Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD
Abstract
1. Introduction
2. Urea Toxicity
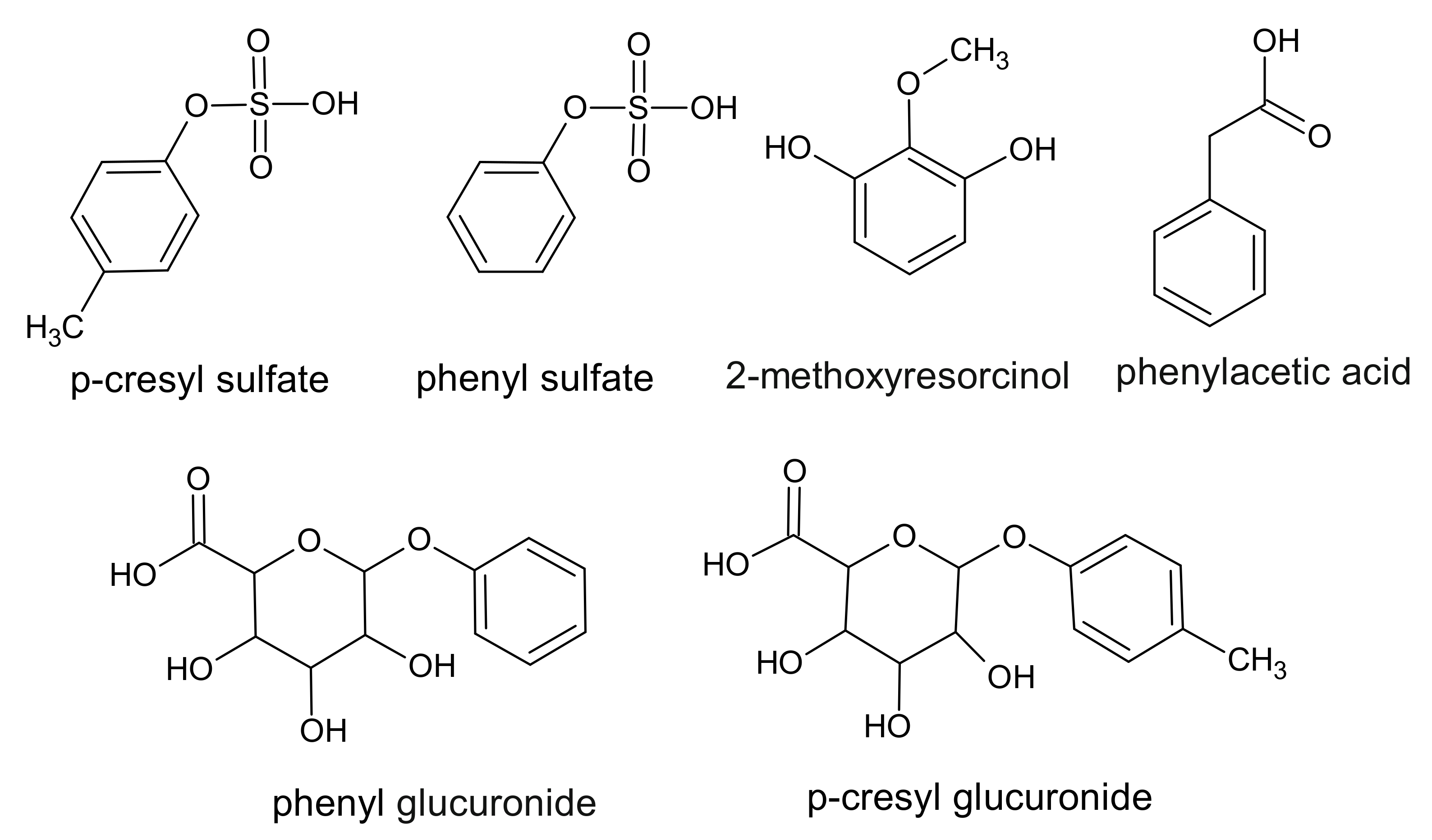
3. Phenol Derivatives
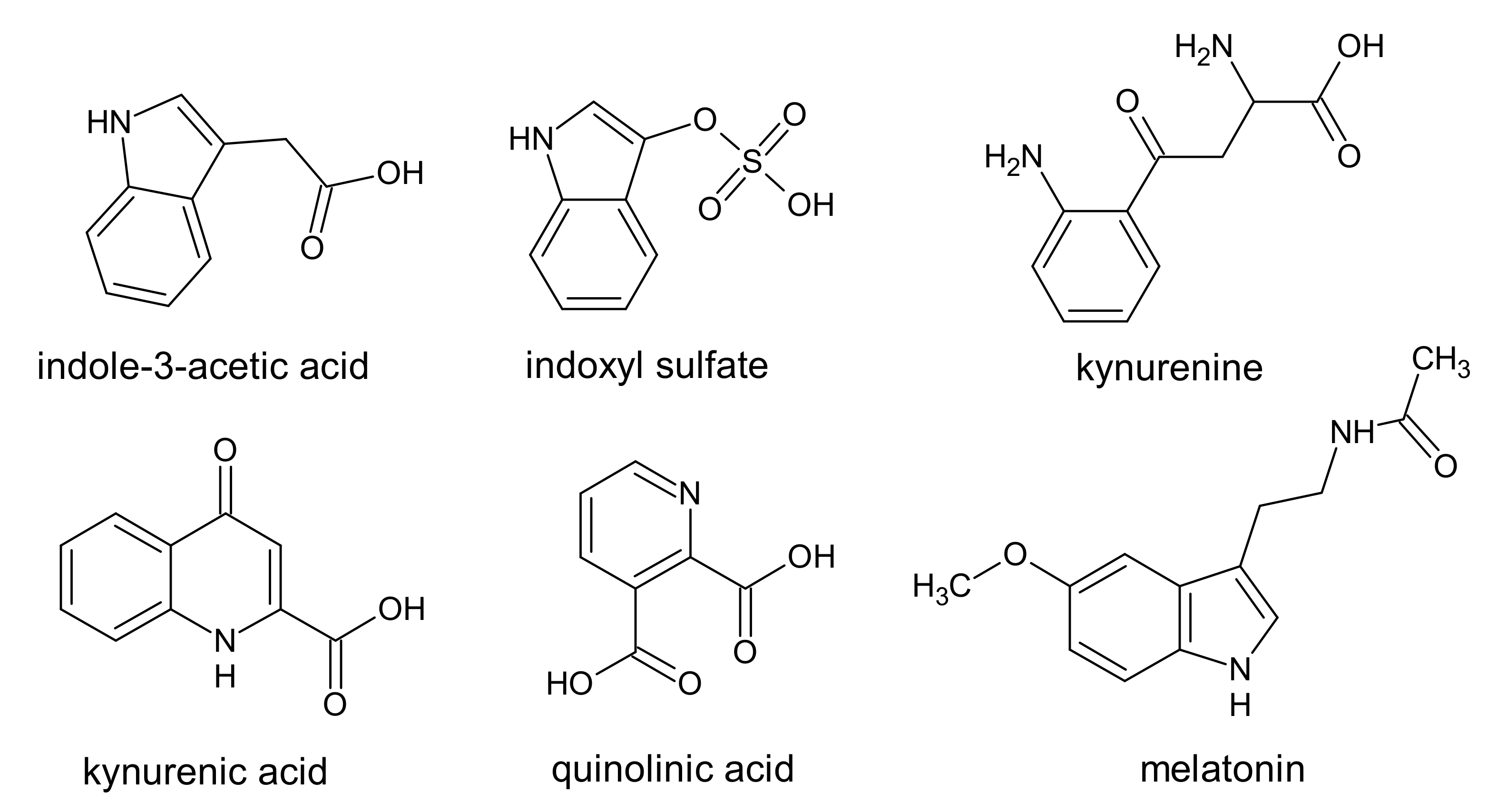
4. Indole Derivatives
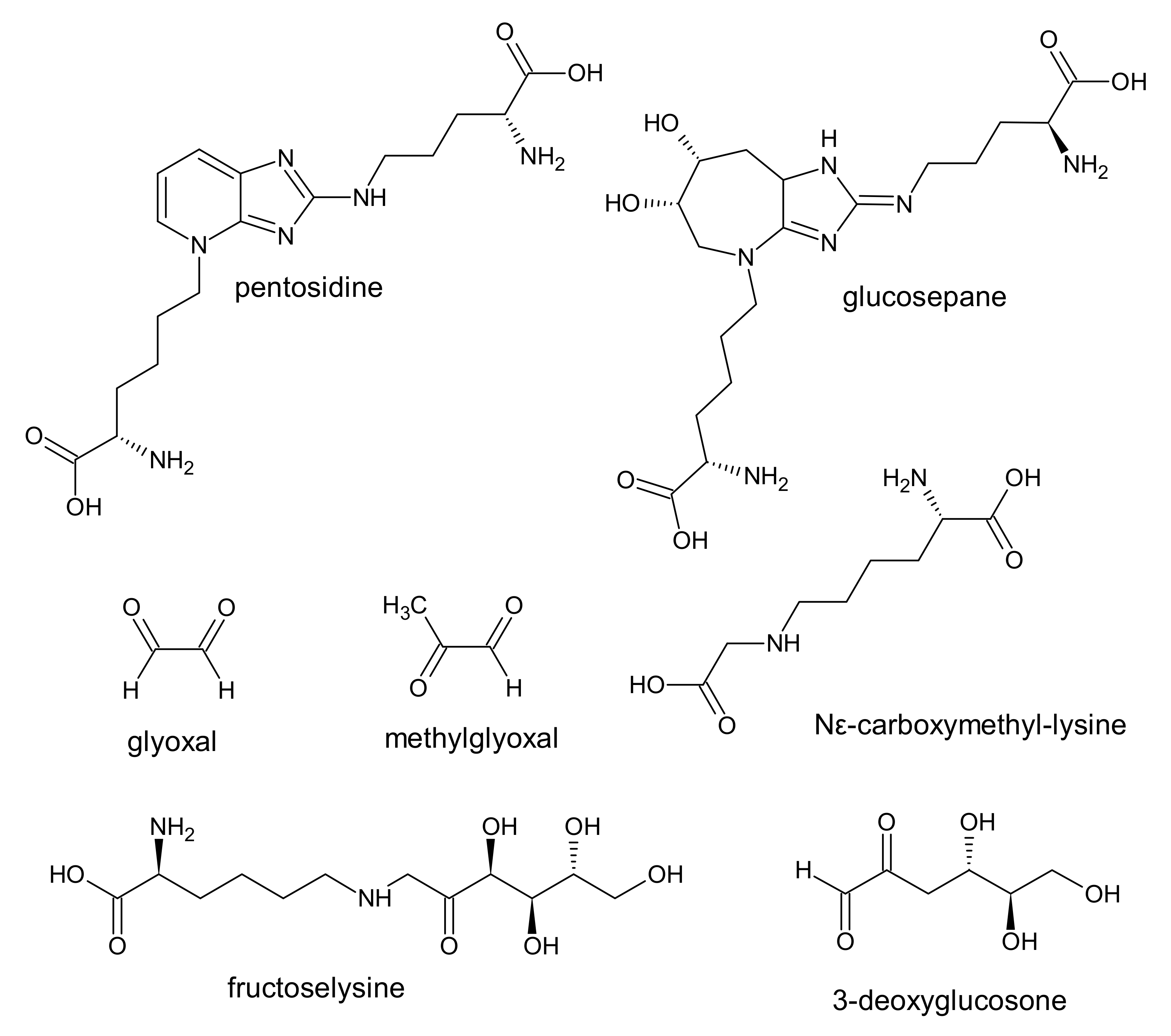
5. Advanced Glycation End Products (AGE)
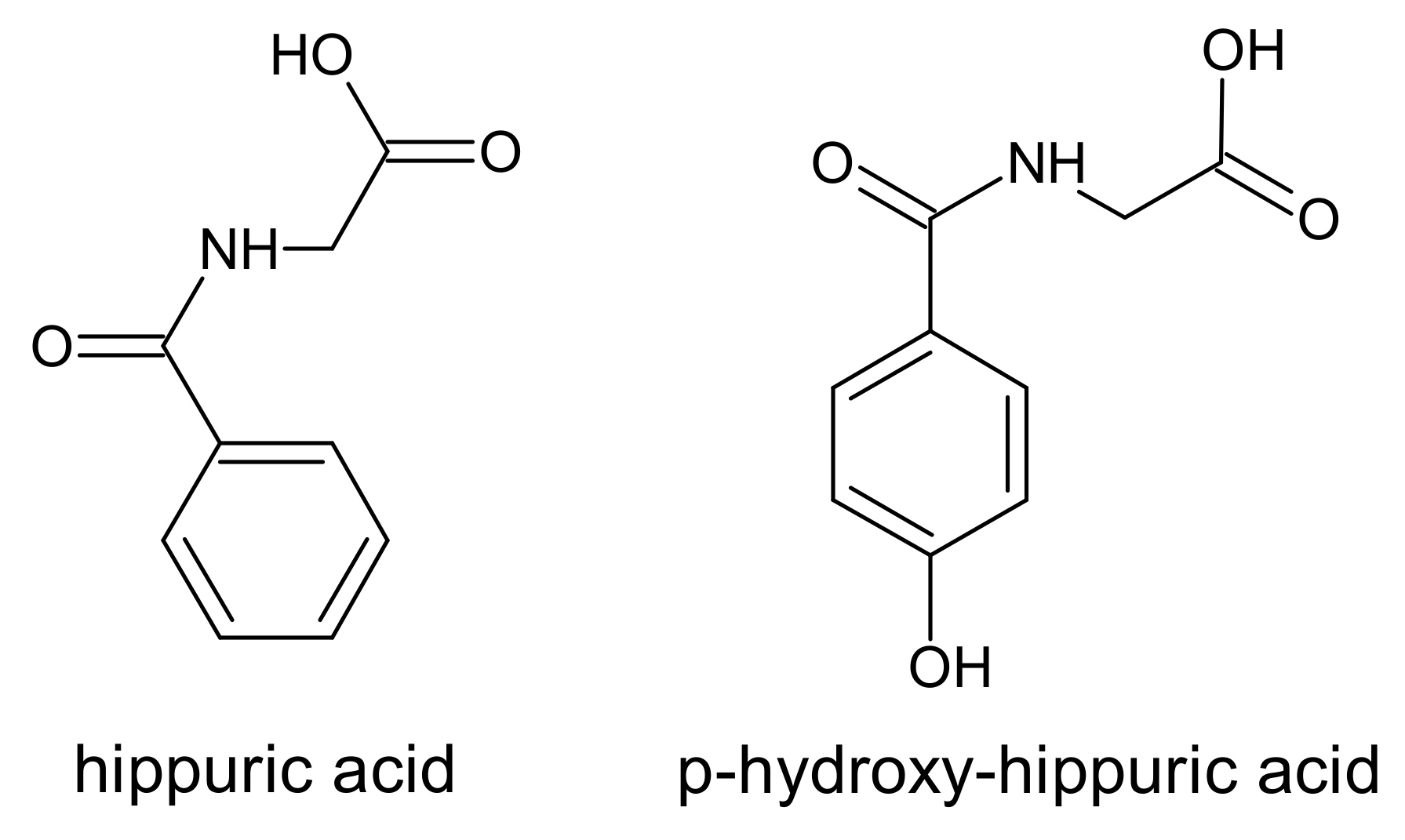
6. Hippurates
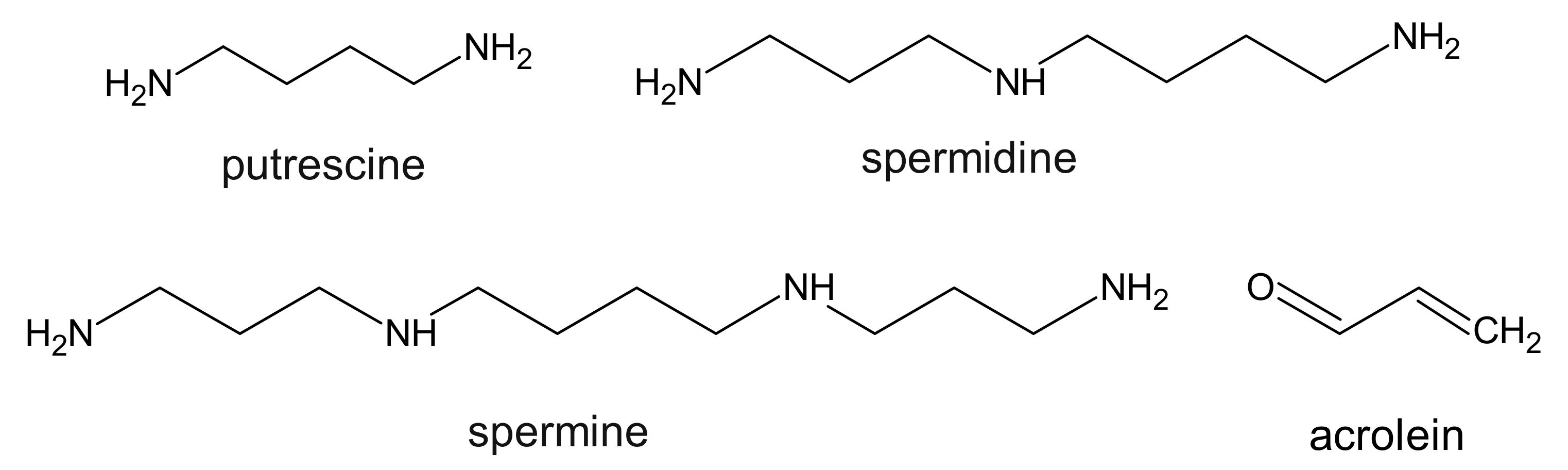
7. Polyamines
8. Other Compounds
9. Implications of Uremic Toxins and Oxidative Stress in Cardiovascular Disease
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| AGE | advanced glycation end products |
| AHR | aryl hydrocarbon receptor |
| Cat | catalase |
| CKD | chronic kidney disease |
| CMPF | 3-carboxy-4-methyl-5-propyl-2-furanopropionic acid |
| ERK ½ | extracellular signal-regulated kinases |
| ESRD | end-stage renal disease |
| GFR | glomerular filtration rate |
| GPx | glutathione peroxidase |
| HDL | high density lipoproteins |
| H-NMR | proton nuclear magnetic resonance |
| IL-18 | Interleukin-18 |
| iNOS | nitric oxide synthase |
| IS | indoxyl sulfate |
| LDL | low density lipoproteins |
| LVH | left ventricular hypertrophy |
| MDA | malondialdehyde |
| O-GlcNAc | O-linked N-acetylglucosamine |
| PAA | phenylacetic acid |
| PC-Acro | protein conjugated acrolein |
| PCS | p-cresyl sulfate |
| RBC | red blood cells |
| RBP4 | retinol-binding protein 4 |
| ROS | reactive oxygen species |
| SOD1 | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
| TGF-β1 | transforming growth factor-β1 |
| TMAO | trimethylamine-N-oxide |
| TNF-α | tumor necrosis factor-α |
References
- Vanholder, R.; de Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; de Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Meert, N.; Schepers, E.; Glorieux, G.; Argiles, A.; Brunet, P.; Cohen, G.; Drüeke, T.; Mischak, H.; Spasovski, G.; et al. Review on uraemic solutes II—variability in reported concentrations: Causes and consequences. Nephrol. Dial. Transpl. 2007, 22, 3115–3121. [Google Scholar] [CrossRef]
- Vanholder, R.; van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; de Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Van Gelder, M.K.; Middel, I.R.; Vernooij, R.W.M.; Bots, M.L.; Verhaar, M.C.; Masereeuw, R.; Grooteman, M.P.; Nubé, M.J.; van den Dorpel, M.A.; Blankestijn, P.J.; et al. Protein-Bound Uremic Toxins in Hemodialysis Patients Relate to Residual Kidney Function, Are Not Influenced by Convective Transport, and Do Not Relate to Outcome. Toxins 2020, 12, 234. [Google Scholar] [CrossRef]
- Tang, W.H.W. Trimethylamine N-Oxide as a Novel Therapeutic Target in CKD. J. Am. Soc. Nephrol. 2016, 27, 8–10. [Google Scholar] [CrossRef]
- Gryp, T.; de Paepe, K.; Vanholder, R.; Kerckhof, F.-M.; van Biesen, W.; van de Wiele, T.; Verbeke, F.; Speeckaert, M.; Joossens, M.; Couttenye, M.M.; et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020, 97, 1230–1242. [Google Scholar] [CrossRef]
- Castillo-Rodríguez, E.; Pizarro-Sánchez, S.; Sanz, A.B.; Ramos, A.M.; Sanchez-Niño, M.D.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Brzeszczynska, J.; Kruszynska, I.; Gwozdzinski, K. Investigation of albumin properties in patients with chronic renal failure. Free Radic. Res. 2009, 43, 1008–1018. [Google Scholar] [CrossRef]
- Kairaitis, L.K.; Yuill, E.; Harris, D.C. Determinants of haemoglobin carbamylation in haemodialysis and peritoneal dialysis patients. Nephrol. Dial. Transpl. 2000, 15, 1431–1437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pieniazek, A.; Gwozdzinski, K. Changes in the conformational state of hemoglobin in hemodialysed patients with chronic renal failure. Oxid. Med. Cell. Longev. 2015, 2015, 783073. [Google Scholar] [CrossRef] [PubMed]
- Jaisson, S.; Kazes, I.; Desmons, A.; Fadel, F.; Oudart, J.-B.; Santos-Weiss, I.C.R.D.; Millart, H.; Touré, F.; Rieu, P.; Gillery, P. Homocitrulline as marker of protein carbamylation in hemodialyzed patients. Clin. Chim. Acta 2016, 460, 5–10. [Google Scholar] [CrossRef]
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research). Nephrol. Dial. Transpl. 2018, 33, 4–12. [Google Scholar] [CrossRef]
- Jaisson, S.; Pietrement, C.; Gillery, P. Carbamylation-derived products: Bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin. Chem. 2011, 57, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Wynckel, A.; Randoux, C.; Millart, H.; Desroches, C.; Gillery, P.; Canivet, E.; Chanard, J. Kinetics of carbamylated haemoglobin in acute renal failure. Nephrol. Dial. Transpl. 2000, 15, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Pieniążek, A.; Gwoździński, K. Karbamylacja białek—mechanizm, przyczyny i skutki. Postepy Hig. Med. Dosw. 2016, 70, 514–521. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Marzocco, S.; Bellasi, A.; de Simone, E.; Dal Piaz, F.; Rocchetti, M.T.; Cosola, C.; Di Micco, L.; Gesualdo, L. Nutritional therapy reduces protein carbamylation through urea lowering in chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 804–813. [Google Scholar] [CrossRef]
- Jin, K. Effects of amino acids and albumin on erythropoietin carbamoylation. Clin. Exp. Nephrol. 2013, 17, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Sirpal, S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin. Sci. 2009, 116, 681–695. [Google Scholar] [CrossRef]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef]
- Pieniazek, A.; Gwozdzinski, K. Carbamylation and oxidation of proteins lead to apoptotic death of lymphocytes. Chem. Biol. Interact. 2017, 270, 24–32. [Google Scholar] [CrossRef]
- Jaisson, S.; Lorimier, S.; Ricard-Blum, S.; Sockalingum, G.D.; Delevallée-Forte, C.; Kegelaer, G.; Manfait, M.; Garnotel, R.; Gillery, P. Impact of carbamylation on type I collagen conformational structure and its ability to activate human polymorphonuclear neutrophils. Chem. Biol. 2006, 13, 149–159. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Gwozdzinski, K.; Czepas, J. EPR study of erythrocyte properties after in vitro treatment with urea and hydrogen peroxide. Int. J. Sci. Res. 2014, 3, 491–494. [Google Scholar] [CrossRef]
- Shaykh, M.; Pegoraro, A.A.; Mo, W.; Arruda, J.; Dunea, G.; Singh, A.K. Carbamylated proteins activate glomerular mesangial cells and stimulate collagen deposition. J. Lab. Clin. Med. 1999, 133, 302–308. [Google Scholar] [CrossRef]
- Desmons, A.; Jaisson, S.; Pietrement, C.; Rieu, P.; Wynckel, A.; Gillery, P. Homocitrulline: A new marker for differentiating acute from chronic renal failure. Clin. Chem. Lab. Med. 2016, 54, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Nyam, E.; Vivot, K.; Manning Fox, J.E.; Dai, X.-Q.; Nguyen, B.N.; Trudel, D.; Attané, C.; Moullé, V.S.; MacDonald, P.E.; et al. Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Investig. 2016, 126, 3598–3612. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Vanholder, R. The gut: The forgotten organ in uremia? Blood Purif. 2010, 29, 130–136. [Google Scholar] [CrossRef]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transpl. 2007, 22, 592–596. [Google Scholar] [CrossRef]
- Vanholder, R.; Bammens, B.; de Loor, H.; Glorieux, G.; Meijers, B.; Schepers, E.; Massy, Z.; Evenepoel, P. Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol. Dial. Transpl. 2011, 26, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.I.; de Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1932–1938. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Edamatsu, T.; Fujieda, A.; Itoh, Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS ONE 2018, 13, e0193342. [Google Scholar] [CrossRef]
- Jankowski, J.; van der Giet, M.; Jankowski, V.; Schmidt, S.; Hemeier, M.; Mahn, B.; Giebing, G.; Tolle, M.; Luftmann, H.; Schluter, H.; et al. Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J. Clin. Investig. 2003, 112, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Böhringer, F.; Jankowski, V.; Gajjala, P.R.; Zidek, W.; Jankowski, J. Release of uremic retention solutes from protein binding by hypertonic predilution hemodiafiltration. ASAIO J. 2015, 61, 55–60. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Yi, D.; Stockler-Pinto, M.B.; Soula, H.A.; Chambert, S.; Fouque, D.; Mafra, D.; Soulage, C.O. Determination of the binding properties of the uremic toxin phenylacetic acid to human serum albumin. Biochimie 2016, 125, 53–58. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Vanholder, R.; Brunet, P. Protein-bound toxins--update 2009. Semin. Dial. 2009, 22, 334–339. [Google Scholar] [CrossRef]
- Schmidt, S.; Westhoff, T.H.; Krauser, P.; Ignatius, R.; Jankowski, J.; Jankowski, V.; Zidek, W.; van der Giet, M. The uraemic toxin phenylacetic acid impairs macrophage function. Nephrol. Dial. Transpl. 2008, 23, 3485–3493. [Google Scholar] [CrossRef]
- Schmidt, S.; Westhoff, T.H.; Krauser, P.; Zidek, W.; van der Giet, M. The uraemic toxin phenylacetic acid increases the formation of reactive oxygen species in vascular smooth muscle cells. Nephrol. Dial. Transpl. 2008, 23, 65–71. [Google Scholar] [CrossRef]
- Morita, M.; Yano, S.; Yamaguchi, T.; Yamauchi, M.; Sugimoto, T. Phenylacetic acid stimulates reactive oxygen species generation and tumor necrosis factor-α secretion in vascular endothelial cells. Ther. Apher. Dial. 2011, 15, 147–150. [Google Scholar] [CrossRef]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-P.; Zhao, F.; Wang, Y.; Zhao, L.-L.; Li, Q.-P.; Liu, H.-W. Antitumor effect of resorcinol derivatives from the roots of Ardisia brevicaulis by inducing apoptosis. J. Asian Nat. Prod. Res. 2011, 13, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Addi, T.; Dou, L.; Burtey, S. Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease. Toxins 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fujigaki, S.; Heyes, M.P.; Shibata, K.; Takemura, M.; Fujii, H.; Wada, H.; Noma, A.; Seishima, M. Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am. J. Physiol. Renal Physiol. 2000, 279, F565–F572. [Google Scholar] [CrossRef]
- Kolodziej, L.R.; Paleolog, E.M.; Williams, R.O. Kynurenine metabolism in health and disease. Amino Acids 2011, 41, 1173–1183. [Google Scholar] [CrossRef]
- Karasek, M.; Szuflet, A.; Chrzanowski, W.; Zylinska, K.; Swietoslawski, J. Decreased melatonin nocturnal concentrations in hemodialyzed patients. Neuro Endocrinol. Lett. 2005, 26, 653–656. [Google Scholar]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Pawlak, D.; Pawlak, K.; Malyszko, J.; Mysliwiec, M.; Buczko, W. Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. Int. Urol. Nephrol. 2001, 33, 399–404. [Google Scholar] [CrossRef]
- Pawlak, K.; Domaniewski, T.; Mysliwiec, M.; Pawlak, D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009, 204, 309–314. [Google Scholar] [CrossRef]
- Lano, G.; Laforêt, M.; von Kotze, C.; Perrin, J.; Addi, T.; Brunet, P.; Poitevin, S.; Burtey, S.; Dou, L. Aryl Hydrocarbon Receptor Activation and Tissue Factor Induction by Fluid Shear Stress and Indoxyl Sulfate in Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 2392. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Small, D.M.; Vesey, D.A.; Johnson, D.W.; Francis, R.; Vitetta, L.; Gobe, G.C.; Morais, C. Indoxyl sulphate and kidney disease: Causes, consequences and interventions. Nephrology 2016, 21, 170–177. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Pieniazek, A.; Gwozdzinski, L.; Hikisz, P.; Gwozdzinski, K. Indoxyl Sulfate Generates Free Radicals, Decreases Antioxidant Defense, and Leads to Damage to Mononuclear Blood Cells. Chem. Res. Toxicol. 2018, 31, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.F.; Bonan, N.B.; Steiner, T.M.; Tozoni, S.S.; Rodrigues, S.; Nakao, L.S.; Kuntsevich, V.; Pecoits Filho, R.; Kotanko, P.; Moreno-Amaral, A.N. Indoxyl Sulfate, a Uremic Toxin, Stimulates Reactive Oxygen Species Production and Erythrocyte Cell Death Supposedly by an Organic Anion Transporter 2 (OAT2) and NADPH Oxidase Activity-Dependent Pathways. Toxins 2018, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Ruocco, M.; Rapa, S.F.; Piaz, F.D.; Di Raffaele Iorio, B.; Popolo, A.; Autore, G.; Nishijima, F.; Pinto, A.; Marzocco, S. Effect of Indoxyl Sulfate on the Repair and Intactness of Intestinal Epithelial Cells: Role of Reactive Oxygen Species’ Release. Int. J. Mol. Sci. 2019, 20, 2280. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, H.; Bae, E.H.; Ma, S.K.; Kim, S.W. Paricalcitol attenuates indoxyl sulfate-induced apoptosis through the inhibition of MAPK, Akt, and NF-kB activation in HK-2 cells. Korean J. Intern. Med. 2019, 34, 146–155. [Google Scholar] [CrossRef]
- Pieniazek, A.; Szczepocki, A. Structural component changes of erythrocytes caused by oxidative stress generated by indoxyl sulfate. Toxicol. In Vitro 2021, 70, 105013. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Bissinger, R.; Abed, M.; Artunc, F. Eryptosis—the Neglected Cause of Anemia in End Stage Renal Disease. Kidney Blood Press. Res. 2017, 42, 749–760. [Google Scholar] [CrossRef]
- Ahmed, M.S.E.; Abed, M.; Voelkl, J.; Lang, F. Triggering of suicidal erythrocyte death by uremic toxin indoxyl sulfate. BMC Nephrol. 2013, 14, 244. [Google Scholar] [CrossRef]
- Chu, S.; Mao, X.; Guo, H.; Wang, L.; Li, Z.; Zhang, Y.; Wang, Y.; Wang, H.; Zhang, X.; Peng, W. Indoxyl sulfate potentiates endothelial dysfunction via reciprocal role for reactive oxygen species and RhoA/ROCK signaling in 5/6 nephrectomized rats. Free Radic. Res. 2017, 51, 237–252. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Yisireyili, M.; Shimizu, H.; Saito, S.; Enomoto, A.; Nishijima, F.; Niwa, T. Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci. 2013, 92, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Stockler-Pinto, M.B.; Saldanha, J.F.; Yi, D.; Mafra, D.; Fouque, D.; Soulage, C.O. The uremic toxin indoxyl sulfate exacerbates reactive oxygen species production and inflammation in 3T3-L1 adipose cells. Free Radic. Res. 2016, 50, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Ikeda, Y.; Watanabe, H.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Imanishi, M.; Zamami, Y.; Takechi, K.; Miyamoto, L.; Ishizawa, K.; et al. The uremic toxin indoxyl sulfate interferes with iron metabolism by regulating hepcidin in chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 586–597. [Google Scholar] [CrossRef]
- Bobot, M.; Thomas, L.; Moyon, A.; Fernandez, S.; McKay, N.; Balasse, L.; Garrigue, P.; Brige, P.; Chopinet, S.; Poitevin, S.; et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020, 31, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.N.; Ponces Freire, A.; Cordeiro, C. The glyoxalase pathway: The first hundred years… and beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Agalou, S.; Ahmed, N.; Babaei-Jadidi, R.; Dawnay, A.; Thornalley, P.J. Profound mishandling of protein glycation degradation products in uremia and dialysis. J. Am. Soc. Nephrol. 2005, 16, 1471–1485. [Google Scholar] [CrossRef]
- Linden, E.; Cai, W.; He, J.C.; Xue, C.; Li, Z.; Winston, J.; Vlassara, H.; Uribarri, J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin. J. Am. Soc. Nephrol. 2008, 3, 691–698. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Matsui, T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Drummond, K.S.; Nelson, R.G.; Howell, S.K.; Szwergold, B.S.; Mauer, M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 2005, 54, 3274–3281. [Google Scholar] [CrossRef]
- Saulnier, P.-J.; Wheelock, K.M.; Howell, S.; Weil, E.J.; Tanamas, S.K.; Knowler, W.C.; Lemley, K.V.; Mauer, M.; Yee, B.; Nelson, R.G.; et al. Advanced Glycation End Products Predict Loss of Renal Function and Correlate with Lesions of Diabetic Kidney Disease in American Indians With Type 2 Diabetes. Diabetes 2016, 65, 3744–3753. [Google Scholar] [CrossRef]
- Tezuka, Y.; Nakaya, I.; Nakayama, K.; Nakayama, M.; Yahata, M.; Soma, J. Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology 2019, 24, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Lees, H.J.; Swann, J.R.; Wilson, I.D.; Nicholson, J.K.; Holmes, E. Hippurate: The natural history of a mammalian-microbial cometabolite. J. Proteome Res. 2013, 12, 1527–1546. [Google Scholar] [CrossRef]
- Phipps, A.N.; Stewart, J.; Wright, B.; Wilson, I.D. Effect of diet on the urinary excretion of hippuric acid and other dietary-derived aromatics in rat. A complex interaction between diet, gut microflora and substrate specificity. Xenobiotica 1998, 28, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Tepel, M.; Stephan, N.; van der Giet, M.; Breden, V.; Zidek, W.; Schlüter, H. Characterization of p-hydroxy-hippuric acid as an inhibitor of Ca2+-ATPase in end-stage renal failure. Kidney Int. Suppl. 2001, 78, S84–S88. [Google Scholar] [CrossRef]
- Cohen, G.; Raupachova, J.; Wimmer, T.; Deicher, R.; Hörl, W.H. The uraemic retention solute para-hydroxy-hippuric acid attenuates apoptosis of polymorphonuclear leukocytes from healthy subjects but not from haemodialysis patients. Nephrol. Dial. Transpl. 2008, 23, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Schools, A.C.; de Vries, P.; Thiemann, R.; Hazejager, W.A.; Visser, S.L.; Oe, P.L. Biochemical and neurophysiological parameters in hemodialyzed patients with chronic renal failure. Clin. Chim. Acta 1989, 185, 91–107. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Liu, X.; Wang, L.; Ren, F.; Wang, X.; Leng, X. Hippuric Acid Promotes Renal Fibrosis by Disrupting Redox Homeostasis via Facilitation of NRF2-KEAP1-CUL3 Interactions in Chronic Kidney Disease. Antioxidants 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br. J. Pharmacol. 2002, 135, 555–563. [Google Scholar] [CrossRef]
- Saito, A.; Takagi, T.; Chung, T.G.; Ohta, K. Serum levels of polyamines in patients with chronic renal failure. Kidney Int. Suppl. 1983, 16, S234–S237. [Google Scholar]
- Sindhu, K.K. Uremic toxins: Some thoughts on acrolein and spermine. Ren. Fail. 2016, 38, 1755–1758. [Google Scholar] [CrossRef]
- Erez, O.; Goldstaub, D.; Friedman, J.; Kahana, C. Putrescine activates oxidative stress dependent apoptotic death in ornithine decarboxylase overproducing mouse myeloma cells. Exp. Cell Res. 2002, 281, 148–156. [Google Scholar] [CrossRef]
- Igarashi, K.; Ueda, S.; Yoshida, K.; Kashiwagi, K. Polyamines in renal failure. Amino Acids 2006, 31, 477–483. [Google Scholar] [CrossRef]
- Gugliucci, A.; Lunceford, N.; Kinugasa, E.; Ogata, H.; Schulze, J.; Kimura, S. Acrolein inactivates paraoxonase 1: Changes in free acrolein levels after hemodialysis correlate with increases in paraoxonase 1 activity in chronic renal failure patients. Clin. Chim. Acta 2007, 384, 105–112. [Google Scholar] [CrossRef]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Igarashi, K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem. Soc. Trans. 2003, 31, 371–374. [Google Scholar] [CrossRef]
- Igarashi, K.; Uemura, T.; Kashiwagi, K. Assessing acrolein for determination of the severity of brain stroke, dementia, renal failure, and Sjögren’s syndrome. Amino Acids 2020, 52, 119–127. [Google Scholar] [CrossRef]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149. [Google Scholar] [CrossRef]
- Yoshida, K.; Yoneda, T.; Kimura, S.; Fujimoto, K.; Okajima, E.; Hirao, Y. Polyamines as an inhibitor on erythropoiesis of hemodialysis patients by in vitro bioassay using the fetal mouse liver assay. Ther. Apher. Dial. 2006, 10, 267–272. [Google Scholar] [CrossRef]
- Yoshida, M.; Tomitori, H.; Machi, Y.; Hagihara, M.; Higashi, K.; Goda, H.; Ohya, T.; Niitsu, M.; Kashiwagi, K.; Igarashi, K. Acrolein toxicity: Comparison with reactive oxygen species. Biochem. Biophys. Res. Commun. 2009, 378, 313–318. [Google Scholar] [CrossRef]
- Sthijns, M.M.J.P.E.; Randall, M.J.; Bast, A.; Haenen, G.R.M.M. Adaptation to acrolein through upregulating the protection by glutathione in human bronchial epithelial cells: The materialization of the hormesis concept. Biochem. Biophys. Res. Commun. 2014, 446, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Bhatnagar, A.; Pierce, W.M. Protein modification by acrolein: Formation and stability of cysteine adducts. Chem. Res. Toxicol. 2009, 22, 708–716. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Hu, Y.; Tang, M. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. USA 2006, 103, 15404–15409. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013, 35, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Liu, Y.; Prentice, K.J.; Sloop, K.W.; Sanders, P.E.; Batchuluun, B.; Hammond, C.D.; Wheeler, M.B.; Durham, T.B. Synthesis and Characterization of Urofuranoic Acids: In Vivo Metabolism of 2-(2-Carboxyethyl)-4-methyl-5-propylfuran-3-carboxylic Acid (CMPF) and Effects on in Vitro Insulin Secretion. J. Med. Chem. 2017, 60, 1860–1875. [Google Scholar] [CrossRef]
- Luce, M.; Bouchara, A.; Pastural, M.; Granjon, S.; Szelag, J.C.; Laville, M.; Arkouche, W.; Fouque, D.; Soulage, C.O.; Koppe, L. Is 3-Carboxy-4-methyl-5-propyl-2-furanpropionate (CMPF) a Clinically Relevant Uremic Toxin in Haemodialysis Patients? Toxins 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Takamatsu, H.; Ideuchi, H.; Oda, M.; Takeda, K.; Saitoh, H. Correlations between Plasma Levels of Anionic Uremic Toxins and Clinical Parameters in Hemodialysis Patients. Yakugaku Zasshi 2016, 136, 1177–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Long, Y.; Nie, J. Homocysteine in Renal Injury. Kidney Dis. 2016, 2, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Matté, C.; Stefanello, F.M.; Mackedanz, V.; Pederzolli, C.D.; Lamers, M.L.; Dutra-Filho, C.S.; Dos Santos, M.F.; Wyse, A.T.S. Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int. J. Dev. Neurosci. 2009, 27, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, K.; Szewczyk, E.M. Microbial Modulation of Coagulation Disorders in Venous Thromboembolism. J. Inflamm. Res. 2020, 13, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-Y.; Xia, G.-H.; Lu, J.-Q.; Chen, M.-X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.-W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transpl. 2006, 21, 1300–1304. [Google Scholar] [CrossRef]
- De Smet, R.; Dhondt, A.; Eloot, S.; Galli, F.; Waterloos, M.A.; Vanholder, R. Effect of the super-flux cellulose triacetate dialyser membrane on the removal of non-protein-bound and protein-bound uraemic solutes. Nephrol. Dial. Transpl. 2007, 22, 2006–2012. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Xu, G.; Luo, K.; Liu, H.; Huang, T.; Fang, X.; Tu, W. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren. Fail. 2015, 37, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tbahriti, H.F.; Meknassi, D.; Moussaoui, R.; Messaoudi, A.; Zemour, L.; Kaddous, A.; Bouchenak, M.; Mekki, K. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J. Nephrol. 2013, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, I.; Inai, K.; Tsutani, Y.; Ueda, T.; Tsutani, H. Binding of monosodium urate crystals with idiotype protein efficiently promote dendritic cells to induce cytotoxic T cells. Cancer Sci. 2008, 99, 2268–2273. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Crawford, A.; Fassett, R.G.; Coombes, J.S.; Kunde, D.A.; Ahuja, K.D.K.; Robertson, I.K.; Ball, M.J.; Geraghty, D.P. Relationship between antioxidant enzyme genotype and activity and kidney function: A case-control study. Clin. Nephrol. 2012, 78, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren Replace Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jingers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Johnson-Davis, K.L.; Fernelius, C.; Eliason, N.B.; Wilson, A.; Beddhu, S.; Roberts, W.L. Blood enzymes and oxidative stress in chronic kidney disease: A cross sectional study. Ann. Clin. Lab. Sci. 2011, 41, 331–339. [Google Scholar]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef]
- Small, D.M.; Gobe, G.C. Oxidative Stress and Antioxidant Therapy in Chronic Kidney and Cardiovascular Disease. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-González, J.A., Ed.; IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1123-8. [Google Scholar]
- Nishino, T.; Okamoto, K.; Kawaguchi, Y.; Hori, H.; Matsumura, T.; Eger, B.T.; Pai, E.F.; Nishino, T. Mechanism of the conversion of xanthine dehydrogenase to xanthine oxidase: Identification of the two cysteine disulfide bonds and crystal structure of a non-convertible rat liver xanthine dehydrogenase mutant. J. Biol. Chem. 2005, 280, 24888–24894. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar] [CrossRef]
- Cohen, G.; Hörl, W.H. Immune dysfunction in uremia—An update. Toxins 2012, 4, 962–990. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Abe, T. (Eds.) Uremic Toxins and Organ Failure, 1st ed.; Springer: Singapore, 2020; ISBN 9789811577932. [Google Scholar]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxid. Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef]
- Pun, P.H.; Smarz, T.R.; Honeycutt, E.F.; Shaw, L.K.; Al-Khatib, S.M.; Middleton, J.P. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009, 76, 652–658. [Google Scholar] [CrossRef]
- Lekawanvijit, S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Martin, C.J.; Murray, D.C.; Barre, P.E. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995, 47, 186–192. [Google Scholar] [CrossRef]
- Roberts, P.R.; Green, D. Arrhythmias in chronic kidney disease. Heart 2011, 97, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Car, S.; Trkulja, V. Higher serum uric acid on admission is associated with higher short-term mortality and poorer long-term survival after myocardial infarction: Retrospective prognostic study. Croat. Med. J. 2009, 50, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chuang, S.-Y.; Chen, H.-J.; Yeh, W.-T.; Pan, W.-H. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: A Chinese cohort study. Arthritis Rheum. 2009, 61, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Korantzopoulos, P.; Filippatos, G.S.; Mihas, C.C.; Markou, V.; Gavrielatos, G.; Efremidis, M.; Sideris, A.; Kardaras, F. Uric acid elevation in atrial fibrillation. Hell. J. Cardiol. 2010, 51, 209–213. [Google Scholar]
- Alani, H.; Tamimi, A.; Tamimi, N. Cardiovascular co-morbidity in chronic kidney disease: Current knowledge and future research needs. World J. Nephrol. 2014, 3, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transpl. 2005, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Abou Dagher, G.; Harmouche, E.; Jabbour, E.; Bachir, R.; Zebian, D.; Bou Chebl, R. Sepsis in hemodialysis patients. BMC Emerg. Med. 2015, 15, 30. [Google Scholar] [CrossRef]
- Pakhchanian, H.; Raiker, R.; Mukherjee, A.; Khan, A.; Singh, S.; Chatterjee, A. Outcomes of COVID-19 in CKD Patients: A Multicenter Electronic Medical Record Cohort Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 785–786. [Google Scholar] [CrossRef]


| Uremic Toxin | ~% Reduction Rate during HD | Reference | ~% Protein Binding | Reference |
|---|---|---|---|---|
| 3-deoxyglucosone | 72 | [73] | ||
| acrolein | 30–32 | [90,93] | ||
| AGE (total) | 45–56 | [108] | 50 | [108] |
| CMPF | −30 −17–(−10) | [32] [99,108] | 100 | [32,99] |
| glyoxal | 45 | [73] | ||
| hippuric acid | 68–70 | [32,108] | 33–48 | [32,108] |
| indole-3-acetic acid | 42–47 | [32,69,108] | 82–94 | [32,69,108] |
| indoxyl glucuronide | 80 | [32] | 60 | [32] |
| indoxyl sulfate | 25–33 | [32,99,108] | 90–98 | [32,33,37,99,108] |
| kynurenic acid | 35 | [50] | ||
| kynurenine | 15 | [50] | ||
| methylglyoxal | 69 | [73] | ||
| Nε-carboxymethyl-lysine | 85 | [73] | ||
| p-cresyl sulfate | 29 | [32] | 93–97 | [32,33,37,99] |
| p-cresyl glucuronide | 81 | [32] | ||
| pentosidine | 69 | [73] | ||
| phenyl glucuronide | 69 | [32] | 76 | [32] |
| phenyl sulfate | 65 | [32] | 91 | [32] |
| phenylacetic acid | 35 | [32] | 59–60 | [32,37] |
| p-hydroxy-hippuric acid | 53 | [81] | ||
| quinolinic acid | 61 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. https://doi.org/10.3390/ijms22126196
Pieniazek A, Bernasinska-Slomczewska J, Gwozdzinski L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. International Journal of Molecular Sciences. 2021; 22(12):6196. https://doi.org/10.3390/ijms22126196
Chicago/Turabian StylePieniazek, Anna, Joanna Bernasinska-Slomczewska, and Lukasz Gwozdzinski. 2021. "Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD" International Journal of Molecular Sciences 22, no. 12: 6196. https://doi.org/10.3390/ijms22126196
APA StylePieniazek, A., Bernasinska-Slomczewska, J., & Gwozdzinski, L. (2021). Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. International Journal of Molecular Sciences, 22(12), 6196. https://doi.org/10.3390/ijms22126196






