Inner Ear and Muscle Developmental Defects in Smpx-Deficient Zebrafish Embryos
Abstract
1. Introduction
2. Results
2.1. Morpholino-Mediated smpx Knockdown
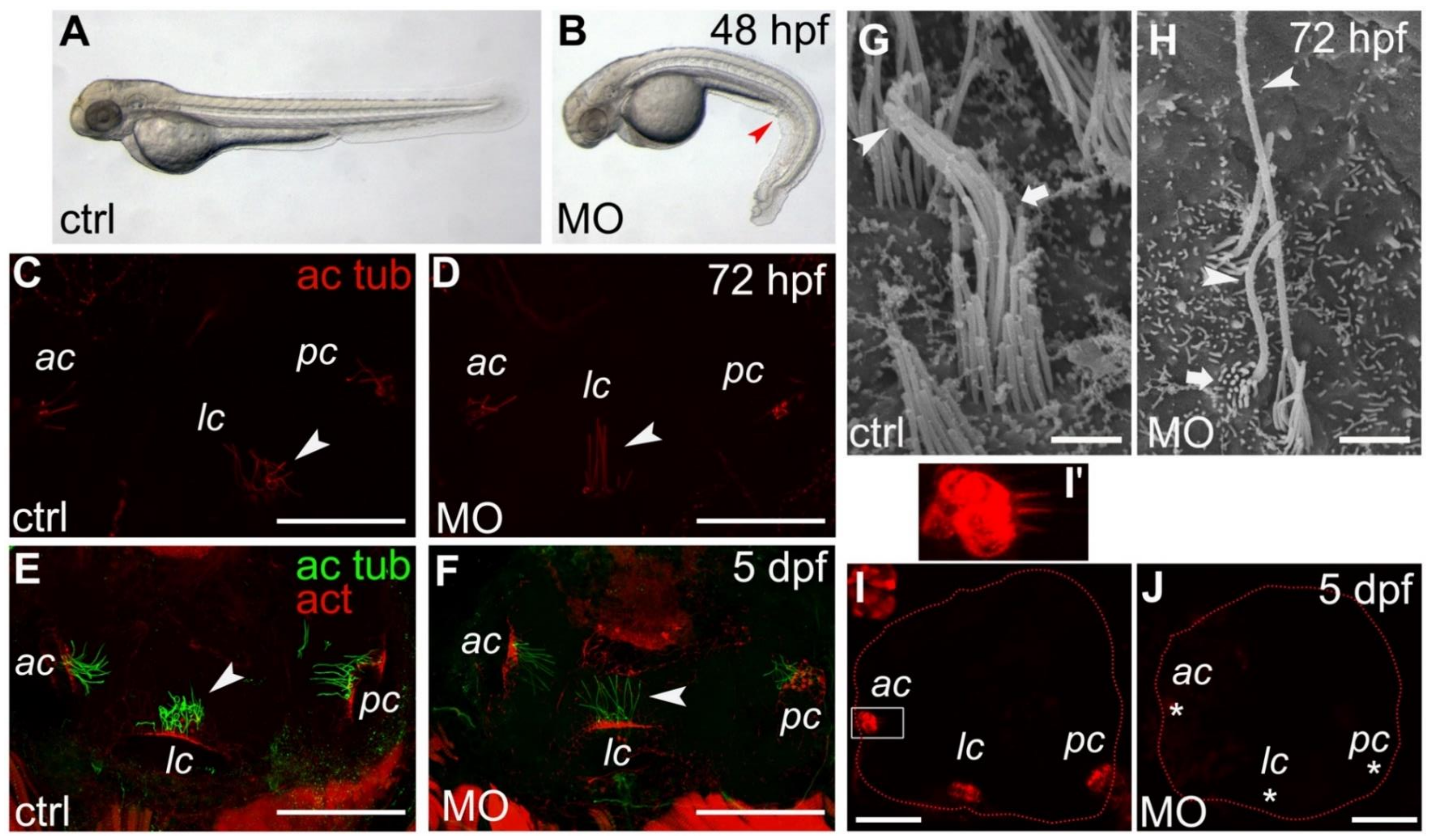
2.2. Morphant Larvae Are Characterized by a Curly Body Axis
2.3. Apical Hair Bundle Alteration in Smpx-Deficient Larvae
2.4. Lack of Smpx Affects Mechanotransduction in Zebrafish Larvae Inner Ear Hair Cells
2.5. Smpx Is Critical for the Proper Organization of the Larvae Muscle Fibers
2.6. Smpx Overexpression and Protein Subcellular Localization
3. Discussion
4. Material and Methods
4.1. Zebrafish Husbandry and Maintenance
4.2. Injections
4.3. RNA Extraction and Quantitative RT-PCR (qRT-PCR)
4.4. Whole-Mount In-Situ Hybridization
4.5. Immunofluorescence and Phalloidin Staining
4.6. FM 4-64 Dye Microinjection: In Vivo Mechanotransduction Assay
4.7. Scanning Electron Microscopy (SEM)
4.8. Touch-Evoked Response Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, Y.; Gu, J.; Qiu, H.; Li, H.; Zhang, Z.; Yin, S.; Mao, Y.; Kong, L.; Liang, B.; Jiang, H.; et al. Whole-exome sequencing identifies a donor splice-site variant in SMPX that causes rare X-linked congenital deafness. Mol. Genet. Genom. Med. 2019, 7, e967. [Google Scholar] [CrossRef] [PubMed]
- Schraders, M.; Haas, S.A.; Weegerink, N.J.; Oostrik, J.; Hu, H.; Hoefsloot, L.H.; Kannan, S.; Huygen, P.L.; Pennings, R.J.; Admiraal, R.J.; et al. Next-generation sequencing identifies mutations of SMPX, which encodes the small muscle protein, X-linked, as a cause of progressive hearing impairment. Am. J. Hum. Genet. 2011, 88, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, D.J.; Kim, M.H.; Bok, J. Identification of genes concordantly expressed with Atoh1 during inner ear development. Anat. Cell. Biol. 2011, 44, 69–78. [Google Scholar] [CrossRef]
- Ghilardi, A.; Diana, A.; Prosperi, L.; Del Giacco, L. Expression pattern of the small muscle protein, X-linked (smpx) gene during zebrafish embryonic and larval developmental stages. Gene Expr. Patterns. 2020, 36, 119110. [Google Scholar] [CrossRef] [PubMed]
- Du, T.T.; Dewey, J.B.; Wagner, E.L.; Cui, R.; Heo, J.; Park, J.J.; Francis, S.P.; Perez-Reyes, E.; Guillot, S.J.; Sherman, N.E.; et al. LMO7 deficiency reveals the significance of the cuticular plate for hearing function. Nat. Commun. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Furness, D.N.; Hackney, C.M.; Evans, M.G. Localisation of the mechanotransducer channels in mammalian cochlear hair cells provides clues to their gating. J. Physiol. 2010, 588, 765–772. [Google Scholar] [CrossRef]
- Schindeler, A.; Lavulo, L.; Harvey, R.P. Muscle costameric protein, Chisel/Smpx, associates with focal adhesion complexes and modulates cell spreading in vitro via a Rac1/p38 pathway. Exp. Cell. Res. 2005, 307, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Riveline, D.; Zamir, E.; Balaban, N.Q.; Schwarz, U.S.; Ishizaki, T.; Narumiya, S.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell. Biol. 2001, 153, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Groves, N.; Schindeler, A.; Yeoh, T.; Biben, C.; Wang, C.C.; Sparrow, D.B.; Barnett, L.; Jenkins, N.A.; Copeland, N.G.; et al. The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an Insulin-like Growth Factor 1–dependent manner. J. Cell Biol. 2001, 5, 985–997. [Google Scholar] [CrossRef]
- Grimsley-Myers, C.M.; Sipe, C.W.; Géléoc, G.S.G.; Lu, X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J. Neurosci. 2009, 29, 15859–15869. [Google Scholar] [CrossRef]
- Sanchez-Calderon, H.; Rodriguez-de la Rosa, L.; Milo, M.; Pichel, J.G.; Holley, M.; Varela-Nieto, I. RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: Implication of MEF2 and FOXM1 transcription factors. PLoS ONE 2010, 5, e8699. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Cediel, R.; Lassaletta, L.; Varela-Nieto, I. The role of insulin-like growth factor-I in the physiopathology of hearing. Front. Mol. Neurosci. 2011, 4, 11. [Google Scholar] [CrossRef]
- Varela-Nieto, I.; Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Lassatetta, L.; Contreras, J. IGF-I deficiency and hearing loss: Molecular clues and clinical implications. Pediatr. Endocrinol. Rev. 2013, 10, 460–472. [Google Scholar] [PubMed]
- Rodríguez-de la Rosa, L.; Sánchez-Calderón, H.; Contreras, J.; Murillo-Cuesta, S.; Falagan, S.; Avendaño, C.; Dopazo, J.; Varela-Nieto, I.; Milo, M. Comparative gene expression study of the vestibular organ of the Igf1 deficient mouse using whole-transcript arrays. Hear. Res. 2015, 330, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, A.; Laos, M.; Anttonen, T.; Pirvola, U. The Rho GTPase Cdc42 regulates hair cell planar polarity and cellular patterning in the developing cochlea. Biol. Open. 2015, 4, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Elkon, R.; Milon, B.; Morrison, L.; Shah, M.; Vijayakumar, S.; Racherla, M.; Leitch, C.C.; Silipino, L.; Hadi, S.; Weiss-Gayet, M.; et al. RFX transcription factors are essential for hearing in mice. Nat. Commun. 2015, 6, 8549. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.N.; Zhang, J.J.; Shi, D.L. Loss of Rbm24a causes defective hair cell development in the zebrafish inner ear and neuromasts. J. Genet. Genom. 2020, 47, 403–406. [Google Scholar] [CrossRef]
- Huebner, A.K.; Gandia, M.; Frommolt, P.; Maak, A.; Wicklein, E.M.; Thiele, H.; Altmüller, J.; Wagner, F.; Viñuela, A.; Aguirre, L.A.; et al. Nonsense mutations in SMPX, encoding a protein responsive to physical force, result in X-chromosomal hearing loss. Am. J. Hum. Genet. 2011, 88, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Weegerink, N.J.; Huygen, P.L.; Schraders, M.; Kremer, H.; Pennings, R.J.; Kunst, H.P. Variable degrees of hearing impairment in a Dutch DFNX4 (DFN6) family. Hear. Res. 2011, 282, 167–177. [Google Scholar] [CrossRef]
- Abdelfatah, N.; Merner, N.; Houston, J.; Benteau, T.; Griffin, A.; Doucette, L.; Stockley, T.; Lauzon, J.L.; Young, T.L. A novel deletion in SMPX causes a rare form of X-linked progressive hearing loss in two families due to a founder effect. Hum. Mutat. 2013, 34, 66–69. [Google Scholar] [CrossRef]
- Stanton, S.G.; Griffin, A.; Stockley, T.L.; Brown, C.; Young, T.L.; Benteau, T.; Abdelfatah, N. X-linked hearing loss: Two gene mutation examples provide generalizable implications for clinical care. Am. J. Audiol. 2014, 23, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Yan, D.; Bressler, S.; Mei, L.; Feng, Y.; Liu, X. A novel splicing mutation in SMPX is linked to nonsyndromic progressive hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Feng, Y.; Mei, L.; Sun, J.; Wang, X.; Wang, J.; Hu, Z.; Dong, Y.; Chen, H.; He, C.; et al. A novel frameshift mutation of SMPX causes a rare form of X-linked nonsyndromic hearing loss in a Chinese family. PLoS ONE 2017, 12, e0178384. [Google Scholar] [CrossRef]
- Deng, Y.; Niu, Z.; Fan, L.; Ling, J.; Chen, H.; Cai, X.; Mei, L.; He, C.; Zhang, X.; Wen, J.; et al. A novel mutation in the SMPX gene associated with X-linked nonsyndromic sensorineural hearing loss in a Chinese family. J. Hum. Genet. 2018, 63, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hao, Y.; Zhang, D.; Xu, H.; Yu, D.; Lv, J.; Fu, Z.; Han, S.; Guo, F.; Bai, J.; et al. A novel missense mutation in SMPX causes a rare form of X-linked postlingual sensorineural hearing loss in a Chinese family. Transl. Pediatr. 2021, 10, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Eftestøl, E.; Alver, T.N.; Gundersen, K.; Bruusgaard, J.C. Overexpression of SMPX in adult skeletal muscle does not change skeletal muscle fiber type or size. PLoS ONE 2014, 9, e99232. [Google Scholar]
- Schwander, M.; Kachar, B.; Müller, U. The cell biology of hearing. J. Cell. Biol. 2010, 190, 9–20. [Google Scholar] [CrossRef]
- Hu, Q.; Guo, L.; Li, J.; Song, C.; Yu, L.; He, D.Z.Z.; Xiong, W. Deletion of Kncn Does Not Affect Kinocilium and Stereocilia Bundle Morphogenesis and Mechanotransduction in Cochlear Hair Cells. Front. Mol. Neurosci. 2018, 11, 326. [Google Scholar] [CrossRef]
- Sun, Z.; Amsterdam, A.; Pazour, G.J.; Cole, D.G.; Miller, M.S.; Hopkins, N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 2004, 131, 4085–4093. [Google Scholar] [CrossRef]
- Chang, M.Y.; Ma, T.L.; Hung, C.C.; Tian, Y.C.; Chen, Y.C.; Yang, C.W.; Cheng, Y.C. Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency. Sci. Rep. 2017, 7, 7161. [Google Scholar] [CrossRef]
- Kindt, K.S.; Finch, G.; Nicolson, T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev. Cell. 2012, 23, 329–341. [Google Scholar] [CrossRef]
- Cai, T.; Jen, H.I.; Kang, H.; Klisch, T.J.; Zoghbi, H.Y.; Groves, A.K. Characterization of the Transcriptome of Nascent Hair Cells and Identification of Direct Targets of the Atoh1 Transcription Factor. J. Neurosci. 2015, 35, 5870–5883. [Google Scholar] [CrossRef] [PubMed]
- Millimaki, B.B.; Sweet, E.M.; Dhason, M.S.; Riley, B.B. Zebrafish atoh1 genes: Classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 2007, 134, 295–305. [Google Scholar] [CrossRef]
- Erickson, T.; Morgan, C.P.; Olt, J.; Hardy, K.; Busch-Nentwich, E.; Maeda, R.; Clemens, R.; Krey, J.F.; Nechiporuk, A.; Barr-Gillespie, P.G.; et al. Integration of Tmc1/2 into the mechanotransduction complex in zebrafish hair cells is regulated by Transmembrane O-methyltransferase (Tomt). eLife 2017, 6, e28474. [Google Scholar] [CrossRef] [PubMed]
- Johari, M.; Savarese, M.; Vihola, A.; Jokela, M.; Torella, A.; Piluso, G.; Jonson, P.; Luque, H.; Magot, A.; Magri, F.; et al. New genes in neuromuscular diseases: O.01 Mutations in the SMPX gene cause the first X-linked recessive form of distal myopathy. Neuromuscul. Disord. 2020, 30, S46. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.J.; Sadusky, T.J.; Simon, M.; Brown, R.; Eastwood, M.; Sassoon, D.A.; Coulton, G.R. Identification of a novel stretch-responsive skeletal muscle gene [Smpx]. Genomics 2001, 72, 260–271. [Google Scholar] [CrossRef]
- Rossi, G.; Messina, G. Comparative myogenesis in teleosts and mammals. Cell. Mol. Life Sci. 2014, 71, 3081–3099. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Thisse, B.; Thisse, C.; Weston, J.A. Novel FGF receptor (Z-FGFR4) is dynamically expressed in mesoderm and neurectoderm during early zebrafish embryogenesis. Dev. Dyn. 1995, 203, 377–391. [Google Scholar] [CrossRef]
- Moore, J.L.; Aros, M.; Steudel, K.G.; Cheng, K.C. Fixation and decalcification of adult zebrafish for histological, immunocytochemical, and genotypic analysis. Biotechniques 2002, 32, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Mastrodonato, V.; Beznoussenko, G.; Mironov, A.; Ferrari, L.; Deflorian, G.; Vaccari, T. A genetic model of CEDNIK syndrome in zebrafish highlights the role of the SNARE protein Snap29 in neuromotor and epidermal development. Sci. Rep. 2019, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Patzak, D.; Zhuchenko, O.; Lee, C.C.; Wehnert, M. Identification, mapping, and genomic structure of a novel X-chromosomal human gene (SMPX) encoding a small muscular protein. Hum. Genet. 1999, 105, 506–512. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghilardi, A.; Diana, A.; Bacchetta, R.; Santo, N.; Ascagni, M.; Prosperi, L.; Del Giacco, L. Inner Ear and Muscle Developmental Defects in Smpx-Deficient Zebrafish Embryos. Int. J. Mol. Sci. 2021, 22, 6497. https://doi.org/10.3390/ijms22126497
Ghilardi A, Diana A, Bacchetta R, Santo N, Ascagni M, Prosperi L, Del Giacco L. Inner Ear and Muscle Developmental Defects in Smpx-Deficient Zebrafish Embryos. International Journal of Molecular Sciences. 2021; 22(12):6497. https://doi.org/10.3390/ijms22126497
Chicago/Turabian StyleGhilardi, Anna, Alberto Diana, Renato Bacchetta, Nadia Santo, Miriam Ascagni, Laura Prosperi, and Luca Del Giacco. 2021. "Inner Ear and Muscle Developmental Defects in Smpx-Deficient Zebrafish Embryos" International Journal of Molecular Sciences 22, no. 12: 6497. https://doi.org/10.3390/ijms22126497
APA StyleGhilardi, A., Diana, A., Bacchetta, R., Santo, N., Ascagni, M., Prosperi, L., & Del Giacco, L. (2021). Inner Ear and Muscle Developmental Defects in Smpx-Deficient Zebrafish Embryos. International Journal of Molecular Sciences, 22(12), 6497. https://doi.org/10.3390/ijms22126497





