Adenocarcinoma. J. Surg. Oncol. 2009, 100, 13–18. [Google Scholar] [CrossRef]
- Felsenstein, M.; Noë, M.; Masica, D.L.; Hosoda, W.; Chianchiano, P.; Fischer, C.G.; Lionheart, G.; Brosens, L.A.A.; Pea, A.; Yu, J.; et al. IPMNs with Co-Occurring Invasive Cancers: Neighbours but Not Always Relatives. Gut 2018, 67, 1652–1662. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International Consensus Guidelines 2012 for the Management of IPMN and MCN of the Pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of Types of Intraductal Papillary-Mucinous Neoplasm of the Pancreas: A Consensus Study. Virchows Arch. 2005, 447, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Noë, M.; Brosens, L.A.A. Gastric- and Intestinal-Type IPMN: Two of a Kind? Virchows Arch. 2020, 477, 17–19. [Google Scholar] [CrossRef]
- Adsay, N.V.; Conlon, K.C.; Zee, S.Y.; Brennan, M.F.; Klimstra, D.S. Intraductal Papillary-Mucinous Neoplasms of the Pancreas: An Analysis of in Situ and Invasive Carcinomas in 28 Patients. Cancer 2002, 94, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Hatori, T.; Fujita, I.; Yamamoto, M.; Kobayashi, M.; Ohike, N.; Morohoshi, T.; Egawa, S.; Unno, M.; Takao, S.; et al. Prognostic Relevance of Morphological Types of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gut 2011, 60, 509–516. [Google Scholar] [CrossRef]
- Kang, M.J.; Lee, K.B.; Jang, J.-Y.; Kwon, W.; Park, J.W.; Chang, Y.R.; Kim, S.-W. Disease Spectrum of Intraductal Papillary Mucinous Neoplasm With an Associated Invasive Carcinoma: Invasive IPMN Versus Pancreatic Ductal Adenocarcinoma-Associated IPMN. Pancreas 2013, 42, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Mino-Kenudson, M.; Ferrone, C.R.; Morales-Oyarvide, V.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F. Patterns of Recurrence After Resection of IPMN: Who, When, and How? Ann. Surg. 2015, 262, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Poultsides, G.A.; Reddy, S.; Cameron, J.L.; Hruban, R.H.; Pawlik, T.M.; Ahuja, N.; Jain, A.; Edil, B.H.; Iacobuzio-Donahue, C.A.; Schulick, R.D.; et al. Histopathologic Basis for the Favorable Survival after Resection of Intraductal Papillary Mucinous Neoplasm-Associated Invasive Adenocarcinoma of the Pancreas. Ann. Surg. 2010, 251, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaee, N.; Barbon, C.; Zaki, A.; He, J.; Salman, B.; Hruban, R.H.; Cameron, J.L.; Herman, J.M.; Ahuja, N.; Lennon, A.M.; et al. Intraductal Papillary Mucinous Neoplasm (IPMN) with High-Grade Dysplasia Is a Risk Factor for the Subsequent Development of Pancreatic Ductal Adenocarcinoma. HPB (Oxford) 2016, 18, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirono, S.; Shimizu, Y.; Ohtsuka, T.; Kin, T.; Hara, K.; Kanno, A.; Koshita, S.; Hanada, K.; Kitano, M.; Inoue, H.; et al. Recurrence Patterns after Surgical Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas; a Multicenter, Retrospective Study of 1074 IPMN Patients by the Japan Pancreas Society. J. Gastroenterol. 2020, 55, 86–99. [Google Scholar] [CrossRef]
- Morales-Oyarvide, V.; Mino-Kenudson, M.; Ferrone, C.R.; Warshaw, A.L.; Lillemoe, K.D.; Sahani, D.V.; Pergolini, I.; Attiyeh, M.A.; Al Efishat, M.; Rezaee, N.; et al. Intraductal Papillary Mucinous Neoplasm of the Pancreas in Young Patients: Tumor Biology, Clinical Features, and Survival Outcomes. J. Gastrointest. Surg. 2018, 22, 226–234. [Google Scholar] [CrossRef]
- Mino-Kenudson, M.; Fernandez-del Castillo, C.; Baba, Y.; Valsangkar, N.P.; Liss, A.S.; Hsu, M.; Correa-Gallego, C.; Ingkakul, T.; Perez Johnston, R.; Turner, B.G.; et al. Prognosis of Invasive Intraductal Papillary Mucinous Neoplasm Depends on Histological and Precursor Epithelial Subtypes. Gut 2011, 60, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Sadakari, Y.; Ohuchida, K.; Nakata, K.; Ohtsuka, T.; Aishima, S.; Takahata, S.; Nakamura, M.; Mizumoto, K.; Tanaka, M. Invasive Carcinoma Derived from the Nonintestinal Type Intraductal Papillary Mucinous Neoplasm of the Pancreas Has a Poorer Prognosis than That Derived from the Intestinal Type. Surgery 2010, 147, 812–817. [Google Scholar] [CrossRef]
- Yamada, S.; Fujii, T.; Shimoyama, Y.; Kanda, M.; Nakayama, G.; Sugimoto, H.; Koike, M.; Nomoto, S.; Fujiwara, M.; Nakao, A.; et al. Clinical Implication of Morphological Subtypes in Management of Intraductal Papillary Mucinous Neoplasm. Ann. Surg. Oncol. 2014, 21, 2444–2452. [Google Scholar] [CrossRef]
- McMillan, M.T.; Lewis, R.S.; Drebin, J.A.; Teitelbaum, U.R.; Lee, M.K.; Roses, R.E.; Fraker, D.L.; Vollmer, C.M. The Efficacy of Adjuvant Therapy for Pancreatic Invasive Intraductal Papillary Mucinous Neoplasm (IPMN): Adjuvant Therapy for Invasive IPMN. Cancer 2016, 122, 521–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, K.; Kanemitsu, S.; Hatori, T.; Maguchi, H.; Shimizu, Y.; Tada, M.; Nakagohri, T.; Hanada, K.; Osanai, M.; Noda, Y.; et al. Pancreatic Ductal Adenocarcinoma Derived From IPMN and Pancreatic Ductal Adenocarcinoma Concomitant With IPMN. Pancreas 2011, 40, 571–580. [Google Scholar] [CrossRef]
- Wasif, N.; Bentrem, D.J.; Farrell, J.J.; Ko, C.Y.; Hines, O.J.; Reber, H.A.; Tomlinson, J.S. Invasive Intraductal Papillary Mucinous Neoplasm versus Sporadic Pancreatic Adenocarcinoma: A Stage-Matched Comparison of Outcomes. Cancer 2010, 116, 3369–3377. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.-X.; Chok, A.-Y.; Zheng, H.-L.; Tan, C.-S.; Goh, B.K.P. Systematic Review and Meta-Analysis Comparing the Surgical Outcomes of Invasive Intraductal Papillary Mucinous Neoplasms and Conventional Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2782–2800. [Google Scholar] [CrossRef]
- Woo, S.M.; Ryu, J.K.; Lee, S.H.; Yoo, J.W.; Park, J.K.; Kim, Y.-T.; Yoon, Y.B. Survival and Prognosis of Invasive Intraductal Papillary Mucinous Neoplasms of the Pancreas: Comparison With Pancreatic Ductal Adenocarcinoma. Pancreas 2008, 36, 50–55. [Google Scholar] [CrossRef]
- Hirono, S.; Kawai, M.; Okada, K.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Ueno, M.; Yanagisawa, A.; Yamaue, H. Factors Associated With Invasive Intraductal Papillary Mucinous Carcinoma of the Pancreas. JAMA Surg. 2017, 152, e165054. [Google Scholar] [CrossRef] [PubMed]
- Turrini, O.; Waters, J.A.; Schnelldorfer, T.; Lillemoe, K.D.; Yiannoutsos, C.T.; Farnell, M.B.; Sarr, M.G.; Schmidt, C.M. Invasive Intraductal Papillary Mucinous Neoplasm: Predictors of Survival and Role of Adjuvant Therapy. HPB 2010, 12, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Hruban, R.H.; Fukushima, N.; Campbell, K.A.; Lillemoe, K.D. Intraductal Papillary Mucinous Neoplasms of the Pancreas: An Updated Experience. Ann. Surg. 2004, 239, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Castillo, C.F.-D.; Bassi, C.; Mantovani, W.; Thayer, S.P.; Crippa, S.; Ferrone, C.R.; Falconi, M.; Pederzoli, P.; Warshaw, A.L.; et al. Invasive Intraductal Papillary Mucinous Carcinomas of the Pancreas: Predictors of Survival and the Role of Lymph Node Ratio. Ann. Surg. 2010, 251, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yopp, A.C.; Katabi, N.; Janakos, M.; Klimstra, D.S.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Brennan, M.F.; Jarnagin, W.R.; Allen, P.J. Invasive Carcinoma Arising in Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Matched Control Study with Conventional Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2011, 253, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, L.; Bengtsson, A.; Torén, W.; Andersson, R.; Ansari, D. Intraductal Papillary Mucinous Carcinoma versus Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Int. J. Surg. 2019, 71, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Nara, S.; Shimada, K.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Minimally Invasive Intraductal Papillary-Mucinous Carcinoma of the Pancreas: Clinicopathologic Study of 104 Intraductal Papillary-Mucinous Neoplasms. Am. J. Surg. Pathol. 2008, 32, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-Y.; Hwang, D.W.; Kim, M.A.; Kang, M.-J.; Lim, C.S.; Lee, S.E.; Kim, S.-W. Analysis of Prognostic Factors and a Proposed New Classification for Invasive Papillary Mucinous Neoplasms. Ann. Surg. Oncol. 2011, 18, 644–650. [Google Scholar] [CrossRef] [PubMed]
- D’Angelica, M.; Brennan, M.F.; Suriawinata, A.A.; Klimstra, D.; Conlon, K.C. Intraductal Papillary Mucinous Neoplasms of the Pancreas: An Analysis of Clinicopathologic Features and Outcome. Ann. Surg. 2004, 239, 400–408. [Google Scholar] [CrossRef]
- Winter, J.M.; Jiang, W.; Basturk, O.; Mino-Kenudson, M.; Fong, Z.V.; Tan, W.P.; Lavu, H.; Vollmer, C.M.; Furth, E.E.; Haviland, D.; et al. Recurrence and Survival After Resection of Small Intraductal Papillary Mucinous Neoplasm-Associated Carcinomas (≤20-Mm Invasive Component): A Multi-Institutional Analysis. Ann. Surg. 2016, 263, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Hank, T.; Qadan, M.; Ciprani, D.; Mino-Kenudson, M.; Weekes, C.D.; Ryan, D.P.; Clark, J.W.; Allen, J.N.; Hong, T.S.; et al. Impact of Adjuvant Therapy in Patients with Invasive Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreatology 2020, 20, 722–728. [Google Scholar] [CrossRef]
- Schlitter, A.M.; Jesinghaus, M.; Jäger, C.; Konukiewitz, B.; Muckenhuber, A.; Demir, I.E.; Bahra, M.; Denkert, C.; Friess, H.; Kloeppel, G.; et al. PT but Not PN Stage of the 8th TNM Classification Significantly Improves Prognostication in Pancreatic Ductal Adenocarcinoma. Eur. J. Cancer 2017, 84, 121–129. [Google Scholar] [CrossRef]
- Park, M.Y.; Shin, S.H.; Song, K.B.; Hwang, D.; Lee, J.H.; Lee, Y.-J.; Kim, S.C. Validation of the Eighth Edition of the American Joint Committee on Cancer Staging System and Proposal of an Improved Staging System for Pancreatic Ductal Adenocarcinoma. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 46. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.W.; Lee, J.; Kim, J.; Woo, S.M.; Lee, W.J.; Han, S.-S.; Park, S.-J.; Choi, K.S.; Cha, H.S.; Yoon, Y.-S.; et al. Validation of the American Joint Committee on Cancer 8th Edition Staging System for the Pancreatic Ductal Adenocarcinoma. Eur. J. Surg. Oncol. 2019, 45, 2159–2165. [Google Scholar] [CrossRef]
- Kwon, W.; Park, T.; He, J.; Higuchi, R.; Son, D.; Lee, S.Y.; Kim, J.; Byun, Y.; Kim, H.; Kim, S.-W.; et al. Is the New T1 Category as Defined in the Eighth Edition of the AJCC Pancreatic Cancer Staging System an Improvement? J. Gastrointest. Surg. 2020, 24, 262–269. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Lillemoe, K.D. Intraductal Papillary Mucinous Neoplasms of the Pancreas: An Increasingly Recognized Clinicopathologic Entity. Ann. Surg. 2001, 234, 313–322. [Google Scholar] [CrossRef]
- Shimada, K.; Sakamoto, Y.; Sano, T.; Kosuge, T.; Hiraoka, N. Invasive Carcinoma Originating in an Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Clinicopathologic Comparison with a Common Type of Invasive Ductal Carcinoma. Pancreas 2006, 32, 281–287. [Google Scholar] [CrossRef]
- Schnelldorfer, T. Experience With 208 Resections for Intraductal Papillary Mucinous Neoplasm of the Pancreas. Arch. Surg. 2008, 143, 639. [Google Scholar] [CrossRef] [Green Version]
- Maire, F. Prognosis of Malignant Intraductal Papillary Mucinous Tumours of the Pancreas after Surgical Resection. Comparison with Pancreatic Ductal Adenocarcinoma. Gut 2002, 51, 717–722. [Google Scholar] [CrossRef]
- Brennan, M.F.; Kattan, M.W.; Klimstra, D.; Conlon, K. Prognostic Nomogram for Patients Undergoing Resection for Adenocarcinoma of the Pancreas. Ann. Surg. 2004, 240, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Pergolini, I.; Javed, A.A.; Honselmann, K.C.; Weiss, M.J.; Di Salvo, F.; Burkhart, R.; Zamboni, G.; Belfiori, G.; Ferrone, C.R.; et al. Implications of Perineural Invasion on Disease Recurrence and Survival After Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma. Ann. Surg. 2021. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Epstein, J.D.; Kozak, G.; Fong, Z.V.; He, J.; Javed, A.A.; Joneja, U.; Jiang, W.; Ferrone, C.R.; Lillemoe, K.D.; Cameron, J.L.; et al. Microscopic Lymphovascular Invasion Is an Independent Predictor of Survival in Resected Pancreatic Ductal Adenocarcinoma. J. Surg. Oncol. 2017, 116, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Duconseil, P.; Périnel, J.; Autret, A.; Adham, M.; Sauvanet, A.; Chiche, L.; Mabrut, J.-Y.; Tuech, J.-J.; Mariette, C.; Régenet, N.; et al. Resectable Invasive IPMN versus Sporadic Pancreatic Adenocarcinoma of the Head of the Pancreas: Should These Two Different Diseases Receive the Same Treatment? A Matched Comparison Study of the French Surgical Association (AFC). Eur. J. Surg. Oncol. 2017, 43, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Hirakawa, A.; Takami, H.; Suenaga, M.; Hayashi, M.; Niwa, Y.; Hattori, N.; Iwata, N.; Kanda, M.; et al. Comparison of the Survival Outcomes of Pancreatic Cancer and Intraductal Papillary Mucinous Neoplasms. Pancreas 2018, 47, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.-C.; Mansour, J.; Mollaee, M.; Wagner, K.-U.; Koduru, P.; Yopp, A.; et al. Whole-Exome Sequencing of Pancreatic Cancer Defines Genetic Diversity and Therapeutic Targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Kuboki, Y.; Tanji, E.; Yoshida, S.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shimizu, K.; Kamatani, N.; Shiratori, K. Whole-Exome Sequencing Uncovers Frequent GNAS Mutations in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Sci. Rep. 2011, 1, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoda, W.; Sasaki, E.; Murakami, Y.; Yamao, K.; Shimizu, Y.; Yatabe, Y. GNAS Mutation Is a Frequent Event in Pancreatic Intraductal Papillary Mucinous Neoplasms and Associated Adenocarcinomas. Virchows Arch. 2015, 466, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS Mutations Define an Unexpected Pathway for Pancreatic Cyst Development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, E.; dal Molin, M.; Mafficini, A.; Yu, J.; Malleo, G.; Rusev, B.; Fassan, M.; Antonello, D.; Sadakari, Y.; Castelli, P.; et al. Targeted Next-Generation Sequencing of Cancer Genes Dissects the Molecular Profiles of Intraductal Papillary Neoplasms of the Pancreas: IPMN Molecular Profile. J. Pathol. 2014, 233, 217–227. [Google Scholar] [CrossRef]
- Gaujoux, S.; Parvanescu, A.; Cesaretti, M.; Silve, C.; Bieche, I.; Rebours, V.; Lévy, P.; Sauvanet, A.; Cros, J. GNAS but Not Extended RAS Mutations Spectrum Are Associated with a Better Prognosis in Intraductal Pancreatic Mucinous Neoplasms. Ann. Surg. Oncol. 2019, 26, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Shimizu, K.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shiratori, K.; Furukawa, T. Molecular Biomarkers for Progression of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2015, 44, 227–235. [Google Scholar] [CrossRef]
- Chang, X.Y.; Wu, Y.; Jiang, Y.; Wang, P.Y.; Chen, J. RNF43 Mutations in IPMN Cases: A Potential Prognostic Factor. Gastroenterol. Res. Pract. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Basturk, O.; Brannon, A.R.; Bhanot, U.; Scott, S.N.; Bouvier, N.; LaFemina, J.; Jarnagin, W.R.; Berger, M.F.; Klimstra, D.; et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J. Am. Coll. Surg. 2015, 220, 845–854. [Google Scholar] [CrossRef] [Green Version]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The Emerging Mutational Landscape of G Proteins and G-Protein-Coupled Receptors in Cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex Roles of CAMP–PKA–CREB Signaling in Cancer. Exp. Hematol. Oncol. 2020, 9. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, L.; Cui, Z.; Tang, J.; Xie, M.; Ren, G. Elevated Expression of GNAS Promotes Breast Cancer Cell Proliferation and Migration via the PI3K/AKT/Snail1/E-Cadherin Axis. Clin. Transl. Oncol. 2019, 21, 1207–1219. [Google Scholar] [CrossRef]
- Beristain, A.G.; Molyneux, S.D.; Joshi, P.A.; Pomroy, N.C.; Di Grappa, M.A.; Chang, M.C.; Kirschner, L.S.; Privé, G.G.; Pujana, M.A.; Khokha, R. PKA Signaling Drives Mammary Tumorigenesis through Src. Oncogene 2015, 34, 1160–1173. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xiao, Y.; Lin, H.-P.; Yang, P.; Humphries, B.; Gao, T.; Yang, C. Integrin A9 Depletion Promotes β-Catenin Degradation to Suppress Triple Negative Breast Cancer Tumor Growth and Metastasis. Int. J. Cancer 2019, 145, 2767–2780. [Google Scholar] [CrossRef]
- Patra, K.C.; Kato, Y.; Mizukami, Y.; Widholz, S.; Boukhali, M.; Revenco, I.; Grossman, E.A.; Ji, F.; Sadreyev, R.I.; Liss, A.S.; et al. Mutant GNAS Drives Pancreatic Tumourigenesis by Inducing PKA-Mediated SIK Suppression and Reprogramming Lipid Metabolism. Nat. Cell Biol. 2018, 20, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.; Choi, J.-W.; Kim, Y.-S. KRAS, GNAS, and RNF43 Mutations in Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Meta-Analysis. SpringerPlus 2016, 5, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonckheere, N.; Vasseur, R.; Van Seuningen, I. The Cornerstone K-RAS Mutation in Pancreatic Adenocarcinoma: From Cell Signaling Network, Target Genes, Biological Processes to Therapeutic Targeting. Crit. Rev. Oncol./Hematol. 2017, 111, 7–19. [Google Scholar] [CrossRef]
- Maertens, O.; Cichowski, K. An Expanding Role for RAS GTPase Activating Proteins (RAS GAPs) in Cancer. Adv. Biol. Regul. 2014, 55, 1–14. [Google Scholar] [CrossRef]
- Chang, X.Y.; Wu, Y.; Li, Y.; Wang, J.; Chen, J. Intraductal Papillary Mucinous Neoplasms of the Pancreas: Clinical Association with KRAS. Mol. Med. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; van de Wetering, M.; van Es, J.H.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; et al. Tumour Suppressor RNF43 Is a Stem-Cell E3 Ligase That Induces Endocytosis of Wnt Receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, H.-X.; Growney, J.D.; Woolfenden, S.; Bottiglio, C.; Ng, N.; Lu, B.; Hsieh, M.H.; Bagdasarian, L.; Meyer, R.; et al. Inactivating Mutations of RNF43 Confer Wnt Dependency in Pancreatic Ductal Adenocarcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 12649–12654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Jiao, Y.; Dal Molin, M.; Maitra, A.; de Wilde, R.F.; Wood, L.D.; Eshleman, J.R.; Goggins, M.G.; Wolfgang, C.L.; Canto, M.I.; et al. Whole-Exome Sequencing of Neoplastic Cysts of the Pancreas Reveals Recurrent Mutations in Components of Ubiquitin-Dependent Pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 21188–21193. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Kuboki, Y.; Hatori, T.; Yamamoto, M.; Sugiyama, M.; Shibata, N.; Shimizu, K.; Shiratori, K.; Furukawa, T. Clinicopathological Significance of Somatic RNF43 Mutation and Aberrant Expression of Ring Finger Protein 43 in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Mod. Pathol. 2015, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Ono, Y.; Kobayashi, T.; Motoi, F.; Karasaki, H.; Mizukami, Y.; Makino, N.; Ueno, Y.; Unno, M.; Furukawa, T. How Does Intestinal-Type Intraductal Papillary Mucinous Neoplasm Emerge? CDX2 Plays a Critical Role in the Process of Intestinal Differentiation and Progression. Virchows Arch. 2020, 477, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Ono, Y.; Tanino, M.; Karasaki, H.; Yamaguchi, H.; Furukawa, T.; Enomoto, K.; Ueda, J.; Sumi, A.; Katayama, J.; et al. Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology 2019, 156, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noë, M.; Niknafs, N.; Fischer, C.G.; Hackeng, W.M.; Beleva Guthrie, V.; Hosoda, W.; Debeljak, M.; Papp, E.; Adleff, V.; White, J.R.; et al. Genomic Characterization of Malignant Progression in Neoplastic Pancreatic Cysts. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Hosoda, W.; Felsenstein, M.; Song, Q.; Reiter, J.G.; Zheng, L.; Beleva Guthrie, V.; Rincon, N.; Dal Molin, M.; Dudley, J.; et al. Multiregion Whole-Exome Sequencing of Intraductal Papillary Mucinous Neoplasms Reveals Frequent Somatic KLF4 Mutations Predominantly in Low-Grade Regions. Gut 2021, 70, 928–939. [Google Scholar] [CrossRef]
- Taki, K.; Ohmuraya, M.; Tanji, E.; Komatsu, H.; Hashimoto, D.; Semba, K.; Araki, K.; Kawaguchi, Y.; Baba, H.; Furukawa, T. GNASR201H and KrasG12D Cooperate to Promote Murine Pancreatic Tumorigenesis Recapitulating Human Intraductal Papillary Mucinous Neoplasm. Oncogene 2016, 35, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Ideno, N.; Yamaguchi, H.; Ghosh, B.; Gupta, S.; Okumura, T.; Steffen, D.J.; Fisher, C.G.; Wood, L.D.; Singhi, A.D.; Nakamura, M.; et al. GNASR201C Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology 2018, 155, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.L.; Dubois, C.L.; Schaeffer, D.F.; Samani, A.; Taghizadeh, F.; Cowan, R.W.; Rhim, A.D.; Stiles, B.L.; Valasek, M.; Sander, M. Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice. Gastroenterology 2018, 154, 1509–1523.e5. [Google Scholar] [CrossRef]
- Collet, L.; Ghurburrun, E.; Meyers, N.; Assi, M.; Pirlot, B.; Leclercq, I.A.; Couvelard, A.; Komuta, M.; Cros, J.; Demetter, P.; et al. Kras and Lkb1 Mutations Synergistically Induce Intraductal Papillary Mucinous Neoplasm Derived from Pancreatic Duct Cells. Gut 2020, 69, 704–714. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International Consensus Fukuoka Guidelines for the Management of IPMN of the Pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Sahora, K.; Crippa, S.; Zamboni, G.; Ferrone, C.; Warshaw, A.L.; Lillemoe, K.; Mino-Kenudson, M.; Falconi, M.; Fernandez-del Castillo, C. Intraductal Papillary Mucinous Neoplasms of the Pancreas with Concurrent Pancreatic and Periampullary Neoplasms. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ideno, N.; Ohtsuka, T.; Kono, H.; Fujiwara, K.; Oda, Y.; Aishima, S.; Ito, T.; Ishigami, K.; Tokunaga, S.; Ohuchida, K.; et al. Intraductal Papillary Mucinous Neoplasms of the Pancreas With Distinct Pancreatic Ductal Adenocarcinomas Are Frequently of Gastric Subtype. Ann. Surg. 2013, 258, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ideno, N.; Ohtsuka, T.; Matsunaga, T.; Kimura, H.; Watanabe, Y.; Tamura, K.; Aso, T.; Aishima, S.; Miyasaka, Y.; Ohuchida, K.; et al. Clinical Significance of GNAS Mutation in Intraductal Papillary Mucinous Neoplasm of the Pancreas with Concomitant Pancreatic Ductal Adenocarcinoma. Pancreas 2015, 44, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.G.; Beleva Guthrie, V.; Braxton, A.M.; Zheng, L.; Wang, P.; Song, Q.; Griffin, J.F.; Chianchiano, P.E.; Hosoda, W.; Niknafs, N.; et al. Intraductal Papillary Mucinous Neoplasms Arise From Multiple Independent Clones, Each With Distinct Mutations. Gastroenterology 2019, 157, 1123–1137. [Google Scholar] [CrossRef] [Green Version]
- Tempero, M.A.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. NCCN Guidelines Insights: Pancreatic Adenocarcinoma, Version 1.2019: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Neuzillet, C.; Gaujoux, S.; Williet, N.; Bachet, J.-B.; Bauguion, L.; Colson Durand, L.; Conroy, T.; Dahan, L.; Gilabert, M.; Huguet, F.; et al. Pancreatic Cancer: French Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig. Liver Dis. 2018, 50, 1257–1271. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of Adjuvant Gemcitabine and Capecitabine with Gemcitabine Monotherapy in Patients with Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Mungo, B.; Croce, C.; Oba, A.; Ahrendt, S.; Gleisner, A.; Friedman, C.; Schulick, R.D.; Del Chiaro, M. Controversial Role of Adjuvant Therapy in Node-Negative Invasive Intraductal Papillary Mucinous Neoplasm. Ann. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Caponi, S.; Vasile, E.; Funel, N.; De Lio, N.; Campani, D.; Ginocchi, L.; Lucchesi, M.; Caparello, C.; Lencioni, M.; Cappelli, C.; et al. Adjuvant Chemotherapy Seems Beneficial for Invasive Intraductal Papillary Mucinous Neoplasms. Eur. J. Surg. Oncol. (EJSO) 2013, 39, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.J.; Hsu, C.C.; Pawlik, T.M.; Winter, J.; Hruban, R.H.; Guler, M.; Schulick, R.D.; Cameron, J.L.; Laheru, D.A.; Wolfgang, C.L.; et al. Adjuvant Chemoradiotherapy After Pancreatic Resection for Invasive Carcinoma Associated With Intraductal Papillary Mucinous Neoplasm of the Pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Marchegiani, G.; Andrianello, S.; Dal Borgo, C.; Secchettin, E.; Melisi, D.; Malleo, G.; Bassi, C.; Salvia, R. Adjuvant Chemotherapy Is Associated with Improved Postoperative Survival in Specific Subtypes of Invasive Intraductal Papillary Mucinous Neoplasms (IPMN) of the Pancreas: It Is Time for Randomized Controlled Data. HPB 2019, 21, 596–603. [Google Scholar] [CrossRef]
- Shaib, W.L.; Narayan, A.S.; Switchenko, J.M.; Kane, S.R.; Wu, C.; Akce, M.; Alese, O.B.; Patel, P.R.; Maithel, S.K.; Sarmiento, J.M.; et al. Role of Adjuvant Therapy in Resected Stage IA Subcentimeter (T1a/T1b) Pancreatic Cancer: Adjuvant Therapy for Subcentimeter PDAC. Cancer 2019, 125, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Chen, L.; Qin, Q.; Sun, X. Salt-Inducible Kinase 2: An Oncogenic Signal Transmitter and Potential Target for Cancer Therapy. Front. Oncol. 2019, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Katsukura, N.; Watanabe, S.; Shirasaki, T.; Hibiya, S.; Kano, Y.; Akahoshi, K.; Tanabe, M.; Kirimura, S.; Akashi, T.; Kitagawa, M.; et al. Intestinal Phenotype Is Maintained by Atoh1 in the Cancer Region of Intraductal Papillary Mucinous Neoplasm. Cancer Sci. 2021, 112, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Tao, Z.; Shi, Q.; Wang, L.; Liu, L.; She, T.; Yi, Q.; Wen, X.; Liu, L.; Li, S.; et al. Selective Photokilling of Colorectal Tumors by Near-Infrared Photoimmunotherapy with a GPA33-Targeted Single-Chain Antibody Variable Fragment Conjugate. Mol. Pharm. 2020, 17, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 Antibody-Functionalized Exosomes for Targeted Delivery of Doxorubicin against Colorectal Cancer. Nanomedicine 2018, 14, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. JCO 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive Characterisation of Pancreatic Ductal Adenocarcinoma with Microsatellite Instability: Histology, Molecular Pathology and Clinical Implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.; Nelson, S.; Bednar, F.; Cho, C.; Nathan, H.; Sahai, V.; di Magliano, M.P.; Frankel, T.L. Immunotherapy for Pancreatic Ductal Adenocarcinoma. J. Surg. Oncol. 2021, 123, 751–759. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Byrum, A.K.; Vindigni, A.; Mosammaparast, N. Defining and Modulating ‘BRCAness’. Trends Cell Biol. 2019, 29, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Skaro, M.; Nanda, N.; Gauthier, C.; Felsenstein, M.; Jiang, Z.; Qiu, M.; Shindo, K.; Yu, J.; Hutchings, D.; Javed, A.A.; et al. Prevalence of Germline Mutations Associated With Cancer Risk in Patients With Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2019, 156, 1905–1913. [Google Scholar] [CrossRef] [PubMed]

| Author, Year [Ref.] | Ethnicity | N | Any Symptom (%) | Abdominal Pain (%) | Weight Loss (%) | Jaundice (%) | AP (%) |
|---|---|---|---|---|---|---|---|
| Hirono 2017 [38] | Asian | 96 | 63 | - | - | - | 23 |
| Rezaee 2016 [27] | Caucasian | 183 | 80 | 46 | 43 | 38 | - |
| Marchegiani 2015 [25] | Caucasian | 84 | 66 | - | - | - | - |
| Mino 2011 [30] | Caucasian | 61 | 72 | 43 | 38 | 28 | - |
| Yopp 2011 [42] | Caucasian | 59 | 88 | 53 | 39 | - | - |
| Partelli 2010 [41] | Caucasian | 104 | 87 | 51 | 53 | 33 | 3 |
| Turrini 2010 [39] | Caucasian | 98 | 90 | 56 | 33 | 34 | 23 |
| Sohn 2004 [40] | Caucasian | 52 | - | 54 | 44 | 33 | 12 |
| Author, Year [Ref.] | N | % Colloid | N+ | PNI | VI | LI | PD | R1 |
|---|---|---|---|---|---|---|---|---|
| Tubular/Colloid | ||||||||
| Poultsides 2010 [26] | 127 | 26 | 59/29 * | 69/48 | 42/7 * | - | 28/11 * | 18/0 |
| Yopp 2011 [42] | 59 | 41 | 49/17 | 17/17 | 20/12 | - | 17/12 | 14/4 |
| Mino 2011 [30] | 54 | 30 | 41/27 | 68/25 | 26/6 | 29/19 | 16/19 | - |
| Waters 2011 [16] | 113 | 24 | 49/29 | - | - | - | - | - |
| Rezaee 2016 [27] | 183 | 24 | 63/20 * | 65/36 * | 40/9 * | - | - | - |
| Morales 2018 [29] | 409 | 33 | 56/20 * | - | - | - | - | - |
| Hirono 2020 [28] | 197 | 29 | 37/16 * | - | - | - | - | - |
| Rodrigues 2020 [48] | 97 | 34 | 42/24 | 53/27 * | - | - | 19/13 | 18/9 |
| Author, Year [Ref.] | Ethnicity | Detection Method | N Total | Intestinal IPMN (%) | N IPMC | Colloid (%) | GNAS Mutation | KRAS Mutation |
|---|---|---|---|---|---|---|---|---|
| IPMN vs. IPMC (%) | ||||||||
| Chang 2020 [69] | Asian | Sequencing | 61 | 48 | 28 | NA | 63 vs. 61 | 52 vs. 64 |
| Gaujoux 2019 [67] | Caucasian | PCR | 159 | 41 | 31 | 22 | 44 vs. 19 | 53 vs. 68 |
| Tan 2015 [70] | Caucasian | Sequencing | 38 | 47 | 38 | 50 | NA vs. 50 | NA vs. 61 |
| Hosoda 2015 [64] | Asian | Sequencing, PCR | 91 | 30 | 30 | 20 | 64 vs. 37 | 59 vs. 77 |
| Kuboki 2015 [68] | Asian | Sequencing | 172 | 33 | 49 | 43 | 50 vs. 41 | 54 vs. 59 |
| Furukawa 2011 [63] | Asian | Sequencing | 118 | 34 | 47 | NA | 42 vs. 28 | 45 vs. 38 |
| Author, Year [Ref.] | N IPMC | N AT (%) | AT Type | Survival Benefit | |||
|---|---|---|---|---|---|---|---|
| Global | N− | N+ | Other Subgroups | ||||
| Mungo 2020 [102] | 492 | 225 (48) | AC+/−RT | - | No | Yes | - |
| Rodrigues 2020 [48] | 103 | 34 (33) | AC+/−RT | No | No | No | - |
| McMillan 2016 [33] | 1220 | 541 (44) | AC+/−RT | Yes | No | Yes | Stage II-IV, PD |
| Caponi 2013 [103] | 64 | 33 (52) | AC+/−RT | Yes | No | Yes | - |
| Swartz 2010 [104] | 70 | 40 (57) | CRT | Yes | - | Yes | R1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas, L.; Lupinacci, R.M.; Cros, J.; Bachet, J.-B.; Coulet, F.; Svrcek, M. Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights. Int. J. Mol. Sci. 2021, 22, 6756. https://doi.org/10.3390/ijms22136756
Mas L, Lupinacci RM, Cros J, Bachet J-B, Coulet F, Svrcek M. Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights. International Journal of Molecular Sciences. 2021; 22(13):6756. https://doi.org/10.3390/ijms22136756
Chicago/Turabian StyleMas, Léo, Renato M. Lupinacci, Jérôme Cros, Jean-Baptiste Bachet, Florence Coulet, and Magali Svrcek. 2021. "Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights" International Journal of Molecular Sciences 22, no. 13: 6756. https://doi.org/10.3390/ijms22136756
APA StyleMas, L., Lupinacci, R. M., Cros, J., Bachet, J.-B., Coulet, F., & Svrcek, M. (2021). Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights. International Journal of Molecular Sciences, 22(13), 6756. https://doi.org/10.3390/ijms22136756







