Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila
Abstract
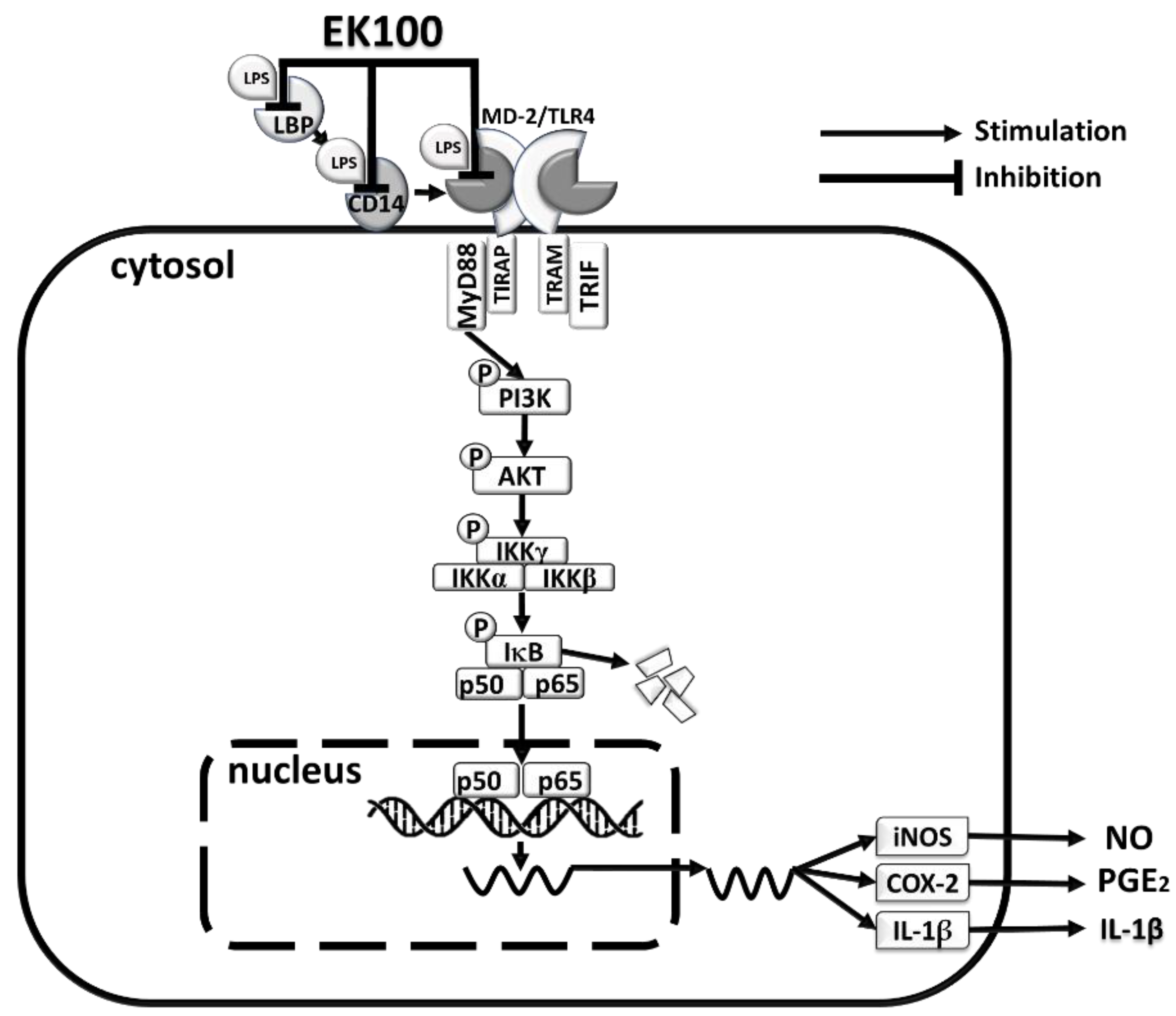
1. Introduction
2. Results
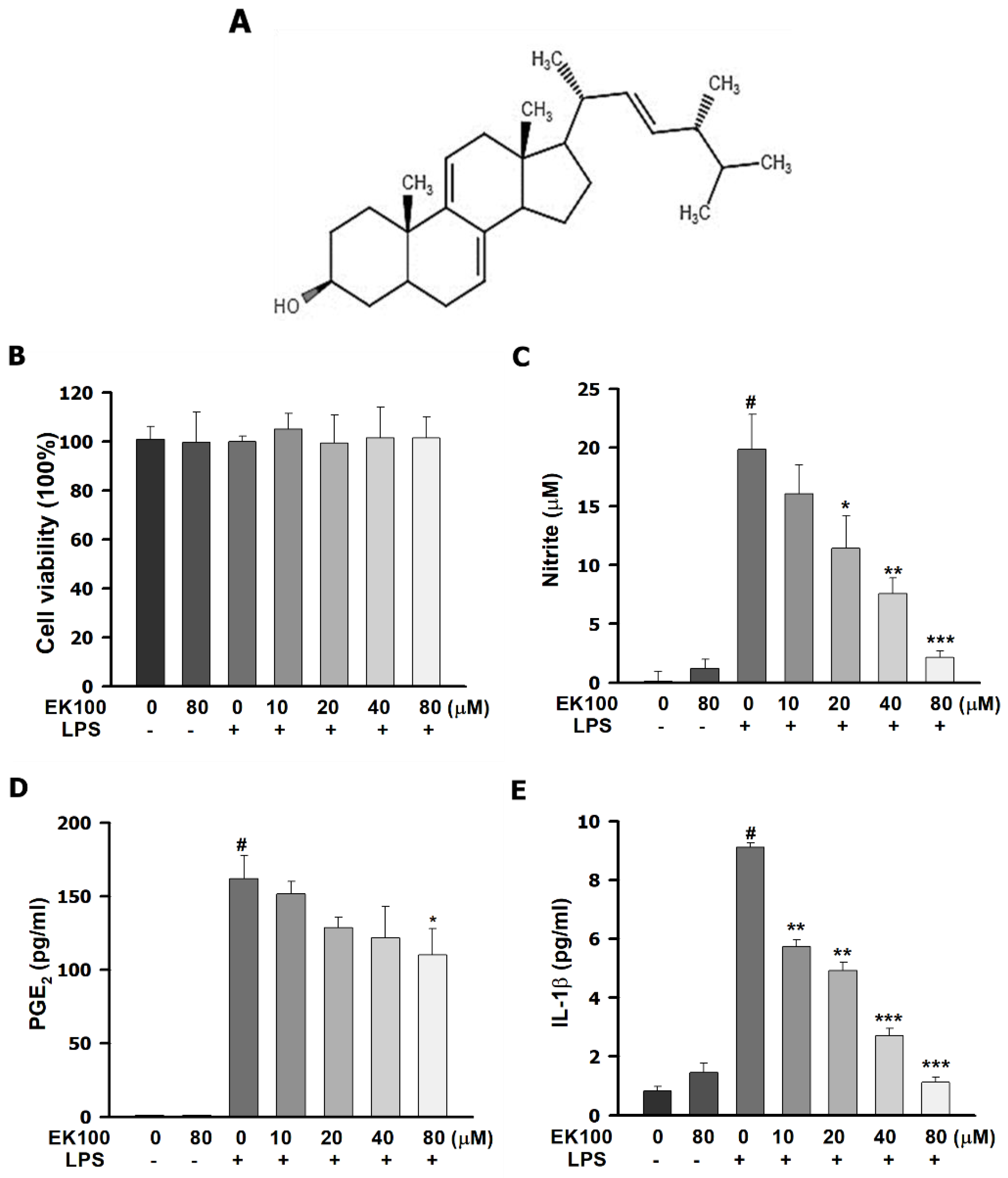
2.1. Effects of EK100 on the Cell Viability and Cytokines Release in LPS-Stimulated RAW264.7 Murine Macrophage Cells
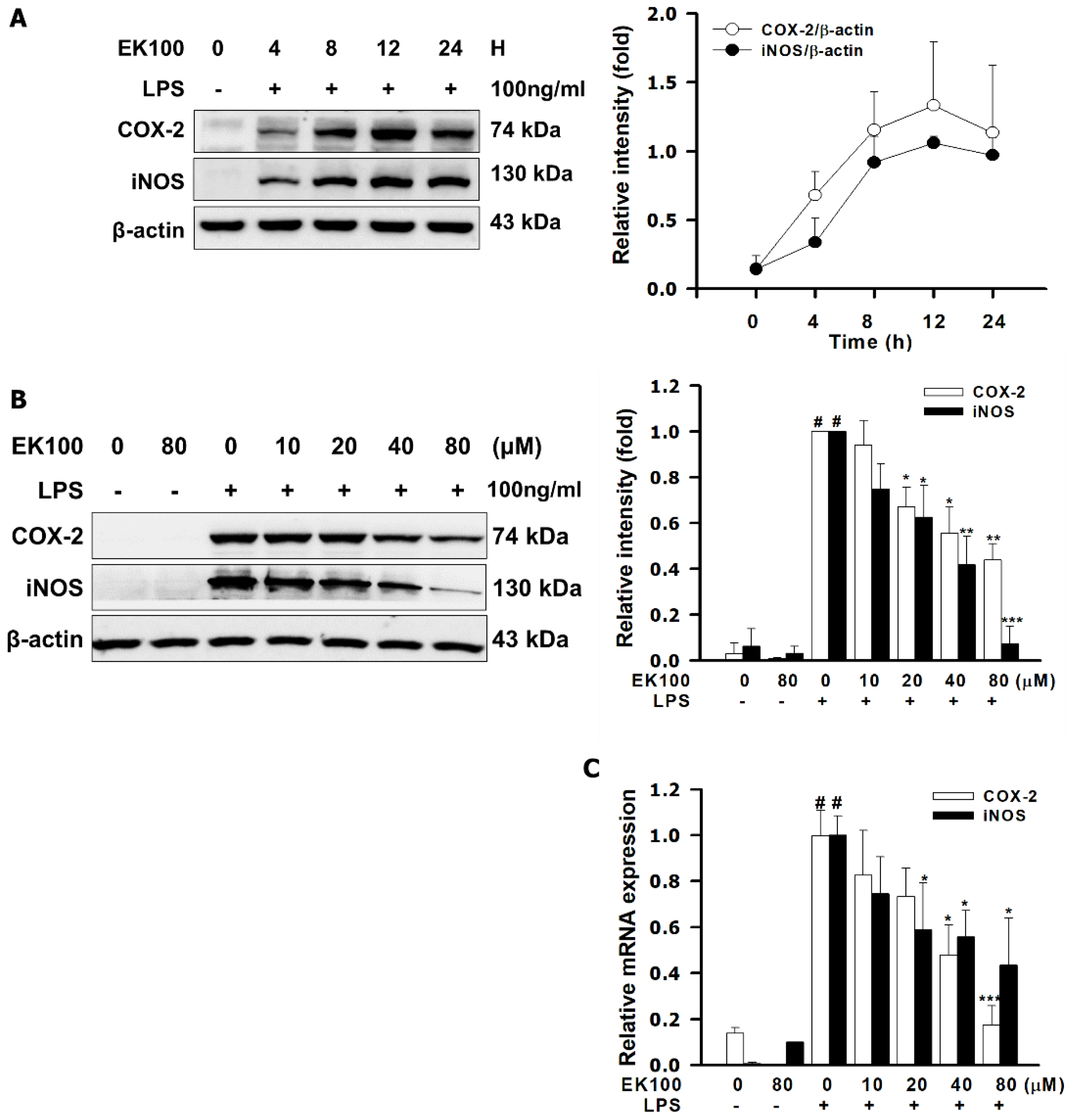
2.2. EK100 Suppressed LPS-Induced mRNA and Protein Expression of Pro-Inflammatory Mediators of iNOS and COX-2 in RAW264.7 Cells
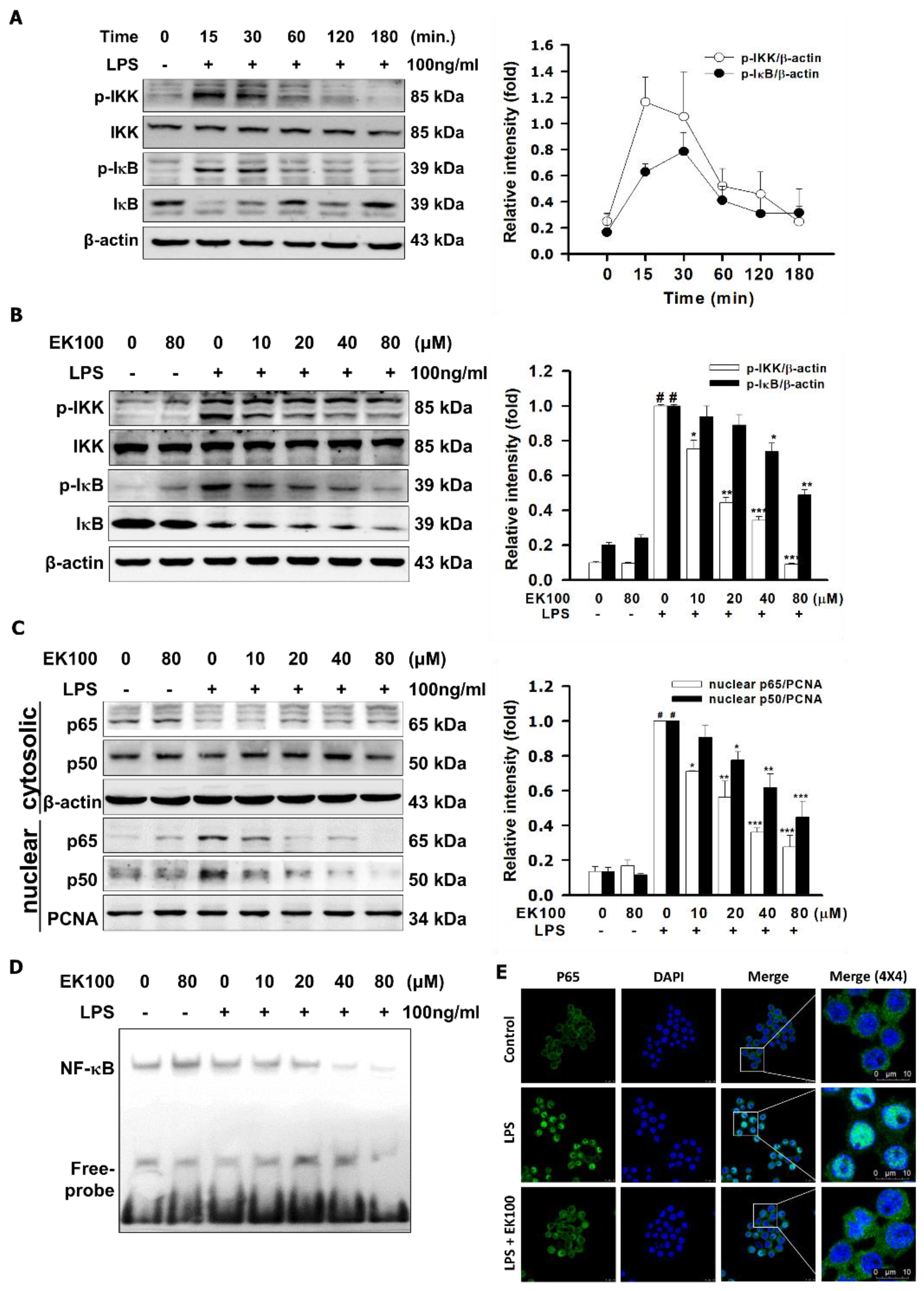
2.3. Effects of EK100 on the Phosphorylation of IKK/IκB and the Translocation of NF-κB in LPS-Induced RAW264.7 Cells
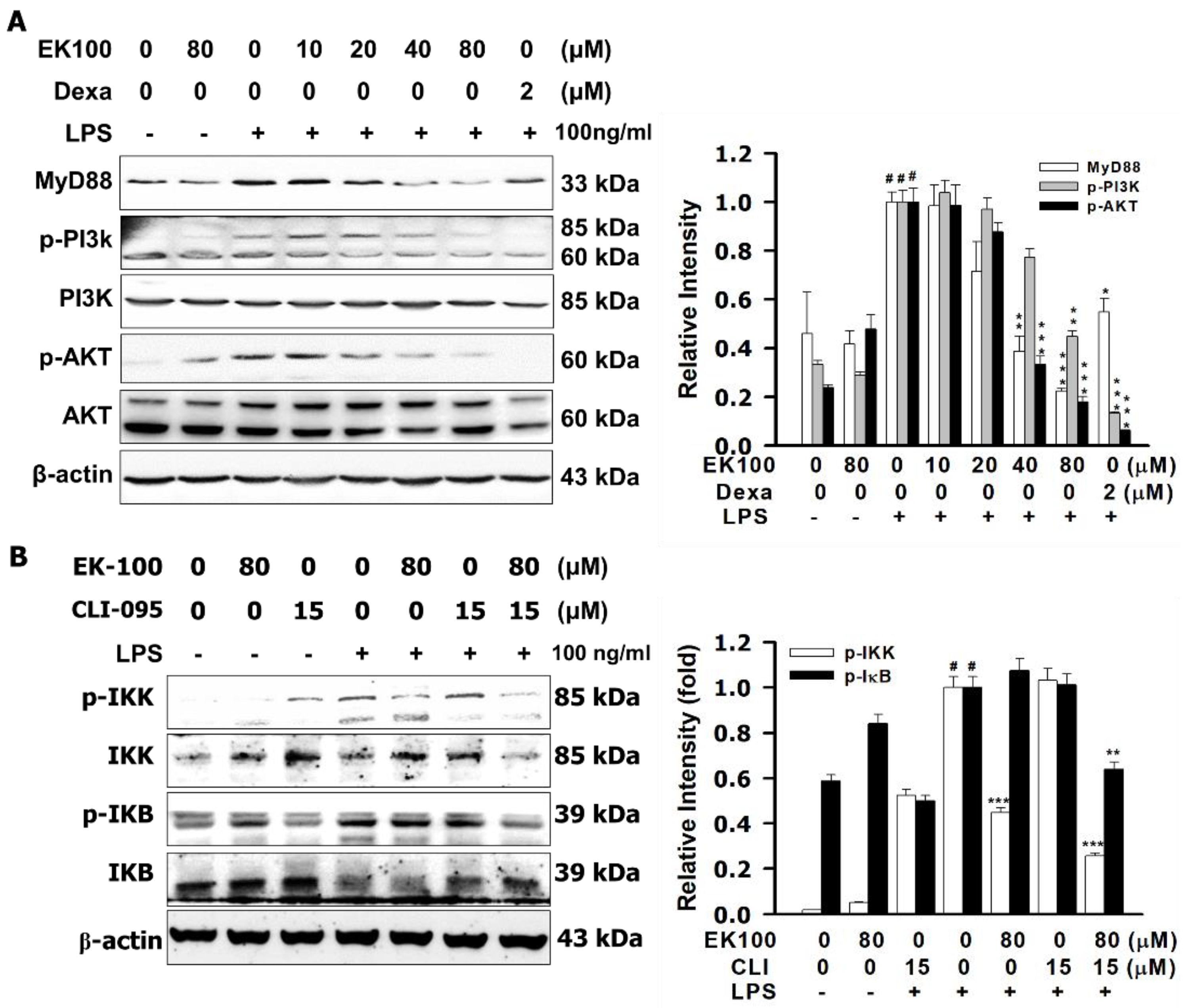
2.4. Effects of EK100 on the PI3K/Akt Signaling Pathway in LPS-Induced RAW264.7 Cells
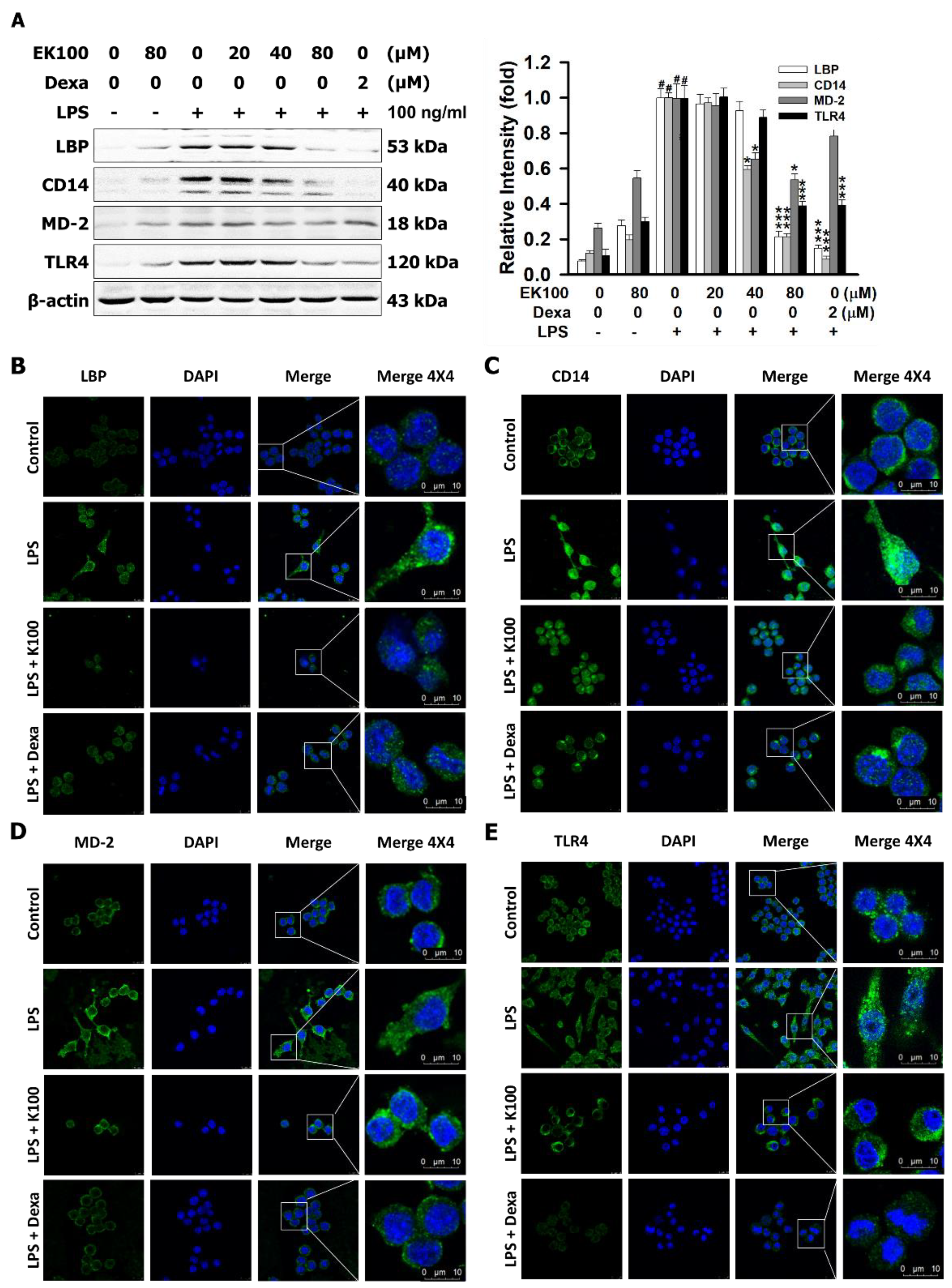
2.5. Effects of EK100 on the Protein Expression of Lipopolysaccharide-Binding Protein (LBP), the Cluster of Differentiation 14 (CD14), and TLR4/Myeloid Differentiation-2 (MD-2) Co-Receptors in LPS-Stimulated RAW264.7 Cells
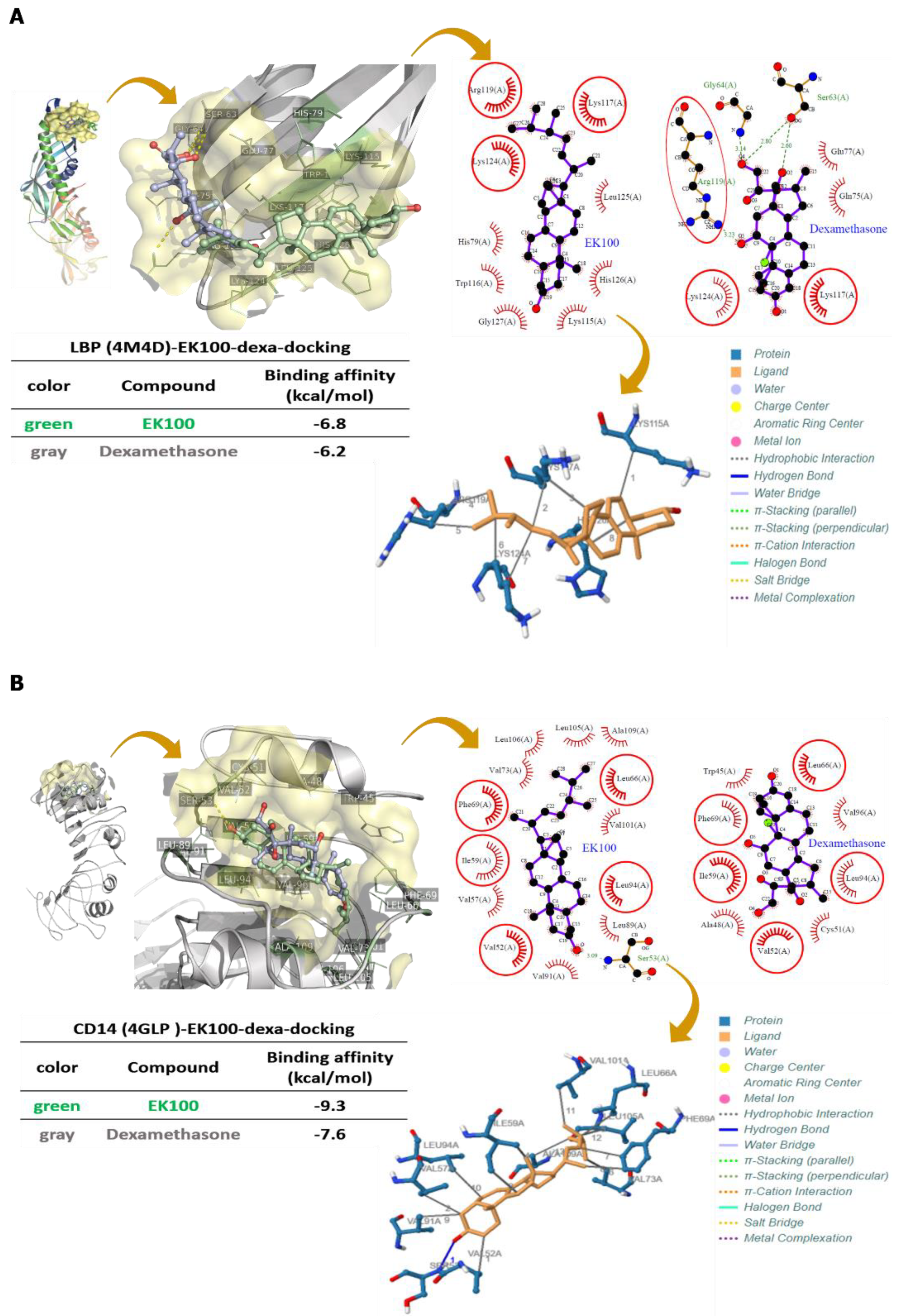
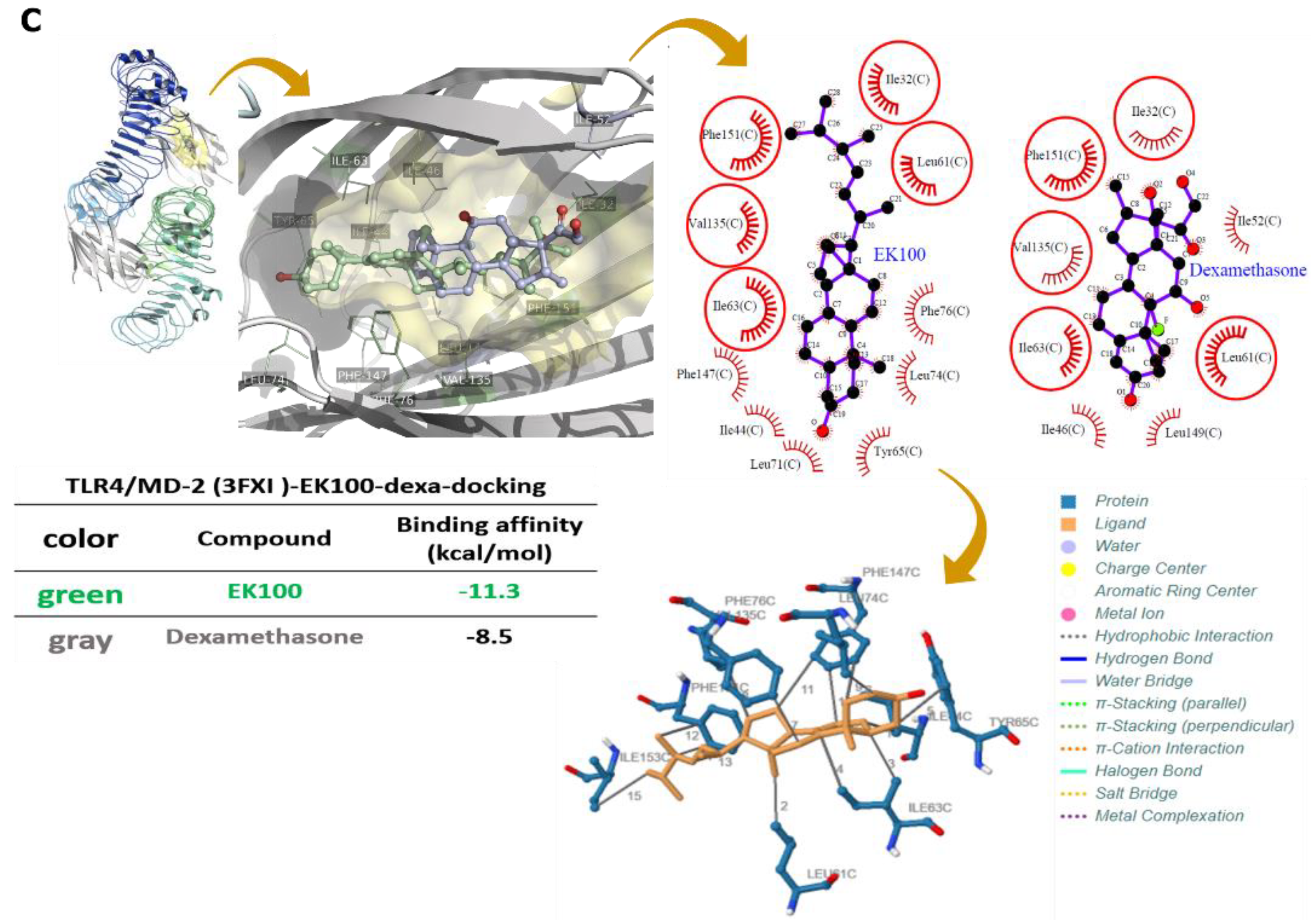
2.6. Effects of EK100 on LPS Binding to LBP, CD14, and TLR4/MD-2 with Protein–Ligand Docking
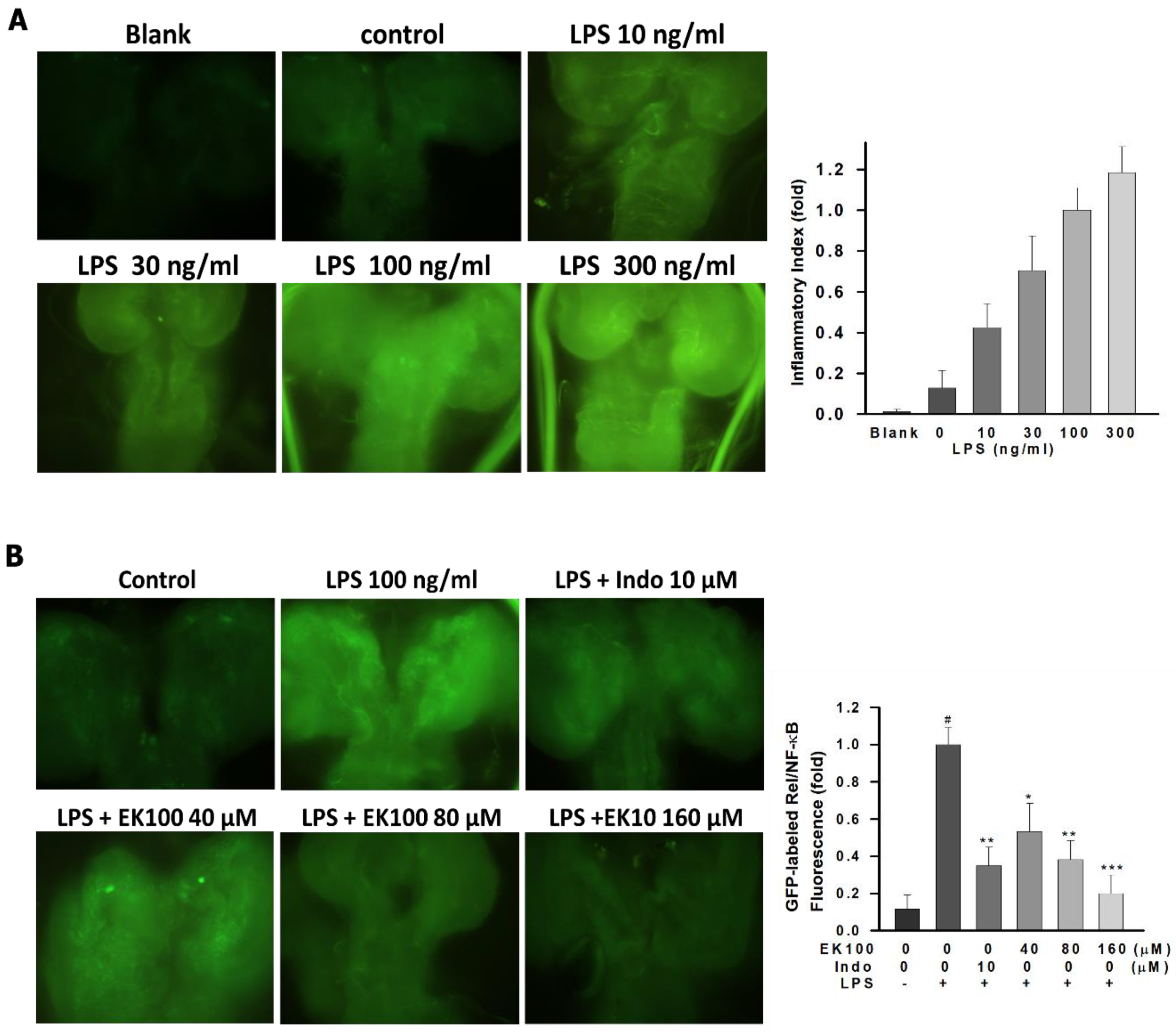
2.7. Effects of EK100 on the Green Fluorescent Protein (GFP)-Labeled Rel/NF-κB Fluorescence Expression in Drosophila
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Line and Culture
4.3. Cell Viability Assay
4.4. Nitrite Assay
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Quantitative Real-Time Polymerase Chain Reaction (Q-PCR)
4.7. Western Blot Assay
4.8. Electrophoretic Mobility Shift Assay (EMSA)
4.9. Immunofluorescence Assay
4.10. Fluorescence Microscopy and NF-κB Activity Analysis in Drosophila
4.11. Protein-Ligand Docking Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| Akt | Protein kinase B |
| BSA | Bovine serum albumin |
| CM | Cordyceps militaris |
| CCD | Charge-coupled Device |
| CD14 | Cluster of differentiation 14 |
| CLI-095 | Resatorvid; TAK-242 |
| COX-2 | Cyclooxygenase-2 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DEHP | Di(2-Ethylhexyl) phthalate |
| Dexa | Dexamethasone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EGFP | Enhanced green fluorescent protein |
| ELISA | Enzyme-linked immunoassay |
| EK100 | Ergosta-7, 9 (11), 22-trien-3β-ol |
| EMSA | Electrophoretic mobility shift assay |
| FBS | Fetal bovine serum |
| GFP | Green fluorescent protein |
| IF | Immunofluorescence |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IκB | Inhibitor kappa B |
| IKK | IκB kinase |
| LBP | Lipopolysaccharide-binding protein |
| LPS | Lipopolysaccharide |
| MD-2 | Myeloid differentiation-2 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MyD88 | Myeloid differentiation factor 88 |
| NF-κB | Nuclear factor-κB |
| OD | optical density |
| p50 | NF-κB p50 |
| p65 | NF-κB p65 |
| PI3K | Phosphatidylinositol-3-kinase |
| p-AKT | Phospho-Protein kinase B |
| p-IκB | Phospho-inhibitor kappa B |
| p-IKK | Phospho-IκB kinase |
| PVDF | Polyvinylidene fluoride |
| Q-PCR | Quantitative real-time polymerase chain reaction |
| SEM | Standard error means |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
References
- Tsai, D.H.; Riediker, M.; Berchet, A.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ. Sci. Pollut. Res. Int. 2019, 26, 19697–19704. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ambesi, A.; McKeown-Longo, P.J. Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin. Cells 2020, 9, 216. [Google Scholar] [CrossRef]
- Strassheim, D.; Asehnoune, K.; Park, J.S.; Kim, J.Y.; He, Q.; Richter, D.; Kuhn, K.; Mitra, S.; Abraham, E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J. Immunol. 2004, 172, 5727–5733. [Google Scholar] [CrossRef]
- Koh, W.; Shin, J.S.; Lee, J.; Lee, I.H.; Lee, S.K.; Ha, I.H.; Chung, H.J. Anti-inflammatory effect of Cortex Eucommiae via modulation of the toll-like receptor 4 pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2017, 209, 255–263. [Google Scholar] [CrossRef]
- Li, Q.; Van Antwerp, D.; Mercurio, F.; Lee, K.F.; Verma, I.M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 1999, 284, 321–325. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Mikenberg, I.; Widera, D.; Kaus, A.; Kaltschmidt, B.; Kaltschmidt, C. Transcription factor NF-kappaB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS ONE 2007, 2, e589. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Bui, B.P.; Lee, H.; Cho, J. A Novel 1,8-Naphthyridine-2-Carboxamide Derivative Attenuates Inflammatory Responses and Cell Migration in LPS-Treated BV2 Cells via the Suppression of ROS Generation and TLR4/Myd88/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 2527. [Google Scholar] [CrossRef]
- Rupa, E.J.; Li, J.F.; Arif, M.H.; Yaxi, H.; Puja, A.M.; Chan, A.J.; Hoang, V.-A.; Kaliraj, L.; Yang, D.C.; Kang, S.C. Cordyceps militaris Fungus Extracts-Mediated Nanoemulsion for Improvement Antioxidant, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2020, 25, 5733. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef]
- Kao, S.T.; Kuo, Y.H.; Wang, S.D.; Hong, H.J.; Lin, L.J. Analogous corticosteroids, 9A and EK100, derived from solid-state-cultured mycelium of Antrodia camphorata inhibit proinflammatory cytokine expression in macrophages. Cytokine 2018, 108, 136–144. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chern, C.M.; Liou, K.T.; Kuo, Y.H.; Shen, Y.C. Ergostatrien-7,9(11),22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-κ-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019, 10, 4725–4738. [Google Scholar] [CrossRef]
- Huang, G.J.; Huang, S.-H.; Lin, S.-S.; Shao, Y.-Y.; Chen, C.-C.; Hou, W.-C.; Kuo, Y.-H. Analgesic Effects and the Mechanisms of Anti-inflammation of Ergostatrien-3??-ol from Antrodia camphorata Submerged Whole Broth in Mice. J. Agric. Food Chem. 2010, 58, 7445–7452. [Google Scholar] [CrossRef]
- Tsai, T.C.; Tung, Y.T.; Kuo, Y.H.; Liao, J.W.; Tsai, H.C.; Chong, K.Y.; Chen, H.L.; Chen, C.M. Anti-inflammatory effects of Antrodia camphorata, a herbal medicine, in a mouse skin ischemia model. J. Ethnopharmacol. 2015, 159, 113–121. [Google Scholar] [CrossRef]
- Chao, T.Y.; Hsieh, C.C.; Hsu, S.M.; Wan, C.H.; Lian, G.T.; Tseng, Y.H.; Kuo, Y.H.; Hsieh, S.C. Ergostatrien-3beta-ol (EK100) from Antrodia camphorata Attenuates Oxidative Stress, Inflammation, and Liver Injury In Vitro and In Vivo. Prev. Nutr. Food Sci. 2021, 26, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef]
- Neuder, L.E.; Keener, J.M.; Eckert, R.E.; Trujillo, J.C.; Jones, S.L. Role of p38 MAPK in LPS induced pro-inflammatory cytokine and chemokine gene expression in equine leukocytes. Vet. Immunol. Immunopathol. 2009, 129, 192–199. [Google Scholar] [CrossRef]
- Küper, C.; Beck, F.X.; Neuhofer, W. Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of pro-inflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Ren. Physiol. 2012, 302, F38–F46. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.C.; Judas, F.; Lopes, M.C.; Mendes, A.F. Nitric oxide synthase isoforms and NF-kappaB activity in normal and osteoarthritic human chondrocytes: Regulation by inducible nitric oxide. Nitric Oxide 2008, 19, 276–283. [Google Scholar] [CrossRef]
- Fukata, M.; Chen, A.; Klepper, A.; Krishnareddy, S.; Arunan, S.; Thomas, L.; Xu, R.; Inoue, H.; Arditi, M.; Dannenberg, A.; et al. Cox-2 Is Regulated by Toll-Like Receptor-4 (TLR4) Signaling: Role in Proliferation and Apoptosis in the Intestine. Gastroenterology 2006, 131, 862–877. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hsiao, Y.-H.; Ng, K.-L.; Kuo, Y.-H.; Lim, Y.-P.; Hsieh, W.-T. Physalin A attenuates inflammation through down-regulating c-Jun NH2 kinase phosphorylation/Activator Protein 1 activation and up-regulating the antioxidant activity. Toxicol. Appl. Pharmacol. 2020, 402, 115115. [Google Scholar] [CrossRef]
- Tseng, P.H.; Chen, I.T. O019 TLR4-mediated IKK and MAPK activation is regulated by differential post-translational modification of TAK1. Cytokine 2012, 59, 505–506. [Google Scholar] [CrossRef]
- Karin, M. How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex. Oncogene 1999, 18, 6867–6874. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Ain, Q.U.; Batool, M.; Choi, S. TLR4-Targeting Therapeutics: Structural Basis and Computer-Aided Drug Discovery Approaches. Molecules 2020, 25, 627. [Google Scholar] [CrossRef]
- Huang, M.H.; Lin, Y.H.; Lyu, P.C.; Liu, Y.C.; Chang, Y.S.; Chung, J.G.; Lin, W.Y.; Hsieh, W.T. Imperatorin Interferes with LPS Binding to the TLR4 Co-Receptor and Activates the Nrf2 Antioxidative Pathway in RAW264.7 Murine Macrophage Cells. Antioxidants 2021, 10, 362. [Google Scholar] [CrossRef]
- Bonin, C.P.; Baccarin, R.Y.; Nostell, K.; Nahum, L.A.; Fossum, C.; de Camargo, M.M. Lipopolysaccharide-induced inhibition of transcription of tlr4 in vitro is reversed by dexamethasone and correlates with presence of conserved NFκB binding sites. Biochem. Biophys. Res. Commun. 2013, 432, 256–261. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kelley, K.; Melichian, D.S.; Tamaki, Z.; Fang, F.; Su, Y.; Feng, G.; Pope, R.M.; Budinger, G.R.S.; Mutlu, G.M.; et al. Toll-Like Receptor 4 Signaling Augments Transforming Growth Factor-β Responses: A Novel Mechanism for Maintaining and Amplifying Fibrosis in Scleroderma. Am. J. Pathol. 2013, 182, 192–205. [Google Scholar] [CrossRef]
- Schumann Ralf, R. Old and new findings on lipopolysaccharide-binding protein: A soluble pattern-recognition molecule. Biochem. Soc. Trans. 2011, 39, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Takeuchi, S.; Kubota, K.; Kobayashi, Y.; Kozakai, S.; Ukai, I.; Shichiku, A.; Okubo, M.; Numasaki, M.; Kanemitsu, Y.; et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKKϵ-IRF3 axis activation. J. Biol. Chem. 2018, 293, 10186–10201. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.K.; Kim, Y.J.; Kim, J.I.; Gürtler, K.; Oh, D.Y.; Sur, S.; Lundvall, L.; Hamann, L.; van der Ploeg, A.; Pickkers, P.; et al. The crystal structure of lipopolysaccharide binding protein reveals the location of a frequent mutation that impairs innate immunity. Immunity 2013, 39, 647–660. [Google Scholar] [CrossRef]
- Kogut, M.H.; He, H.; Kaiser, P. Lipopolysaccharide binding protein/CD14/TLR4-dependent recognition of salmonella LPS induces the functional activation of chicken heterophils and up-regulation of pro-inflammatory cytokine and chemokine gene expression in these cells. Anim. Biotechnol. 2005, 16, 165–181. [Google Scholar] [CrossRef]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Kimoto, M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef]
- Foley, J.F. Inflamed by TLR4 internalization. Science 2020, 367, 1438–1440. [Google Scholar]
- Ganesan, S.; Aggarwal, K.; Paquette, N.; Silverman, N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 2011, 349, 25–60. [Google Scholar]
- Hetru, C.; Hoffmann, J.A. NF-kappaB in the immune response of Drosophila. Cold Spring Harb. Perspect. Biol. 2009, 1, a000232. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.N.; Wu, K.C.; Chung, W.S.; Zheng, L.C.; Juan, T.K.; Hsiao, Y.T.; Peng, S.F.; Yang, J.L.; Ma, Y.S.; Wu, R.S.; et al. Etomidate Suppresses Invasion and Migration of Human A549 Lung Adenocarcinoma Cells. Anticancer Res. 2019, 39, 215–223. [Google Scholar] [CrossRef]
- Vian, L.; Vincent, J.; Maurin, J.; Fabre, I.; Giroux, J.; Cano, J.P. Comparison of three in vitro cytotoxicity assays for estimating surfactant ocular irritation. Toxicol. In Vitro 1995, 9, 185–190. [Google Scholar] [CrossRef]
- Waltz, P.; Escobar, D.; Botero, A.M.; Zuckerbraun, B.S. Nitrate/Nitrite as Critical Mediators to Limit Oxidative Injury and Inflammation. Antioxid. Redox Signal. 2015, 23, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Mandon, J.; Cristescu, S.M.; Harren, F.J.; Prats, E. Methods of nitric oxide detection in plants: A commentary. Plant Sci. 2011, 181, 509–519. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 1972, 109, 129–135. [Google Scholar]
- Xiang, L.; Jiang, Y.; Liu, W.; Bi, Y.; Zhao, F.; Huo, G. Real-time RT PCR with DNA subtraction for relative quantification of gene expression in Staphylococcus aureus. Wei Sheng Wu Xue Bao Acta Microbiol. Sin. 2008, 48, 526–531. [Google Scholar]
- Hsieh, W.T.; Lin, H.Y.; Chen, J.H.; Lin, W.C.; Kuo, Y.H.; Wood, W.G.; Lu, H.F.; Chung, J.G. Latex of Euphorbia antiquorum-induced S-phase arrest via active ATM kinase and MAPK pathways in human cervical cancer HeLa cells. Environ. Toxicol. 2015, 30, 1205–1215. [Google Scholar] [CrossRef]
- Islam, K.N.; Koch, W.J. Involvement of nuclear factor κB (NF-κB) signaling pathway in regulation of cardiac G protein-coupled receptor kinase 5 (GRK5) expression. J. Biol. Chem. 2012, 287, 12771–12778. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Wei, D.; Zheng, N.N.; Chi, Z.H.; Xin, N.; Ma, T.X.; Zheng, L.Y.; Sumi, R.; Sun, L. Coccomyxa Gloeobotrydiformis Polysaccharide Inhibits Lipopolysaccharide-Induced Inflammation in RAW 264.7 Macrophages. Cell Physiol. Biochem. 2018, 51, 2523–2535. [Google Scholar] [CrossRef]
- Buszczak, M.; Paterno, S.; Lighthouse, D.; Bachman, J.; Planck, J.; Owen, S.; Skora, A.D.; Nystul, T.G.; Ohlstein, B.; Allen, A.; et al. The carnegie protein trap library: A versatile tool for Drosophila developmental studies. Genetics 2007, 175, 1505–1531. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.L.; Lukk, T.; Nair, S.K.; Tapping, R.I. The crystal structure of human soluble CD14 reveals a bent solenoid with a hydrophobic amino-terminal pocket. J. Immunol. 2013, 190, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, W.-T.; Hsu, M.-H.; Lin, W.-J.; Xiao, Y.-C.; Lyu, P.-C.; Liu, Y.-C.; Lin, W.-Y.; Kuo, Y.-H.; Chung, J.-G. Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila. Int. J. Mol. Sci. 2021, 22, 6511. https://doi.org/10.3390/ijms22126511
Hsieh W-T, Hsu M-H, Lin W-J, Xiao Y-C, Lyu P-C, Liu Y-C, Lin W-Y, Kuo Y-H, Chung J-G. Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila. International Journal of Molecular Sciences. 2021; 22(12):6511. https://doi.org/10.3390/ijms22126511
Chicago/Turabian StyleHsieh, Wen-Tsong, Min-Hsien Hsu, Wen-Jen Lin, Yi-Cheng Xiao, Ping-Chiang Lyu, Yi-Chung Liu, Wei-Yong Lin, Yueh-Hsiung Kuo, and Jing-Gung Chung. 2021. "Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila" International Journal of Molecular Sciences 22, no. 12: 6511. https://doi.org/10.3390/ijms22126511
APA StyleHsieh, W.-T., Hsu, M.-H., Lin, W.-J., Xiao, Y.-C., Lyu, P.-C., Liu, Y.-C., Lin, W.-Y., Kuo, Y.-H., & Chung, J.-G. (2021). Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila. International Journal of Molecular Sciences, 22(12), 6511. https://doi.org/10.3390/ijms22126511






