An Update on the Pathophysiology and Diagnosis of Inappropriate Secretion of Thyroid-Stimulating Hormone
Abstract
1. Introduction
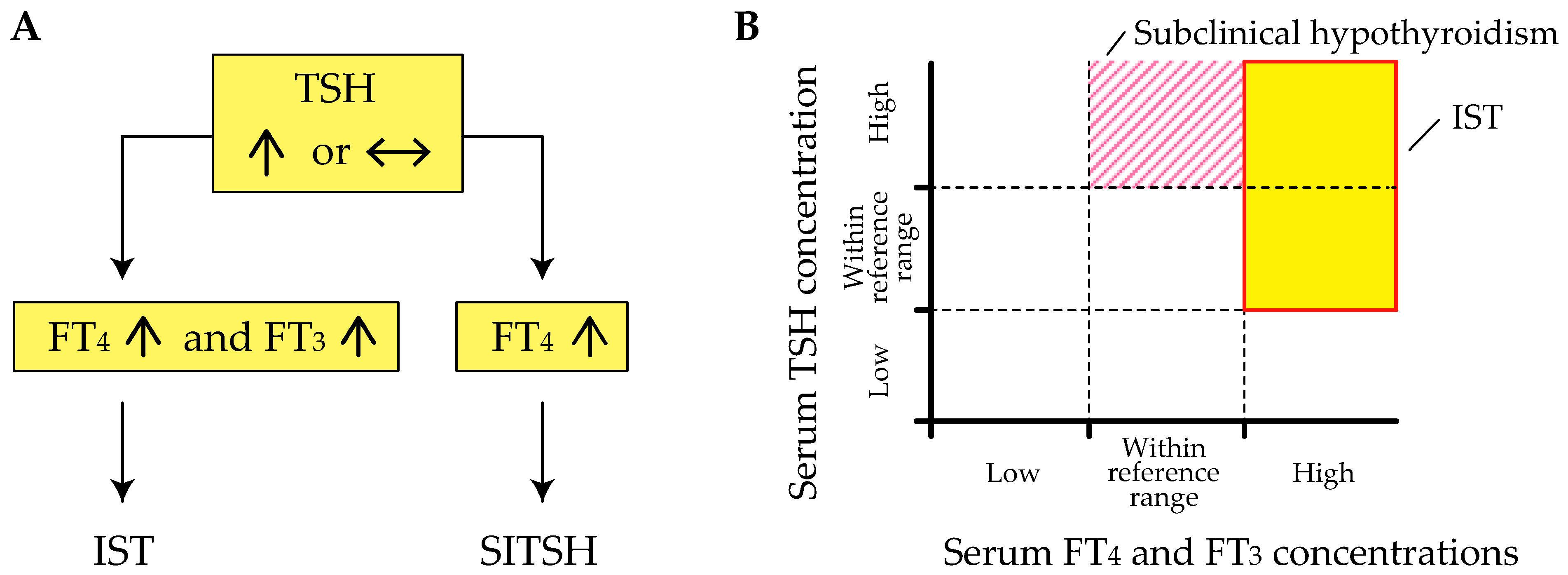
2. Definition of IST
3. Pathophysiology of IST
3.1. Overview
3.2. Causes Associated with the Classification Proposed by Gershengorn and Weintraub
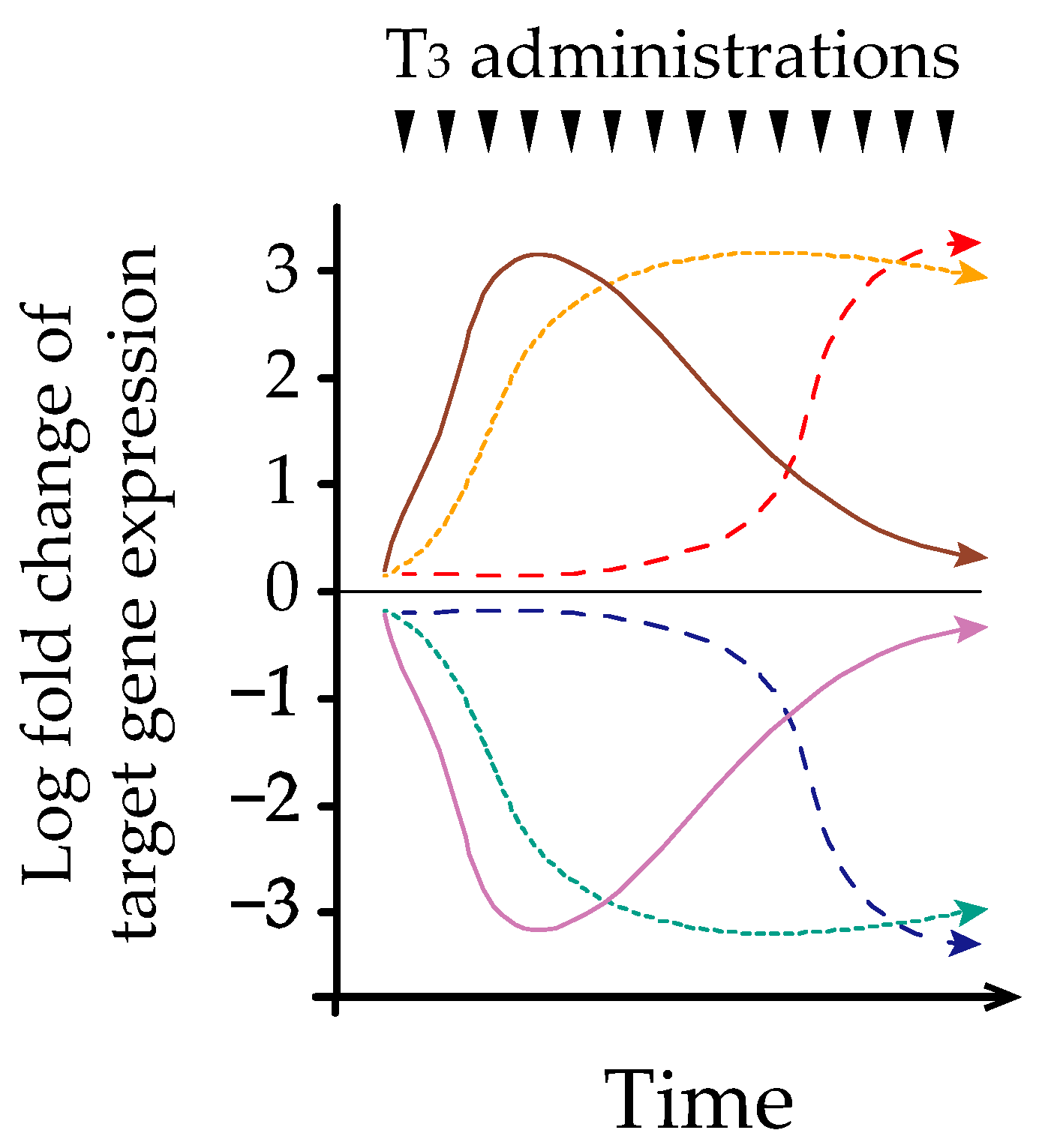
3.3. IST Associated with Methodological Interference and Phenomenon of Hysteresis
3.4. Possible Mechanism of Hysteresis Involving the HPT-Axis
4. Epidemiology of IST
5. Clinical Characteristics of IST
6. Differential Diagnosis of IST
6.1. Confirmation of Genuine IST
6.2. Differential Diagnosis of Genuine IST
6.3. Differential Diagnosis in Problematic Cases of IST
7. Treatment
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDH | Familial dysalbuminemic hyperthyroxinemia |
| HPT-axis | Hypothalamus-pituitary-thyroid axis |
| HSA | Human serum albumin |
| IST | Inappropriate secretion of thyroid-stimulating hormone |
| JTA | Japan Thyroid Association |
| L-T3 | Liothyronine |
| Macro-TSH | Macro-thyrotropin |
| MCT8 | Monocarboxylate transporter 8 |
| Non-TR-RTH | Resistance to thyroid hormone without mutations in the thyroid hormone receptor β gene |
| RTHβ | Resistance to thyroid hormone β |
| SBP2 | Selenocysteine insertion sequence-binding protein 2 |
| SITSH | Syndrome of inappropriate secretion of thyroid-stimulating hormone |
| SSA | Somatostatin analog |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TR | Thyroid hormone receptor |
| TRα | α isoform of thyroid hormone receptor |
| TRβ | β isoform of thyroid hormone receptor |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| TSHoma | Thyrotropin-secreting pituitary adenoma |
References
- Hollenberg, A.N. Regulation of Thyrotropin Secretion. In The Thyroid A Fundamental and Clinical Text, 10th ed.; Braverman, L.E., Cooper, D.S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 169–182. [Google Scholar]
- Sasaki, S.; Matsushita, A.; Kuroda, G.; Nakamura, H.M.; Oki, Y.; Suda, T. The Mechanism of Negative Transcriptional Regulation by Thyroid Hormone: Lessons from the Thyrotropin β Subunit Gene. Vitam. Horm. 2018, 106, 97–127. [Google Scholar] [PubMed]
- Weintraub, B.; Gershengorn, M.; Kourides, I.; Fein, H. Inappropriate Secretion of Thyroid-Stimulating Hormone. Ann. Intern. Med. 1981, 95, 339–351. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Brucker-Davis, F.; Persani, L.; Smallridge, R.C.; Weintraub, B.D. Thyrotropin-Secreting Pituitary Tumors. Endocr. Rev. 1996, 17, 610–638. [Google Scholar] [PubMed]
- Campi, I.; Covelli, D.; Moran, C.; Fugazzola, L.; Cacciatore, C.; Orlandi, F.; Gallone, G.; Chatterjee, K.; Beck-Peccoz, P.; Persani, L. The Differential Diagnosis of Discrepant Thyroid Function Tests: Insistent Pitfalls and Updated Flow-Chart Based on a Long-Standing Experience. Front. Endocrinol. Lausanne 2020, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Gershengorn, M.C.; Weintraub, B.D. Thyrotropin-Induced Hyperthyroidism Caused by Selective Pituitary Resistance to Thyroid Hormone. A New Syndrome of “Inappropriate Secretion of TSH”. J. Clin. Investig. 1975, 56, 633–642. [Google Scholar] [CrossRef][Green Version]
- Dumitrescu, A.M.; Refetoff, S. Impaired Sensitivity to Thyroid Hormone: Defects of Transport, Metabolism and Action. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Japan Thyroid Association. Diagnosis of the Resistance to Thyroid Hormone Beta (RTHβ). 2016. Available online: http://www.japanthyroid.jp/doctor/img/hormone03.pdf (accessed on 28 April 2021).
- Gessl, A.; Blueml, S.; Bieglmayer, C.; Marculescu, R. Anti-Ruthenium Antibodies Mimic Macro-TSH in Electrochemiluminescent Immunoassay. Clin. Chem. Lab. Med. 2014, 52, 1589–1594. [Google Scholar] [CrossRef]
- Kara, C.; Ocal, G.; Berberoğlu, M.; Siklar, Z.; Adiyaman, P. Persistently Raised Thyroid Stimulating Hormone in Adequately Treated Congenital Hypothyroidism on Long-Term Follow-Up. J. Pediatr. Endocrinol. Metab. 2008, 21, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Takayama, T.; Matsunaga, H.; Matsushita, A.; Sasaki, S.; Oki, Y.; Ozono, S.; Nakamura, H. Inappropriate Elevation of Serum Thyrotropin Levels in Patients Treated with Axitinib. Thyroid 2013, 23, 443–448. [Google Scholar] [CrossRef]
- Sakai, H.; Fukuda, G.; Suzuki, N.; Watanabe, C.; Odawara, M. Falsely Elevated Thyroid-Stimulating Hormone (TSH) Level due to Macro-TSH. Endocr. J. 2009, 56, 435–440. [Google Scholar] [CrossRef]
- Hattori, N.; Ishihara, T.; Matsuoka, N.; Saito, T.; Shimatsu, A. Anti-Thyrotropin Autoantibodies in Patients with Macro-Thyrotropin and Long-Term Changes in Macro-Thyrotropin and Serum Thyrotropin Levels. Thyroid 2017, 27, 138–146. [Google Scholar] [CrossRef]
- Ohba, K.; Maekawa, M.; Iwahara, K.; Suzuki, Y.; Matsushita, A.; Sasaki, S.; Oki, Y.; Nakamura, H. Abnormal Thyroid Hormone Response to TRH in a Case of Macro-TSH and the Cut-Off Value for Screening Cases of Inappropriate TSH Elevation. Endocr. J. 2020, 67, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Fukata, S.; Brent, G.A.; Sugawara, M. Resistance to Thyroid Hormone in Hashimoto’s Thyroiditis. N. Engl. J. Med. 2005, 352, 517–518. [Google Scholar] [CrossRef]
- Cooper, D.S.; Wenig, B.M. Hyperthyroidism Caused by an Ectopic TSH-Secreting Pituitary Tumor. Thyroid 1996, 6, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Trummer, C.; Reiher, H.; Theiler-Schwetz, V.; Pandis, M.; Gstettner, C.; Potzinger, P.; Keck, T.; Pieber, T.R.; Lax, S.; Haybaeck, J.; et al. Secondary Hyperthyroidism due to an Ectopic Thyrotropin-Secreting Neuroendocrine Pituitary Tumor: A Case Report. Eur. Thyroid J. 2020, 9, 106–112. [Google Scholar] [CrossRef]
- Refetoff, S.; Bassett, J.H.; Beck-Peccoz, P.; Bernal, J.; Brent, G.; Chatterjee, K.; De Groot, L.J.; Dumitrescu, A.M.; Jameson, J.L.; Kopp, P.A.; et al. Classification and Proposed Nomenclature for Inherited Defects of Thyroid Hormone Action, Cell Transport, and Metabolism. Eur. Thyroid J. 2014, 3, 7–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onigata, K.; Szinnai, G. Resistance to Thyroid Hormone. Endocr. Dev. 2014, 26, 118–129. [Google Scholar]
- Kaplan, M.M.; Taft, J.A.; Reichlin, S.; Munsat, T.L. Sustained Rises in Serum Thyrotropin, Thyroxine, and Triiodothyronine during Long Term, Continuous Thyrotropin-Releasing Hormone Treatment in Patients with Amyotrophic Lateral Sclerosis. J. Clin. Endocrinol. Metab. 1986, 63, 808–814. [Google Scholar] [CrossRef]
- Ohba, K.; Shirakawa, K.; Okawa, Y.; Iwaki, H.; Matsunaga, H.; Suzuki, S.; Matsushita, A.; Morita, H.; Sasaki, S.; Oki, Y.; et al. Syndrome of Inappropriate Secretion of Thyrotropin Associated with Thymoma-Related Peripheral Nerve Hyperexcitability. Endocr. J. 2011, 58, 597–602. [Google Scholar] [CrossRef]
- Monga, V.; Meena, C.L.; Kaur, N.; Jain, R. Chemistry and Biology of Thyrotropin-Releasing Hormone (TRH) and its Analogs. Curr. Med. Chem. 2008, 15, 2718–2733. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Murakami, M.; Satoh, T.; Monden, T.; Yamada, M.; Iriuchijima, T.; Kazihara, A.; Mori, M. Alteration by Prolonged Oral Administration of a Thyrotropin-Releasing Hormone Analog, TA-0910, of the Pituitary-Thyroid axis in Subjects with Brain Stroke. Intern. Med. 1993, 32, 681–685. [Google Scholar] [CrossRef][Green Version]
- Tamada, D.; Onodera, T.; Kitamura, T.; Yamamoto, Y.; Hayashi, Y.; Murata, Y.; Otsuki, M.; Shimomura, I. Hyperthyroidism due to Thyroid-Stimulating Hormone Secretion after Surgery for Cushing’s Syndrome: A Novel Cause of the Syndrome of Inappropriate Secretion of Thyroid-Stimulating Hormone. J. Clin. Endocrinol. Metab. 2013, 98, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Burlacu, M.C.; Maiter, D.; Gruson, D. Interferences with Thyroid Function Immunoassays: Clinical Implications and Detection Algorithm. Endocr. Rev. 2018, 39, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Noh, J.Y.; Unno, T.; Satoh, T.; Iwahara, K.; Matsushita, A.; Sasaki, S.; Oki, Y.; Nakamura, H. Falsely Elevated Thyroid Hormone Levels Caused by Anti-Ruthenium Interference in the Elecsys Assay Resembling the Syndrome of Inappropriate Secretion of Thyrotropin. Endocr. J. 2012, 59, 663–667. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yoshimura Noh, J.; Shimizu, T.; Sato, T.; Kurihara, I.; Sugino, K.; Itoh, H.; Ito, K. A Case of Familial Dysalbuminemic Hyperthyroxinemia (FDH) in Japan: FDH as a Possible Differential Diagnosis of Syndrome of Inappropriate Secretion of Thyroid-Stimulating Hormone (SITSH). Endocr. J. 2017, 64, 207–212. [Google Scholar] [CrossRef]
- Nagano, H.; Nakagawa, Y.; Ishikawa, N.; Watanabe, H.; Miyabayashi, Y.; Nakayama, A.; Fujimoto, M.; Komai, E.; Shiga, A.; Tamura, A.; et al. Seven Familial Dysalbuminemic Hyperthyroxinemia Cases in Three Unrelated Japanese Families and High-Performance Liquid Chromatography Analysis of the Thyroxine Binding Profile. Endocr. Pract. 2017, 23, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.K. A Review of the Phenomenon of Hysteresis in the Hypothalamus-Pituitary-Thyroid Axis. Front. Endocrinol. Lausanne 2016, 7, 64. [Google Scholar] [CrossRef]
- Ewing, J.A. On Hysteresis in the Relation of Strain to Stress; British Association Reports; British Association: London, UK, 1889; pp. 502–504. Available online: https://archive.org/details/reportofbritisha90brit/page/502/mode/2up (accessed on 28 April 2021).
- Leow, M.K. A Mathematical Model of Pituitary-Thyroid Interaction to Provide an Insight into the Nature of the Thyrotropin--Thyroid Hormone Relationship. J. Theor. Biol. 2007, 248, 275–287. [Google Scholar] [CrossRef]
- Flores-Morales, A.; Gullberg, H.; Fernandez, L.; Ståhlberg, N.; Lee, N.H.; Vennström, B.; Norstedt, G. Patterns of Liver Gene Expression Governed by TRbeta. Mol. Endocrinol. 2002, 16, 1257–1268. [Google Scholar]
- Ohba, K.; Leow, M.K.; Singh, B.K.; Sinha, R.A.; Lesmana, R.; Liao, X.H.; Ghosh, S.; Refetoff, S.; Sng, J.C.; Yen, P.M. Desensitization and Incomplete Recovery of Hepatic Target Genes After Chronic Thyroid Hormone Treatment and Withdrawal in Male Adult Mice. Endocrinology 2016, 157, 1660–1672. [Google Scholar] [CrossRef]
- Umezawa, R.; Yamada, M.; Horiguchi, K.; Ishii, S.; Hashimoto, K.; Okada, S.; Satoh, T.; Mori, M. Aberrant Histone Modifications at the Thyrotropin-Releasing Hormone Gene in Resistance to Thyroid Hormone: Analysis of F455S Mutant Thyroid Hormone Receptor. Endocrinology 2009, 150, 3425–3432. [Google Scholar] [CrossRef]
- Ohba, K.; Sinha, R.A.; Singh, B.K.; Iannucci, L.F.; Zhou, J.; Kovalik, J.P.; Liao, X.H.; Refetoff, S.; Sng, J.C.G.; Leow, M.K.; et al. Changes in Hepatic TRβ Protein Expression, Lipogenic Gene Expression, and Long-Chain Acylcarnitine Levels During Chronic Hyperthyroidism and Triiodothyronine Withdrawal in a Mouse Model. Thyroid 2017, 27, 852–860. [Google Scholar] [CrossRef]
- Ohye, H. The Differential Diagnosis of the Syndrome of Inappropriate Secretion of TSH (SITSH). Mod. Physician 2015, 35, 1119–1121. (In Japanese) [Google Scholar]
- Murata, Y. Diagnostic Criteria Making of the Resistance to Thyroid Hormone. J. Jpn. Thyroid Assoc. 2012, 3, 10–14. (In Japanese) [Google Scholar]
- Macchia, E.; Lombardi, M.; Raffaelli, V.; Piaggi, P.; Macchia, L.; Scattina, I.; Martino, E. Clinical and Genetic Characteristics of a Large Monocentric Series of Patients Affected by Thyroid Hormone (Th) Resistance and Suggestions for Differential Diagnosis in Patients without Mutation of Th Receptor β. Clin. Endocrinol. 2014, 81, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Lafranchi, S.H.; Snyder, D.B.; Sesser, D.E.; Skeels, M.R.; Singh, N.; Brent, G.A.; Nelson, J.C. Follow-Up of Newborns with Elevated Screening T4 Concentrations. J. Pediatr. 2003, 143, 296–301. [Google Scholar] [CrossRef]
- Tajima, T.; Jo, W.; Fujikura, K.; Fukushi, M.; Fujieda, K. Elevated Free Thyroxine Levels Detected by a Neonatal Screening System. Pediatr. Res. 2009, 66, 312–316. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Lania, A.; Beckers, A.; Chatterjee, K.; Wemeau, J.L. 2013 European Thyroid Association Guidelines for the Diagnosis and Treatment of Thyrotropin-Secreting Pituitary Tumors. Eur. Thyroid J. 2013, 2, 76–82. [Google Scholar] [CrossRef]
- Teng, X.; Jin, T.; Brent, G.A.; Wu, A.; Teng, W.; Shan, Z. A Patient with a Thyrotropin-Secreting Microadenoma and Resistance to Thyroid Hormone (P453T). J. Clin. Endocrinol. Metab. 2015, 100, 2511–2514. [Google Scholar] [CrossRef]
- Pappa, T.; Ferrara, A.M.; Refetoff, S. Inherited Defects of Thyroxine-Binding Proteins. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 735–747. [Google Scholar] [CrossRef]
- DeCosimo, D.R.; Fang, S.L.; Braverman, L.E. Prevalence of Familial Dysalbuminemic Hyperthyroxinemia in Hispanics. Ann. Intern. Med. 1987, 107, 780–781. [Google Scholar] [CrossRef]
- Arevalo, G. Prevalence of Familial Dysalbuminemic Hyperthyroxinemia in Serum Samples Received for Thyroid Testing. Clin. Chem. 1991, 37, 1430–1431. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L.; Lania, A. Thyrotropin-Secreting Pituitary Adenomas. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Yamada, S.; Fukuhara, N.; Horiguchi, K.; Yamaguchi-Okada, M.; Nishioka, H.; Takeshita, A.; Takeuchi, Y.; Ito, J.; Inoshita, N. Clinicopathological Characteristics and Therapeutic Outcomes in Thyrotropin-Secreting Pituitary Adenomas: A Single-Center Study of 90 Cases. J. Neurosurg. 2014, 121, 1462–1473. [Google Scholar] [CrossRef]
- Tjörnstrand, A.; Nyström, H.F. Diagnosis of Endocrine Disease: Diagnostic Approach to TSH-Producing Pituitary Adenoma. Eur. J. Endocrinol. 2017, 177, R183–R197. [Google Scholar] [CrossRef]
- Ueda, Y.; Tagami, T.; Tamanaha, T.; Kakita, M.; Tanase-Nakao, K.; Nanba, K.; Usui, T.; Naruse, M.; Shimatsu, A. A Family of RTHbeta with p.R316C Mutation Presenting Occasional Syndrome of Inappropriate Secretion of TSH. Endocr. J. 2015, 62, 251–260. [Google Scholar] [CrossRef]
- Okuma, H.; Hashimoto, K.; Ohashi, T.; Mihara, M.; Minami, I.; Izumiyama, H.; Sasaki, S.; Inoshita, N.; Nishioka, H.; Yamada, S.; et al. A Case of TSH-Secreting Pituitary Adenoma with Cyclic Fluctuations in Serum TSH Levels. Endocr. J. 2018, 65, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Luciano, M.G.; Doppman, J.L.; Patronas, N.J.; Oldfield, E.H. Pituitary Magnetic Resonance Imaging in Normal Human Volunteers: Occult Adenomas in the General Population. Ann. Intern. Med. 1994, 120, 817–820. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L.; Mannavola, D.; Campi, I. Pituitary tumours: TSH-Secreting Adenomas. Best. Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 597–606. [Google Scholar] [CrossRef]
- Refetoff, S.; Weiss, R.E.; Usala, S.J. The Syndromes of Resistance to Thyroid Hormone. Endocr. Rev. 1993, 14, 348–399. [Google Scholar] [PubMed]
- Mannavola, D.; Persani, L.; Vannucchi, G.; Zanardelli, M.; Fugazzola, L.; Verga, U.; Facchetti, M.; Beck-Peccoz, P. Different Responses to Chronic Somatostatin Analogues in Patients with Central Hyperthyroidism. Clin. Endocrinol. 2005, 62, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Shen, L.; Zhang, J.; Xie, J.; Fang, W.; Sun, Q.; Bian, L.; Zhou, Y.; Wang, S.; Ning, G.; et al. Diagnosing Thyrotropin-Secreting Pituitary Adenomas by Short-Term Somatostatin Analogue Test. Thyroid 2020, 30, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Socin, H.V.; Chanson, P.; Delemer, B.; Tabarin, A.; Rohmer, V.; Mockel, J.; Stevenaert, A.; Beckers, A. The Changing Spectrum of TSH-Secreting Pituitary Adenomas: Diagnosis and Management in 43 Patients. Eur. J. Endocrinol. 2003, 148, 433–442. [Google Scholar] [CrossRef]
- Ohba, K.; Okada, E.; Goto, Y.; Suzuki, S.; Machii, M.; Nonaka, D.; Matsushita, A.; Sasaki, S.; Suda, T.; Oki, Y.; et al. Influence of Thyroid Dysfunction on Brain Natriuretic Peptide Level in Health Examination Participants. Endocr. J. 2020, 67, 449–454. [Google Scholar] [CrossRef]
- Shimatsu, A.; Nakamura, A.; Takahashi, Y.; Fujio, S.; Satoh, F.; Tahara, S.; Nishioka, H.; Takano, K.; Yamashita, M.; Arima, H.; et al. Preoperative and Long-Term Efficacy and Safety of Lanreotide Autogel in Patients with Thyrotropin-Secreting Pituitary Adenoma: A Multicenter, Single-Arm, Phase 3 Study in Japan. Endocr. J. 2021, in press. [Google Scholar] [CrossRef]
- Singh, B.K.; Yen, P.M. A Clinician’s Guide to Understanding Resistance to Thyroid Hormone due to Receptor Mutations in the TRα and TRβ Isoforms. Clin. Diabetes Endocrinol. 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Yamada, M.; Horiguchi, K.; Satoh, T.; Hashimoto, K.; Tokuhiro, E.; Onigata, K.; Mori, M. Resistance to Thyroid Hormone due to a Novel Thyroid Hormone Receptor Mutant in a Patient with Hypothyroidism Secondary to Lingual Thyroid and Functional Characterization of the Mutant Receptor. Thyroid 2010, 20, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, M.; Otsuka, F.; Tozaki, T.; Ban, Y. Thyroid Profiles in a Patient with Resistance to Thyroid Hormone and Episodes of Thyrotoxicosis, Including Repeated Painless Thyroiditis. Thyroid 2013, 23, 898–901. [Google Scholar] [CrossRef]
- Nakao, T.; Fukata, S.; Hisakado, M.; Kasahara, T.; Kudo, T.; Nishihara, E.; Ito, M.; Nishikawa, M.; Nakamura, H.; Miyauchi, A. A Patient with Resistance to Thyroid Hormon and Macro-TSH. Folia Endocrinol. Jpn. 2017, 93, 35–37. Available online: https://www.jstage.jst.go.jp/article/endocrine/93/S.Update/93_35/_pdf (accessed on 28 April 2021). (In Japanese).
|
1. Causes associated with the classification proposed by Gershengorn and Weintraub [6] I. Neoplastic production of thyroid-stimulating hormone (TSH) Thyrotropin-secreting pituitary adenoma (TSHoma) * Ectopic TSHoma * II. Non-neoplastic pituitary hypersecretion of TSH Resistance to thyroid hormone β (RTHβ) * Non-TR-RTH * Thyrotropin-releasing hormone (TRH) administration Cushing’s syndrome after surgical resection 2. Methodological interference Heterophilic interference Thyroid hormone autoantibodies Assay-specific interference Familial dysalbuminemic hyperthyroxinemia (FDH) 3. Hysteresis involving the hypothalamus-pituitary-thyroid axis (lagging TSH recovery) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohba, K. An Update on the Pathophysiology and Diagnosis of Inappropriate Secretion of Thyroid-Stimulating Hormone. Int. J. Mol. Sci. 2021, 22, 6611. https://doi.org/10.3390/ijms22126611
Ohba K. An Update on the Pathophysiology and Diagnosis of Inappropriate Secretion of Thyroid-Stimulating Hormone. International Journal of Molecular Sciences. 2021; 22(12):6611. https://doi.org/10.3390/ijms22126611
Chicago/Turabian StyleOhba, Kenji. 2021. "An Update on the Pathophysiology and Diagnosis of Inappropriate Secretion of Thyroid-Stimulating Hormone" International Journal of Molecular Sciences 22, no. 12: 6611. https://doi.org/10.3390/ijms22126611
APA StyleOhba, K. (2021). An Update on the Pathophysiology and Diagnosis of Inappropriate Secretion of Thyroid-Stimulating Hormone. International Journal of Molecular Sciences, 22(12), 6611. https://doi.org/10.3390/ijms22126611






