Roles of Key Ion Channels and Transport Proteins in Age-Related Hearing Loss
Abstract
1. Introduction
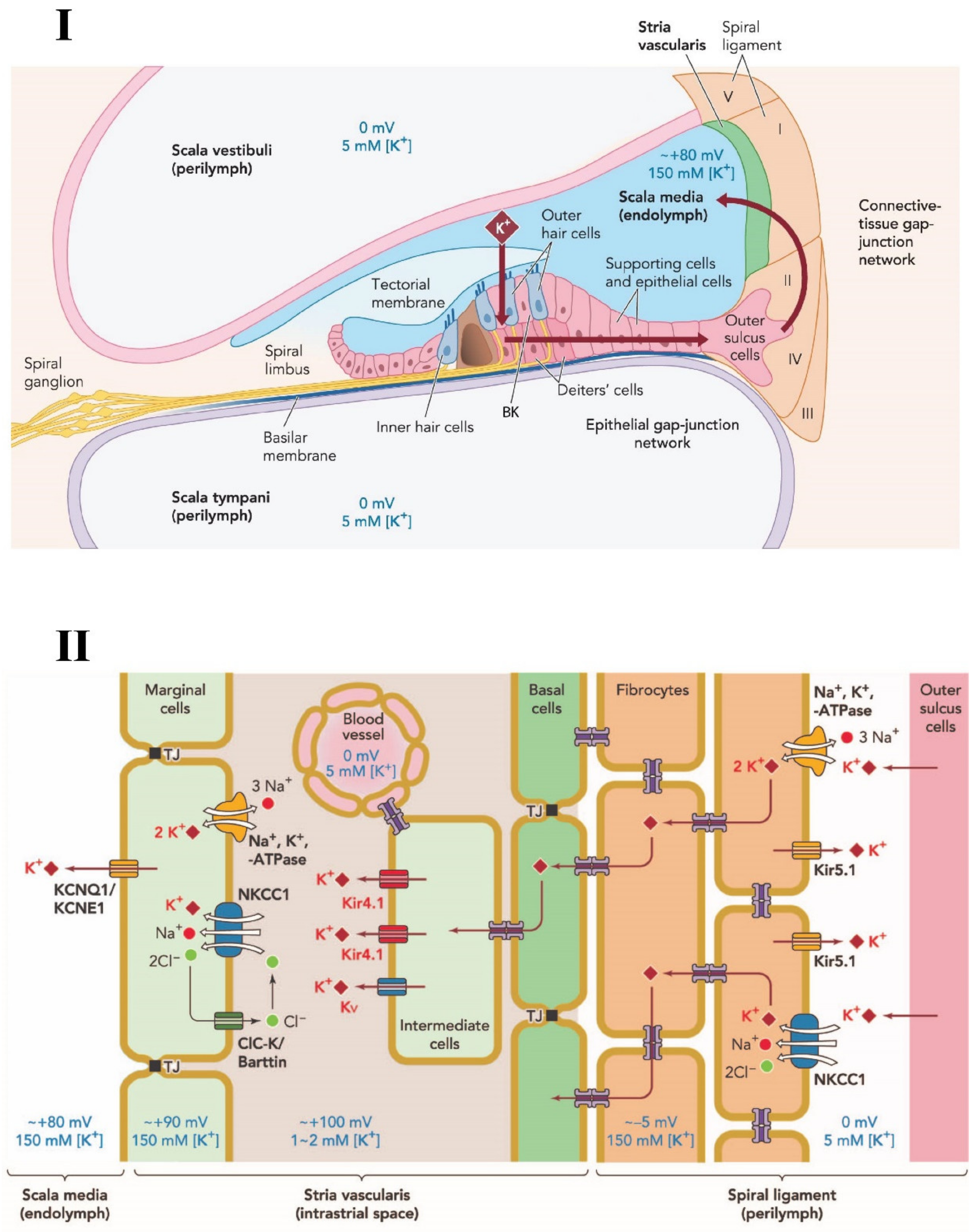
2. Sodium-Potassium-Chloride Cotransporter
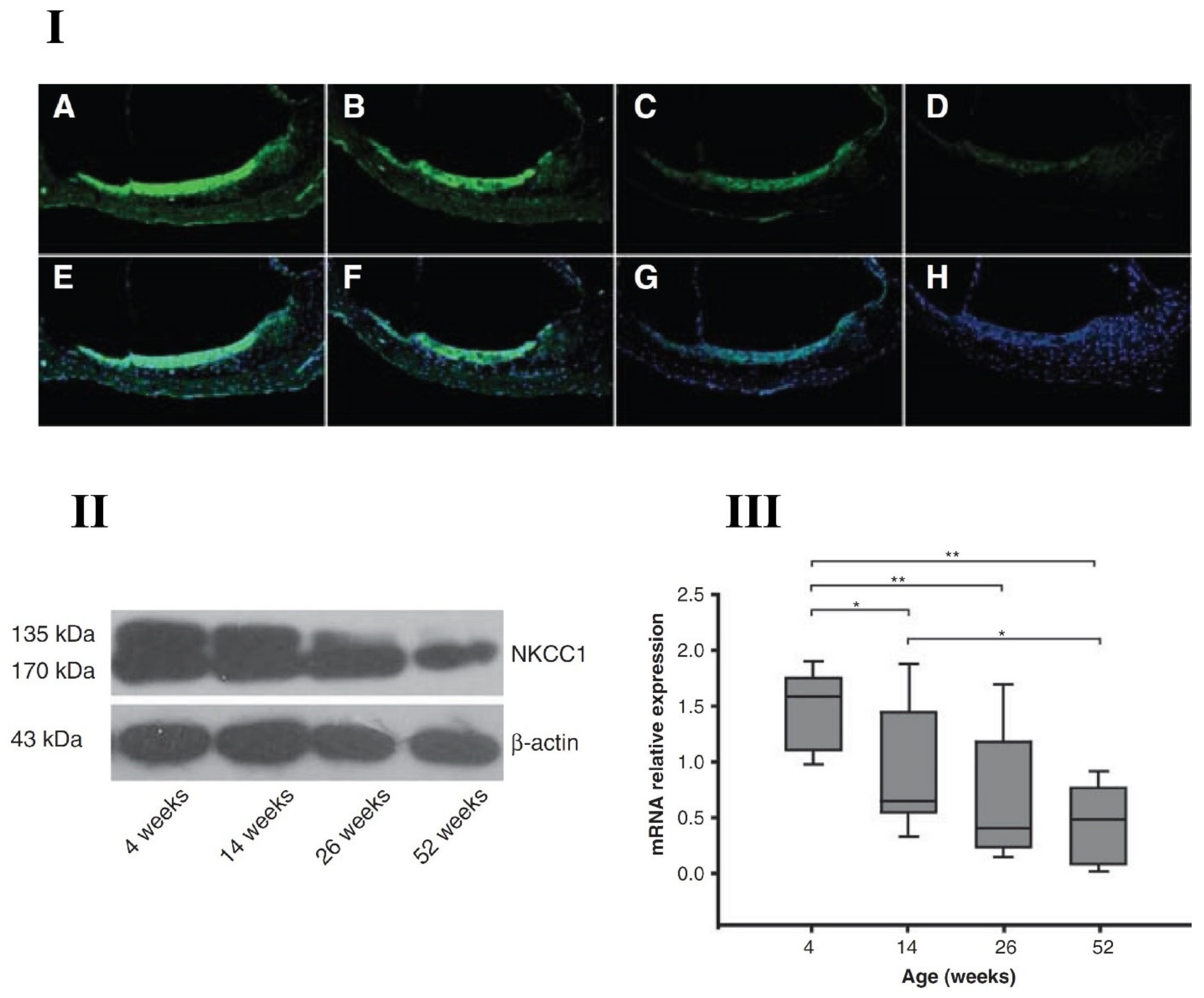
Presbycusis and NKCC1 (Sodium-Potassium-Chloride Cotransporter)
3. Na+ K+-ATPase
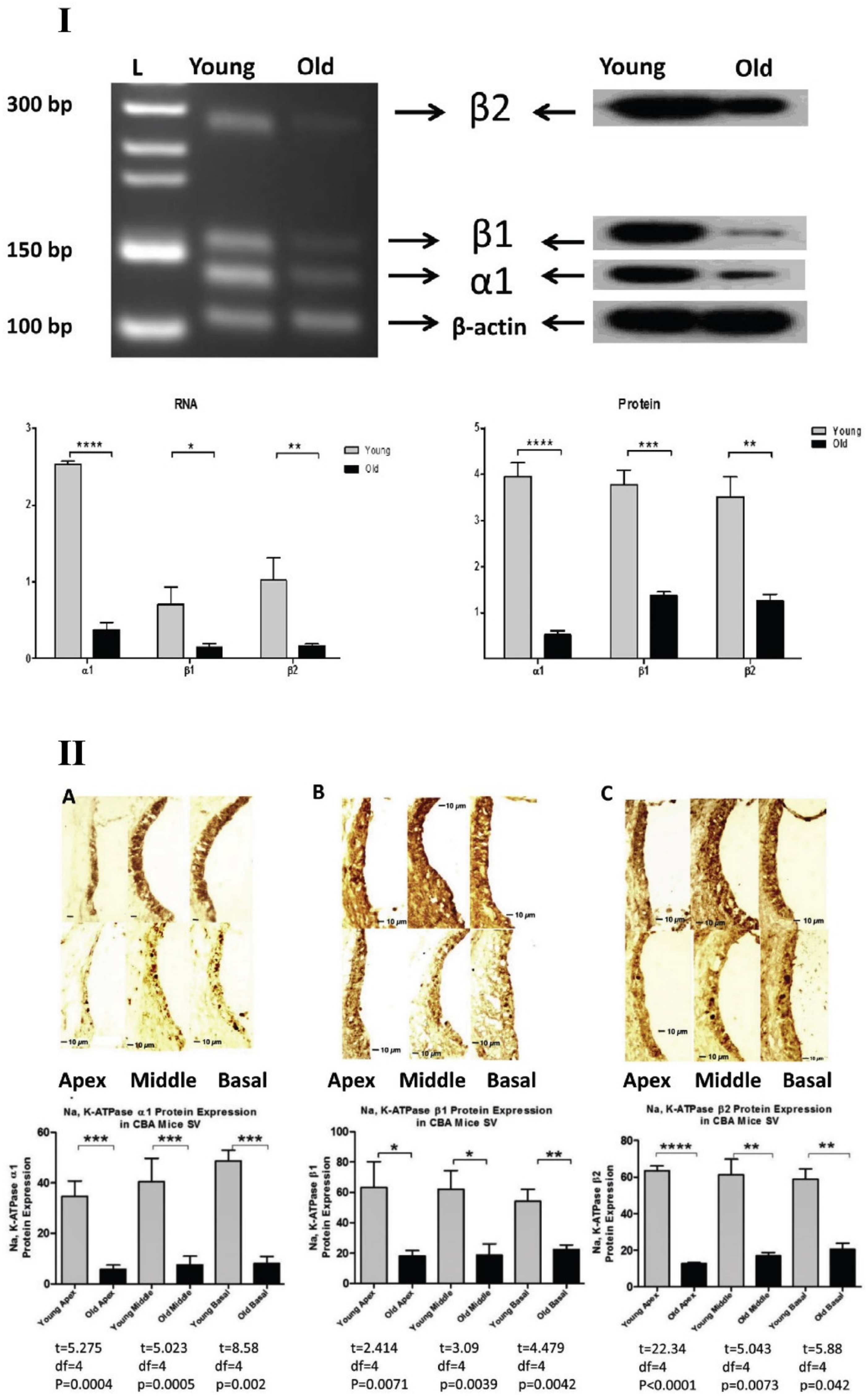
Presbycusis and Na+, K+-ATPase
4. Potassium Channels
4.1. KCNQ Channels
KCNQ and Presbycusis
4.2. Inward Rectifying Channels
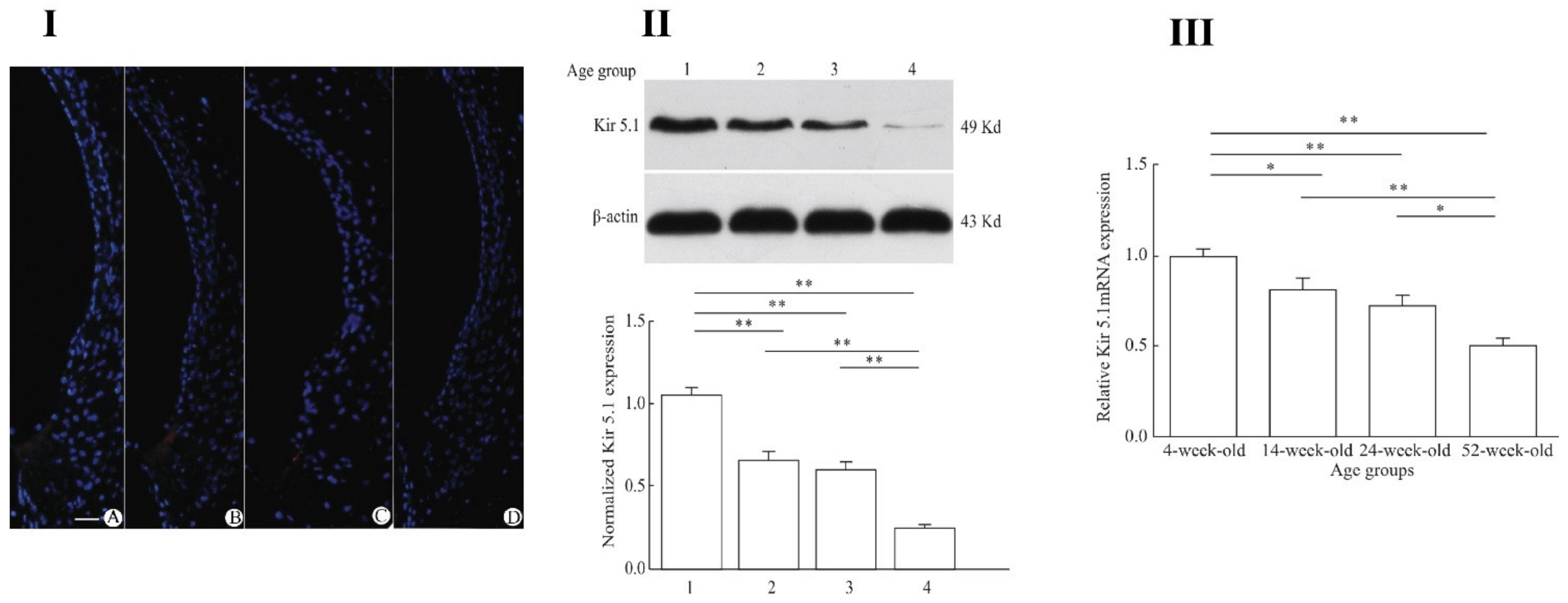
KCNJ10 and Presbycusis
4.3. Ca2+-Activated K+ (BK) Channels
BK Channels and Presbycusis
4.4. Other Potassium Channels Related to Presbycusis
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Frisina, D.R.; Frisina, R.D. Speech recognition in noise and presbycusis: Relations to possible neural mechanisms. Hear. Res. 1997, 106, 95–104. [Google Scholar] [CrossRef]
- Dalton, D.S.; Cruickshanks, K.J.; Klein, B.E.; Klein, R.; Wiley, T.L. The Impact of Hearing Loss on Quality of Life in Older Adults. Gerontologist 2003, 43, 661–668. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, J. Age-related hearing loss or presbycusis. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.E.; Kapteyn, T.S.; Kuik, D.J. The Association of Hearing Impairment and Chronic Diseases with Psychosocial Health Status in Older Age. J. Aging Health 2002, 14, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Ferrucci, L.; Metter, E.J.; An, Y.; Zonderman, A.B. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011, 25, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, D. Ageing and hearing loss. J. Pathol. 2007, 211, 188–197. [Google Scholar] [CrossRef]
- Strawbridge, W.J.; Wallhagen, M.I.; Shema, S.J.; Kaplan, G.A. Negative Consequences of Hearing Impairment in Old Age: A Longitudinal Analysis. Gerontologist 2000, 40, 320–326. [Google Scholar] [CrossRef]
- Frisina, R.D.; Williamson, T.T.; Bazard, P.; Zhu, X.; Ding, B. 2.44—The Aging Cochlea. In The Senses: A Comprehensive Reference, 2nd ed.; Fritzsch, B., Ed.; Elsevier: Oxford, UK, 2020; pp. 871–883. [Google Scholar]
- Gates, G.A.; Cooper, J.C. Incidence of Hearing Decline in the Elderly. Acta Oto-Laryngol. 1991, 111, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef]
- Howarth, A.; Shone, G.R. Ageing and the auditory system. Postgrad. Med. J. 2006, 82, 166. [Google Scholar] [CrossRef] [PubMed]
- Willott, J.F. Anatomic and physiologic aging: A behavioral neuroscience perspective. J. Am. Acad. Audiol. 1996, 7, 141–151. [Google Scholar]
- Lee, K.-Y. Pathophysiology of Age-Related Hearing Loss (Peripheral and Central). Korean J. Audiol. 2013, 17, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N. Schuknecht’s Pathology of the Ear, 3rd ed.; PMPH: Shelton, CT, USA, 2010. [Google Scholar]
- Schuknecht, H.F.; Gacek, M.R. Cochlear Pathology in Presbycusis. Ann. Otol. Rhinol. Laryngol. 1993, 102 (Suppl. S1), 1–16. [Google Scholar] [CrossRef]
- Gates, G.A.; Mills, D.; Nam, B.-h.; D’Agostino, R.; Rubel, E.W. Effects of age on the distortion product otoacoustic emission growth functions. Hear. Res. 2002, 163, 53–60. [Google Scholar] [CrossRef]
- Bhattacharyya, T.K.; Dayal, V.S. Age-Related Cochlear Hair Cell Loss in the Chinchilla. Ann. Otol. Rhinol. Laryngol. 1985, 94, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.K.; Dayal, V.S. Influence of age on hair cell loss in the rabbit cochlea. Hear. Res. 1989, 40, 179–183. [Google Scholar] [CrossRef]
- Spongr, V.P.; Flood, D.G.; Frisina, R.D.; Salvi, R.J. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J. Acoust. Soc. Am. 1997, 101, 3546–3553. [Google Scholar] [CrossRef]
- Wu, P.-z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J. Neurosci. 2020, 40, 6357–6366. [Google Scholar] [CrossRef]
- Hudspeth, A.J. How the ear’s works work. Nature 1989, 341, 397–404. [Google Scholar] [CrossRef]
- Uetsuka, S.; Ogata, G.; Nagamori, S.; Isozumi, N.; Nin, F.; Yoshida, T.; Komune, S.; Kitahara, T.; Kikkawa, Y.; Inohara, H.; et al. Molecular architecture of the stria vascularis membrane transport system, which is essential for physiological functions of the mammalian cochlea. Eur. J. Neurosci. 2015, 42, 1984–2002. [Google Scholar] [CrossRef]
- Hibino, H.; Nin, F.; Tsuzuki, C.; Kurachi, Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflügers Archiv—Eur. J. Physiol. 2010, 459, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Hibino, H.; Kurachi, Y. Molecular and Physiological Bases of the K+ Circulation in the Mammalian Inner Ear. Physiology 2006, 21, 336–345. [Google Scholar] [CrossRef]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Orlov, S.N.; Koltsova, S.V.; Kapilevich, L.V.; Gusakova, S.V.; Dulin, N.O. NKCC1 and NKCC2: The pathogenetic role of cation-chloride cotransporters in hypertension. Genes & Diseases. Genes Dis. 2015, 2, 186–196. [Google Scholar]
- Russell, J.M. Sodium-Potassium-Chloride Cotransport. Physiol. Rev. 2000, 80, 211–276. [Google Scholar] [CrossRef]
- Delpire, E.; Rauchman, M.I.; Beier, D.R.; Hebert, S.C.; Gullans, S.R. Molecular cloning and chromosome localization of a putative basolateral Na(+)-K(+)-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J. Biol. Chem. 1994, 269, 25677–25683. [Google Scholar] [CrossRef]
- Park, J.H.; Saier, J.M.H. Phylogenetic, Structural and Functional Characteristics of the Na-K-Cl Cotransporter Family. J. Membr. Biol. 1996, 149, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G.; Miyanoshita, A.; Lombardi, M.; Lytton, J.; Lee, W.S.; Hediger, M.A.; Hebert, S.C. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 1994, 269, 17713–17722. [Google Scholar] [CrossRef]
- Markadieu, N.; Delpire, E. Physiology and pathophysiology of SLC12A1/2 transporters. Pflügers Archiv—Eur. J. Physiol. 2014, 466, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Moore-Hoon, M.L.; Turner, R.J. The Structural Unit of the Secretory Na+−K+−2Cl- Cotransporter (NKCC1) Is a Homodimer. Biochemistry 2000, 39, 3718–3724. [Google Scholar] [CrossRef]
- Starremans, P.G.J.F.; Kersten, F.F.J.; Knoers, N.V.A.M.; van den Heuvel, L.P.W.J.; Bindels, R.J.M. Mutations in the Human Na-K-2Cl Cotransporter (NKCC2) Identified in Bartter Syndrome Type I Consistently Result in Nonfunctional Transporters. J. Am. Soc. Nephrol. 2003, 14, 1419–1426. [Google Scholar] [CrossRef]
- Monette, M.Y.; Forbush, B. Regulatory activation is accompanied by movements in the Cterminus of the Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 2012, 26 (Suppl. S1), 604.3. [Google Scholar]
- Gamba, G. Molecular Physiology and Pathophysiology of Electroneutral Cation-Chloride Cotransporters. Physiol. Rev. 2005, 85, 423–493. [Google Scholar] [CrossRef] [PubMed]
- Amteshwar Singh, J.; Aalamjeet, K.; Anjana, B.; Nirmal, S. Expanding Spectrum of Sodium Potassium Chloride Co-transporters in the Pathophysiology of Diseases. Curr. Neuropharmacol. 2015, 13, 369–388. [Google Scholar]
- Plotkin, M.D.; Snyder, E.Y.; Hebert, S.C.; Delpire, E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 1997, 33, 781–795. [Google Scholar] [CrossRef]
- Adragna, N.C.; Fulvio, M.D.; Lauf, P.K. Regulation of K-Cl Cotransport: From Function to Genes. J. Membr. Biol. 2004, 201, 109–137. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.N.; Pokudin, N.I.; Kotelevtsev, Y.V.; Gulak, P.V. Volume-dependent regulation of ion transport and membrane phosphorylation in human and rat erythrocytes. J. Membr. Biol. 1989, 107, 105–117. [Google Scholar] [CrossRef]
- Orlov, S.N.; Tremblay, J.; Hamet, P. Cell volume in vascular smooth muscle is regulated by bumetanide-sensitive ion transport. Am. J. Physiol. Cell Physiol. 1996, 270, C1388–C1397. [Google Scholar] [CrossRef]
- Haas, M. The Na-K-Cl cotransporters. J. Bioenerg. Biomembr. 1994, 267, C869–C885. [Google Scholar] [CrossRef]
- Kregenow, F.M. The response of duck erythrocytes to hypertonic media. Further evidence for a volume-controlling mechanism. J. Gen. Physiol. 1971, 58, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Busch, G.L.; Ritter, M.; Völkl, H.; Waldegger, S.; Gulbins, E.; Häussinger, D. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef] [PubMed]
- Mongin, A.A.; Orlov, S.N. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 2001, 8, 77–88. [Google Scholar] [CrossRef]
- Bazard, P.; Ding, B.; Chittam, H.K.; Zhu, X.; Parks, T.A.; Taylor-Clark, T.E.; Bhethanabotla, V.R.; Frisina, R.D.; Walton, J.P. Aldosterone up-regulates voltage-gated potassium currents and NKCC1 protein membrane fractions. Sci. Rep. 2020, 10, 15604. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.; Hinton, A.S.; Frisina, R.D.; Ding, B.; Zhu, X.; Walton, J.P. Long-term treatment with aldosterone slows the progression of age-related hearing loss. Hear. Res. 2016, 336, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.D.; Ding, B.; Zhu, X.; Walton, J.P. Age-related hearing loss: Prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging 2016, 8, 2081–2099. [Google Scholar] [CrossRef]
- Ding, B.; Frisina, R.D.; Zhu, X.; Sakai, Y.; Sokolowski, B.; Walton, J.P. Direct control of Na+-K+-2Cl−-cotransport protein (NKCC1) expression with aldosterone. Am. J. Physiol. Cell Physiol. 2014, 306, C66–C75. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, W.B.; Kim, Y.-B.; Lee, Y.; Kim, Y.S.; Shen, F.-Y.; Lee, S.W.; Park, D.; Choi, H.-J.; Hur, J.; et al. Chronic Hyperosmotic Stress Converts GABAergic Inhibition into Excitation in Vasopressin and Oxytocin Neurons in the Rat. J. Neurosci. 2011, 31, 13312–13322. [Google Scholar] [CrossRef]
- Cho, H.-M.; Lee, H.-A.; Kim, H.Y.; Han, H.S.; Kim, I.K. Expression of Na+-K+-2Cl− Cotransporter 1 Is Epigenetically Regulated During Postnatal Development of Hypertension. Am. J. Hypertens. 2011, 24, 1286–1293. [Google Scholar] [CrossRef][Green Version]
- Orlov, S.N.; Tremblay, J.; Hamet, P. NKCC1 and hypertension: A novel therapeutic target involved in the regulation of vascular tone and renal function. Curr. Opin. Nephrol. Hypertens. 2010, 19, 163–168. [Google Scholar] [CrossRef]
- Brandt, C.; Nozadze, M.; Heuchert, N.; Rattka, M.; Löscher, W. Disease-Modifying Effects of Phenobarbital and the NKCC1 Inhibitor Bumetanide in the Pilocarpine Model of Temporal Lobe Epilepsy. J. Neurosci. 2010, 30, 8602–8612. [Google Scholar] [CrossRef]
- Sen, A.; Martinian, L.; Nikolic, M.; Walker, M.C.; Thom, M.; Sisodiya, S.M. Increased NKCC1 expression in refractory human epilepsy. Epilepsy Res. 2007, 74, 220–227. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, H.; Chen, J.; Zhou, L.; Chen, Q.; Yu, Y.; Wu, Z.; Wang, S.; Lai, Y.; Pan, C.; et al. Age-related change in the expression of NKCC1 in the cochlear lateral wall of C57BL/6J mice. Acta Oto Laryngol. 2014, 134, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Lu, J.; England, R.; Dull, C.; Thorne, T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat. Genet. 1999, 22, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.; Gazzard, J.; Chaudhry, S.S.; Sampson, N.; Schulte, B.A.; Steel, K.P. Mutation of the Na-K-Cl Co-Transporter Gene Slc12a2 Results in Deafness in Mice. Hum. Mol. Genet. 1999, 8, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Flagella, M.; Clarke, L.L.; Miller, M.L.; Erway, L.C.; Giannella, R.A.; Andringa, A.; Gawenis, L.R.; Kramer, J.; Duffy, J.J.; Doetschman, T.; et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J. Biol. Chem. 1999, 274, 26946–26955. [Google Scholar] [CrossRef] [PubMed]
- Schmiedt, R.A.; Lang, H.; Okamura, H.-o.; Schulte, B.A. Effects of Furosemide Applied Chronically to the Round Window: A Model of Metabolic Presbyacusis. J. Neurosci. 2002, 22, 9643–9650. [Google Scholar] [CrossRef]
- Sewell, W.F. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear. Res. 1984, 14, 305–314. [Google Scholar] [CrossRef]
- Mills, D.M. Determining the Cause of Hearing Loss: Differential Diagnosis Using a Comparison of Audiometric and Otoacoustic Emission Responses. Ear Hear. 2006, 27, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.D.; Bazard, P.; Bauer, M.; Pineros, J.; Zhu, X.; Ding, B. Translational implications of the interactions between hormones and age-related hearing loss. Hear. Res. 2021, 402, 108093. [Google Scholar] [CrossRef]
- Axelsen, K.B.; Palmgren, M.G. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 1998, 46, 84–101. [Google Scholar] [CrossRef]
- Geering, K. Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 2008, 17, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef]
- Brown, P.D.; Davies, S.L.; Speake, T.; Millar, I.D. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 2004, 129, 955–968. [Google Scholar] [CrossRef]
- Caplan, M.J.; Anderson, H.C.; Palade, G.E.; Jamieson, J.D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell 1986, 46, 623–631. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, D.; Orlowski, J.; Rodriguez-Boulan, E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J. Cell Biol. 1991, 112, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Azizan, E.A.B.; Poulsen, H.; Tuluc, P.; Zhou, J.; Clausen, M.V.; Lieb, A.; Maniero, C.; Garg, S.; Bochukova, E.G.; Zhao, W.; et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013, 45, 1055–1060. [Google Scholar] [CrossRef]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45, 440–444. [Google Scholar] [CrossRef]
- Juhaszova, M.; Blaustein, M.P. Distinct Distribution of Different Na+ Pump α Subunit Isoforms in Plasmalemma. Ann. N. Y. Acad. Sci. 1997, 834, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Habeck, M.; Tokhtaeva, E.; Nadav, Y.; Ben Zeev, E.; Ferris, S.P.; Kaufman, R.J.; Bab-Dinitz, E.; Kaplan, J.H.; Dada, L.A.; Farfel, Z.; et al. Selective Assembly of Na,K-ATPase α2β2 Heterodimers in the Heart: Distinct functional properties and isoform-selective inhibitors. J. Biol. Chem. 2016, 291, 23159–23174. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Petrov, A.M.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Benziane, B.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Distinct α2 Na,K-ATPase membrane pools are differently involved in early skeletal muscle remodeling during disuse. J. Gen. Physiol. 2016, 147, 175–188. [Google Scholar] [CrossRef] [PubMed]
- StanStanley, C.M.; Gagnon, D.G.; Bernal, A.; Meyer, D.J.; Rosenthal, J.J.; Artigas, P. Importance of the Voltage Dependence of Cardiac Na/K ATPase Isozymes. Biophys. J. 2015, 109, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Morth, J.P.; Poulsen, H.; Toustrup-Jensen, M.S.; Rodacker Schack, V.; Egebjerg, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. The structure of the Na+,K+ATPase and mapping of isoform differences and disease-related mutations. Philos. Trans. R. Soc. B 2009, 364, 217–227. [Google Scholar] [CrossRef]
- Spiller, S.; Friedrich, T. Functional analysis of human Na(+)/K(+)-ATPase familial or sporadic hemiplegic migraine mutations expressed in Xenopus oocytes. World J. Biol. Chem. 2014, 5, 240–253. [Google Scholar]
- Bøttger, P.; Doğanlı, C.; Lykke-Hartmann, K. Migraine- and dystonia-related disease-mutations of Na+/K+-ATPases: Relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci. Biobehav. Rev. 2012, 36, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Fusco, M.D.; Marconi, R.; Silvestri, L.; Atorino, L.; Rampoldi, L.; Morgante, L.; Ballabio, A.; Aridon, P.; Casari, G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003, 33, 192–196. [Google Scholar] [CrossRef]
- Pelzer, N.; Blom, D.; Stam, A.; Vijfhuizen, L.; Hageman, A.; van Vliet, J.; Ferrari, M.; van den Maagdenberg, A.; Haan, J.; Terwindt, G. Recurrent coma and fever in familial hemiplegic migraine type 2. A prospective 15-year follow-up of a large family with a novel ATP1A2 mutation. Cephalalgia Int. Headache Soc. 2017, 37, 737–755. [Google Scholar] [CrossRef]
- Bøttger, P.; Tracz, Z.; Heuck, A.; Nissen, P.; Romero-Ramos, M.; Lykke-Hartmann, K. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J. Comp. Neurol. 2011, 519, 376–404. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, M.; Stimers, J.R. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front. Biosci. 2005, 10, 2373–2396. [Google Scholar] [CrossRef]
- Rose, C.R.; Konnerth, A. NMDA Receptor-Mediated Na+ Signals in Spines and Dendrites. J. Neurosci. 2001, 21, 4207–4214. [Google Scholar] [CrossRef]
- De Carvalho Aguiar, P.; Sweadner, K.J.; Penniston, J.T.; Zaremba, J.; Liu, L.; Caton, M.; Linazasoro, G.; Borg, M.; Tijssen, M.A.J.; Bressman, S.B.; et al. Mutations in the Na+/K+-ATPase α3 Gene ATP1A3 Are Associated with Rapid-Onset Dystonia Parkinsonism. Neuron 2004, 43, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Heinzen, E.L.; Swoboda, K.J.; Hitomi, Y.; Gurrieri, F.; Nicole, S.; de Vries, B.; Tiziano, F.D.; Fontaine, B.; Walley, N.M.; Heavin, S.; et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat. Genet. 2012, 44, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Rosewich, H.; Thiele, H.; Ohlenbusch, A.; Maschke, U.; Altmüller, J.; Frommolt, P.; Zirn, B.; Ebinger, F.; Siemes, H.; Nürnberg, P.; et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: A whole-exome sequencing gene-identification study. Lancet Neurol. 2012, 11, 764–773. [Google Scholar] [CrossRef]
- Brashear, A.; Cook, J.F.; Hill, D.F.; Amponsah, A.; Snively, B.M.; Light, L.; Boggs, N.; Suerken, C.K.; Stacy, M.; Ozelius, L.; et al. Psychiatric disorders in rapid-onset dystonia-parkinsonism. Neurology 2012, 79, 1168–1173. [Google Scholar] [CrossRef]
- Heinzen, E.L.; Arzimanoglou, A.; Brashear, A.; Clapcote, S.J.; Gurrieri, F.; Goldstein, D.B.; Jóhannesson, S.H.; Mikati, M.A.; Neville, B.; Nicole, S.; et al. Distinct neurological disorders with ATP1A3 mutations. Lancet Neurol. 2014, 13, 503–514. [Google Scholar] [CrossRef]
- Heimer, G.; Sadaka, Y.; Israelian, L.; Feiglin, A.; Ruggieri, A.; Marshall, C.R.; Scherer, S.W.; Ganelin-Cohen, E.; Marek-Yagel, D.; Tzadok, M.; et al. CAOS—Episodic Cerebellar Ataxia, Areflexia, Optic Atrophy, and Sensorineural Hearing Loss: A Third Allelic Disorder of the ATP1A3 Gene. J. Child Neurol. 2015, 30, 1749–1756. [Google Scholar] [CrossRef]
- McDermott, J.P.; Sánchez, G.; Chennathukuzhi, V.; Blanco, G. Green fluorescence protein driven by the Na,K-ATPase α4 isoform promoter is expressed only in male germ cells of mouse testis. J. Assist. Reprod. Genet. 2012, 29, 1313–1325. [Google Scholar] [CrossRef]
- Clausen, M.; Nissen, J.; Poulsen, H. The pumps that fuel a sperm’s journey. Biochem. Soc. Trans. 2011, 39, 741–745. [Google Scholar] [CrossRef]
- Voldsgaard Clausen, M.; Nissen, P.; Poulsen, H. The α4 isoform of the Na+,K+-ATPase is tuned for changing extracellular environments. FAEBS J. 2016, 283, 282–293. [Google Scholar] [CrossRef]
- Geering, K. The Functional Role of β Subunits in Oligomeric P-Type ATPases. J. Bioenerg. Biomembr. 2001, 33, 425–438. [Google Scholar] [CrossRef]
- Vasallo, M. Biología molecular, biogénesis y regulación de a bomba de sodio. Nefrología 1989, 9, 7–14. [Google Scholar]
- Rasmussen, H.H.; Hamilton, E.J.; Liu, C.-C.; Figtree, G.A. Reversible Oxidative Modification: Implications for Cardiovascular Physiology and Pathophysiology. Trends Cardiovasc. Med. 2010, 20, 85–90. [Google Scholar] [CrossRef]
- Magyar, J.P.; Bartsch, U.; Wang, Z.Q.; Howells, N.; Aguzzi, A.; Wagner, E.F.; Schachner, M. Degeneration of neural cells in the central nervous system of mice deficient in the gene for the adhesion molecule on Glia, the beta 2 subunit of murine Na,K-ATPase. J. Cell Biol. 1994, 127, 835–845. [Google Scholar] [CrossRef]
- Jones, D.H.; Li, T.Y.; Arystarkhova, E.; Barr, K.J.; Wetzel, R.K.; Peng, J.; Markham, K.; Sweadner, K.J.; Fong, G.-H.; Kidder, G.M. Na,K-ATPase from Mice Lacking the γ Subunit (FXYD2) Exhibits Altered Na+ Affinity and Decreased Thermal Stability. J. Biol. Chem. 2005, 280, 19003–19011. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, Y.; Petrovich, E.; Haviv, H.; Goldshleger, R.; Tal, D.M.; Garty, H.; Karlish, S.J.D. Purification of the Human α2 Isoform of Na,K-ATPase Expressed in Pichia pastoris. Stabilization by Lipids and FXYD1. Biochemistry 2007, 46, 14937–14950. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Grosdidier, A.; Crambert, G.; Horisberger, J.-D.; Michielin, O.; Geering, K. Structural and Functional Interaction Sites between Na,K-ATPase and FXYD Proteins. J. Biol. Chem. 2004, 279, 38895–38902. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.R.; Kennington, E.; Fuller, W.; Dighe, K.; Donoghue, P.; Clark, J.E.; Jia, L.-G.; Tucker, A.L.; Moorman, J.R.; Marber, M.S.; et al. Characterization of the phospholemman knockout mouse heart: Depressed left ventricular function with increased Na-K-ATPase activity. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H613–H621. [Google Scholar] [CrossRef]
- Chen, L.-S.K.; Lo, C.F.; Numann, R.; Cuddy, M. Characterization of the Human and Rat Phospholemman (PLM) cDNAs and Localization of the Human PLM Gene to Chromosome 19q13.1. Genomics 1997, 41, 435–443. [Google Scholar] [CrossRef]
- Cheung, J.Y.; Rothblum, L.I.; Moorman, J.R.; Tucker, A.L.; Song, J.; Ahlers, B.A.; Carl, L.L.; Wang, J.; Zhang, X.-Q. Regulation of Cardiac Na+/Ca2+ Exchanger by Phospholemman. Ann. N. Y. Acad. Sci. 2007, 1099, 119–134. [Google Scholar] [CrossRef]
- Pavlovic, D.; Fuller, W.; Shattock, M.J. The intracellular region of FXYD1 is sufficient to regulate cardiac Na/K ATPase. FASEB J. 2007, 21, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Moorman, J.R.; Ahlers, B.A.; Carl, L.L.; Lake, D.E.; Song, J.; Mounsey, J.P.; Tucker, A.L.; Chan, Y.-m.; Rothblum, L.I.; et al. Phospholemman overexpression inhibits Na+-K+-ATPase in adult rat cardiac myocytes: Relevance to decreased Na+ pump activity in postinfarction myocytes. J. Appl. Physiol. 2006, 100, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. FXYD proteins: New regulators of Na-K-ATPase. Am. J. Physiol. Ren. Physiol. 2006, 290, F241–F250. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, I.; Grahammer, F.; Warth, R.; Schulz-Baldes, A.; Garty, H.; Greger, R.; Bleich, M. Kidney and Colon Electrolyte Transport in CHIF Knockout Mice. Cell. Physiol. Biochem. 2004, 14, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Yoshida, T.; Nin, F.; Ogata, G.; Yamaguchi, S.; Suzuki, T.; Komune, S.; Hisa, Y.; Hibino, H.; Kurachi, Y. The mechanism underlying maintenance of the endocochlear potential by the K+ transport system in fibrocytes of the inner ear. J. Physiol. 2013, 591, 4459–4472. [Google Scholar] [CrossRef]
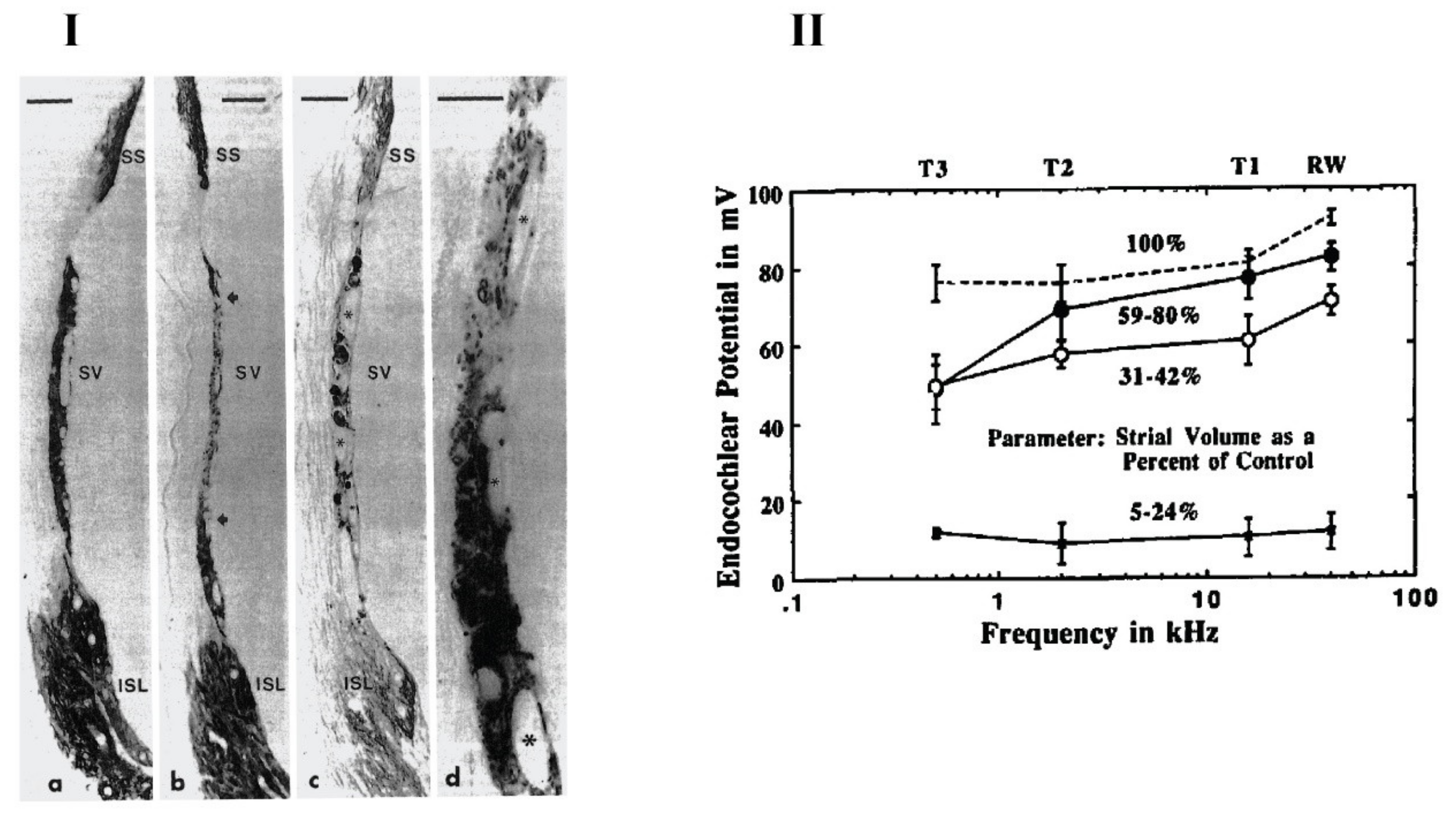
- Mills, J.H.; Schmiedt, R.A.; Schulte, B.A.; Dubno, J.R. Age-Related Hearing Loss: A Loss of Voltage, Not Hair Cells. Semin. Hear. 2006, 27, 228–236. [Google Scholar] [CrossRef]
- Pauler, M.; Schuknecht, H.F.; White, J.A. Atrophy of the stria vascularis as a cause of sensorineural hearing loss. Laryngoscope 1988, 98, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H.F. Pathology of the Ear; Harvard University Press: Cambridge, MA, USA, 1974; pp. 576–577. Volume 85. [Google Scholar]
- Schulte, B.A.; Adams, J.C. Distribution of immunoreactive Na+, K+-ATPase in gerbil cochlea. J. Histochem. Cytochem. 1989, 37, 127–134. [Google Scholar] [CrossRef]
- Johnsson, L.-G.; Hawkins, J.E. Sensory and Neural Degeneration with Aging, as Seen in Microdissections of the Human Inner Ear. Ann. Oto. Rhi. Lary. 1972, 81, 179–193. [Google Scholar] [CrossRef]
- Nadol, J.B., Jr. The aging peripheral hearing mechanism. Aging Commun. Process. Disord. 1980, 4, 63–85. [Google Scholar]
- Schuknecht, H.F. Presbycusis. Laryngoscope 1955, 65, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H.F. Further Observations on the Pathology of Presbycusis. Arch. Otolaryngol. 1964, 80, 369–382. [Google Scholar] [CrossRef]
- Gacek, R.R.; Schuknecht, H.F. Pathology of presbycusis. Int. Audiol. 1969, 8, 199–209. [Google Scholar] [CrossRef]
- Takahashi, T. The Ultrastructure of the Pathologic Stria Vascularis and Spiral Prominence in Man. Ann. Oto. Rhin. Laryngo. 1971, 80, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.A.; Schmiedt, R.A. Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear. Res. 1992, 61, 35–46. [Google Scholar] [CrossRef]
- Hellier, W.P.L.; Wagstaff, S.A.; O’Leary, S.J.; Shepherd, R.K. Functional and morphological response of the stria vascularis following a sensorineural hearing loss. Hear. Res. 2002, 172, 127–136. [Google Scholar] [CrossRef]
- Lippincott, L.; Rarey, K.E. Status of cochlear Na,K-ATPase in the aged SHR rat and its possible role in hearing loss. Eur. Arch. Oto Rhino Laryngol. 1997, 254, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Walton, J.P.; Zhu, X.; Frisina, R.D. Age-related changes in Na, K-ATPase expression, subunit isoform selection and assembly in the stria vascularis lateral wall of mouse cochlea. Hear. Res. 2018, 367, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Schulte, B.A.; Schmiedt, R.A. Ouabain Induces Apoptotic Cell Death in Type I Spiral Ganglion Neurons, but not Type II Neurons. J. Assoc. Res. Otolaryngol. 2005, 6, 63–74. [Google Scholar] [CrossRef]
- Diaz, R.C.; Vazquez, A.E.; Dou, H.; Wei, D.; Cardell, E.L.; Lingrel, J.; Shull, G.E.; Doyle, K.J.; Yamoah, E.N. Conservation of Hearing by Simultaneous Mutation of Na,K-ATPase and NKCC1. J. Assoc. Res. Otolaryngol. 2007, 8, 422–434. [Google Scholar] [CrossRef]
- Kuo, M.M.-C.; Haynes, W.J.; Loukin, S.H.; Kung, C.; Saimi, Y. Prokaryotic K+ channels: From crystal structures to diversity. FEMS Microbiol. Rev. 2005, 29, 961–985. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Kidd, J.F.; Law, R.J.; Franks, C.J.; Sattelle, D.B. Structure and function of two-pore-domain K+ channels: Contributions from genetic model organisms. Trends Pharmacol. Sci. 2005, 26, 361–367. [Google Scholar] [CrossRef]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, R. Potassium channels. FEBS Lett. 2003, 555, 62–65. [Google Scholar] [CrossRef]
- Sansom, M.S.P.; Shrivastava, I.H.; Bright, J.N.; Tate, J.; Capener, C.E.; Biggin, P.C. Potassium channels: Structures, models, simulations. Biochim. Biophys. Acta Biomembr. 2002, 1565, 294–307. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, A.; Chen, J.; Ruta, V.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a voltage-dependent K+ channel. Nature 2003, 423, 33–41. [Google Scholar] [CrossRef]
- Imai, S.; Osawa, M.; Takeuchi, K.; Shimada, I. Structural basis underlying the dual gate properties of KcsA. PNAS 2010, 107, 6216–6221. [Google Scholar] [CrossRef]
- Ben-Abu, Y.; Zhou, Y.; Zilberberg, N.; Yifrach, O. Inverse coupling in leak and voltage-activated K+ channel gates underlies distinct roles in electrical signaling. Nat. Struct. Mol. Biol. 2009, 16, 71–79. [Google Scholar] [CrossRef]
- Wang, D.T.; Hill, A.P.; Mann, S.A.; Tan, P.S.; Vandenberg, J.I. Mapping the sequence of conformational changes underlying selectivity filter gating in the Kv11.1 potassium channel. Nat. Struct. Mol. Biol. 2011, 18, 35–41. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Sulphonylurea action revisited: The post-cloning era. Diabetologia 2003, 46, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Tomuschat, C.; O’Donnell, A.M.; Coyle, D.; Dreher, N.; Kelly, D.; Puri, P. Altered expression of ATP-sensitive K+ channels in Hirschsprung’s disease. J. Pediatric Surg. 2016, 51, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, F.; Schmidt, C.; Lugenbiel, P.; Staudacher, I.; Rahm, A.K.; Seyler, C.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Therapeutic targeting of two-pore-domain potassium (K(2P)) channels in the cardiovascular system. Clin. Sci. 2016, 130, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Berkefeld, H.; Sailer, C.A.; Bildl, W.; Rohde, V.; Thumfart, J.-O.; Eble, S.; Klugbauer, N.; Reisinger, E.; Bischofberger, J.; Oliver, D.; et al. BKCa-Cav Channel Complexes Mediate Rapid and Localized Ca2-Activated K+ Signaling. Science 2006, 314, 615–620. [Google Scholar] [CrossRef]
- Wang, L.; Sigworth, F.J. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature 2009, 461, 292–295. [Google Scholar] [CrossRef]
- Delmas, P.; Brown, D.A. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci. 2005, 6, 850–862. [Google Scholar] [CrossRef]
- Robbins, J. KCNQ potassium channels: Physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 2001, 90, 1–19. [Google Scholar] [CrossRef]
- Wang, J.-J.; Li, Y. KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol. Sin. 2016, 37, 25–33. [Google Scholar] [CrossRef]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Jespersen, T.; Grunnet, M.; Olesen, S.-P. The KCNQ1 Potassium Channel: From Gene to Physiological Function. Physiology 2005, 20, 408–416. [Google Scholar] [CrossRef]
- Tyson, J.; Tranebjærg, L.; McEntagart, M.; Larsen, L.; Christiansen, M.; Whiteford, M.; Bathen, J.; Aslaksen, B.; Sørland, S.; Lund, O.; et al. Mutational spectrum in the cardioauditory syndrome of Jervell and Lange-Nielsen. Hum. Genet. 2000, 107, 499–503. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Marrion, N.V.; Smart, T.G.; Marsh, S.J.; Brown, D.A. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br. J. Pharmacol. 1989, 98, 557–573. [Google Scholar] [CrossRef]
- Wang, H.-S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 Potassium Channel Subunits: Molecular Correlates of the M-Channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef]
- Marrion, N.V. Control of m-current. Annu. Rev. Physiol. 1997, 59, 483–504. [Google Scholar] [CrossRef]
- Biervert, C.; Schroeder, B.C.; Kubisch, C.; Berkovic, S.F.; Propping, P.; Jentsch, T.J.; Steinlein, O.K. A Potassium Channel Mutation in Neonatal Human Epilepsy. Science 1998, 279, 403–406. [Google Scholar] [CrossRef]
- Charlier, C.; Singh, N.A.; Ryan, S.G.; Lewis, T.B.; Reus, B.E.; Leach, R.J.; Leppert, M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 1998, 18, 53–55. [Google Scholar] [CrossRef]
- Heidenreich, M.; Lechner, S.G.; Vardanyan, V.; Wetzel, C.; Cremers, C.W.; De Leenheer, E.M.; Aránguez, G.; Moreno-Pelayo, M.Á.; Jentsch, T.J.; Lewin, G.R. KCNQ4 K+ channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat. Neurosci. 2012, 15, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kharkovets, T.; Hardelin, J.-P.; Safieddine, S.; Schweizer, M.; El-Amraoui, A.; Petit, C.; Jentsch, T.J. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. PNAS 2000, 97, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Passmore, G.M.; Selyanko, A.A.; Mistry, M.; Al-Qatari, M.; Marsh, S.J.; Matthews, E.A.; Dickenson, A.H.; Brown, T.A.; Burbidge, S.A.; Main, M.; et al. KCNQ/M Currents in Sensory Neurons: Significance for Pain Therapy. J. Neurosci. 2003, 23, 7227–7236. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Iannotti, C.A.; Dargis, P.; Christian, E.P.; Aiyar, J. Differential Expression of KCNQ2 Splice Variants: Implications to M Current Function during Neuronal Development. J. Neurosci. 2001, 21, 1096–1103. [Google Scholar] [CrossRef]
- Huang, H.; Trussell, L.O. KCNQ5 channels control resting properties and release probability of a synapse. Nat. Neurosci. 2011, 14, 840–847. [Google Scholar] [CrossRef]
- Gamper, N.; Li, Y.; Shapiro, M.S. Structural Requirements for Differential Sensitivity of KCNQ K+ Channels to Modulation by Ca2+/Calmodulin. Mol. Biol. Cell 2005, 16, 3538–3551. [Google Scholar] [CrossRef]
- Wen, H.; Jurkovicova, D.; Pickel, V.M.; Gioio, A.E.; Kaplan, B.B. Identification of a novel membrane-associated protein expressed in neurons of the squid and rodent nervous systems. Neuroscience 2002, 114, 995–1004. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Zaika, O.; Tolstykh, G.P.; Shapiro, M.S. Regulation of neural KCNQ channels: Signalling pathways, structural motifs and functional implications. J. Physiol. 2008, 586, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Craciun, L.C.; Mirshahi, T.; Rohács, T.; Lopes, C.M.B.; Jin, T.; Logothetis, D.E. PIP2 Activates KCNQ Channels, and Its Hydrolysis Underlies Receptor-Mediated Inhibition of M Currents. Neuron 2003, 37, 963–975. [Google Scholar] [CrossRef]
- Hoshi, N.; Zhang, J.-S.; Omaki, M.; Takeuchi, T.; Yokoyama, S.; Wanaverbecq, N.; Langeberg, L.K.; Yoneda, Y.; Scott, J.D.; Brown, D.A.; et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat. Neurosci. 2003, 6, 564–571. [Google Scholar] [CrossRef]
- Li, Y.; Langlais, P.; Gamper, N.; Liu, F.; Shapiro, M.S. Dual Phosphorylations Underlie Modulation of Unitary KCNQ K+ Channels by Src Tyrosine Kinase. J. Biol. Chem. 2004, 279, 45399–45407. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J. Neuronal KCNQ potassium channels: Physislogy and role in disease. Nat. Rev. Neurosci. 2000, 1, 21–30. [Google Scholar] [CrossRef]
- Kubisch, C.; Schroeder, B.C.; Friedrich, T.; Lütjohann, B.; El-Amraoui, A.; Marlin, S.; Petit, C.; Jentsch, T.J. KCNQ4, a Novel Potassium Channel Expressed in Sensory Outer Hair Cells, Is Mutated in Dominant Deafness. Cell 1999, 96, 437–446. [Google Scholar] [CrossRef]
- Van Camp, G.; Coucke, P.J.; Akita, J.; Fransen, E.; Abe, S.; De Leenheer, E.M.R.; Huygen, P.L.M.; Cremers, C.W.R.J.; Usami, S.-I. A mutational hot spot in the KCNQ4 gene responsible for autosomal dominant hearing impairment. Hum. Mutat. 2002, 20, 15–19. [Google Scholar] [CrossRef]
- Van Eyken, E.; Van Laer, L.; Fransen, E.; Topsakal, V.; Lemkens, N.; Laureys, W.; Nelissen, N.; Vandevelde, A.; Wienker, T.; Van De Heyning, P.; et al. KCNQ4: A gene for age-related hearing impairment? Hum. Mutat. 2006, 27, 1007–1016. [Google Scholar] [CrossRef]
- Jung, J.; Lin, H.; Koh, Y.I.; Ryu, K.; Lee, J.S.; Rim, J.H.; Choi, H.J.; Lee, H.J.; Kim, H.-Y.; Yu, S.; et al. Rare KCNQ4 variants found in public databases underlie impaired channel activity that may contribute to hearing impairment. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Beisel, K.W.; Rocha-Sanchez, S.M.; Morris, K.A.; Nie, L.; Feng, F.; Kachar, B.; Yamoah, E.N.; Fritzsch, B. Differential expression of KCNQ4 in inner hair cells and sensory neurons is the basis of progressive high-frequency hearing loss. J. Neurosci. 2005, 25, 9285–9293. [Google Scholar] [CrossRef]
- Kharkovets, T.; Dedek, K.; Maier, H.; Schweizer, M.; Khimich, D.; Nouvian, R.; Vardanyan, V.; Leuwer, R.; Moser, T.; Jentsch, T.J. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006, 25, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.-T.; Demêmes, D.; Martin, A.; Kupershmidt, S.; Barhanin, J. KCNQ1/KCNE1 potassium channels in mammalian vestibular dark cells. Hear. Res. 2001, 153, 132–145. [Google Scholar] [CrossRef]
- Vetter, D.E.; Mann, J.R.; Wangemann, P.; Liu, J.; McLaughlin, K.J.; Lesage, F.; Marcus, D.C.; Lazdunski, M.; Heinemann, S.F.; Barhanin, J. Inner Ear Defects Induced by Null Mutationof the isk Gene. Neuron 1996, 17, 1251–1264. [Google Scholar] [CrossRef]
- Hille, B. Ionic Channels in Excitable Membranes; Sinauer Ass: Sunderland, MA, USA, 2001. [Google Scholar]
- Hebert, S.C.; Desir, G.; Giebisch, G.; Wang, W. Molecular Diversity and Regulation of Renal Potassium Channels. Physiol. Rev. 2005, 85, 319–371. [Google Scholar] [CrossRef]
- Lewis, D.L.; Ikeda, S.R.; Aryee, D.; Joho, R.H. Expression of an inwardly rectifying K+ channel from rat basophilic leukemia cell mRNA in Xenopus oocytes. FEBS Lett. 1991, 290, 17–21. [Google Scholar] [CrossRef]
- Nobles, M.; Benians, A.; Tinker, A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. PNAS 2005, 102, 18706–18711. [Google Scholar] [CrossRef]
- Sims, S.M.; Dixon, S.J. Inwardly rectifying K+ current in osteoclasts. Am. J. Physiol. Cell Physiol. 1989, 256, C1277–C1282. [Google Scholar] [CrossRef]
- Takahashi, T. Inward rectification in neonatal rat spinal motoneurones. J. Physiol. 1990, 423, 47–62. [Google Scholar] [CrossRef]
- Fakler, B.; Ruppersberg, J.P. Functional and Molecular Diversity Classifies the Family of Inward-Rectifier K+ Channels. Cell. Physiol. Biochem. 1996, 6, 195–209. [Google Scholar] [CrossRef]
- Stanfield, R.; Nakajima, S.; Nakajima, Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2. 0 and Kir3. 0. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 47–179. [Google Scholar]
- Chen, J.; Zhao, H.B. The role of an inwardly rectifying K+ channel (Kir4.1) in the inner ear and hearing loss. Neuroscience 2014, 265, 137–146. [Google Scholar] [CrossRef]
- Hibino, H.; Horio, Y.; Fujita, A.; Inanobe, A.; Doi, K.; Gotow, T.; Uchiyama, Y.; Kubo, T.; Kurachi, Y. Expression of an inwardly rectifying K(+) channel, Kir4.1, in satellite cells of rat cochlear ganglia. Am. J. Physiol. Cell Physiol. 1999, 277, C638–C644. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Horio, Y.; Inanobe, A.; Doi, K.; Ito, M.; Yamada, M.; Gotow, T.; Uchiyama, Y.; Kawamura, M.; Kubo, T.; et al. An ATP-Dependent Inwardly Rectifying Potassium Channel, KAB-2 (Kir4.1), in Cochlear Stria Vascularis of Inner Ear: Its Specific Subcellular Localization and Correlation with the Formation of Endocochlear Potential. J. Neurosci. 1997, 17, 4711–4721. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ando, M. Inwardly rectifying K+ currents in intermediate cells in the cochlea of gerbils: A possible contribution to the endocochlear potential. Neurosci. Lett. 1998, 247, 175–178. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ando, M.; Sato, T.; Kakigi, A. Three-dimensional and ultrastructural relationships between intermediate cells and capillaries in the gerbil stria vascularis. Hear. Res. 2001, 155, 103–112. [Google Scholar] [CrossRef]
- Ando, M.; Takeuchi, S. Immunological identification of an inward rectifier K+ channel (Kir4.1) in the intermediate cell (melanocyte) of the cochlear stria vascularis of gerbils and rats. Cell Tissue Res. 1999, 298, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Morizono, T. Electrochemical profiles for monovalent ions in the stria vascularis: Cellular model of ion transport mechanisms. Hear. Res. 1989, 39, 279–286. [Google Scholar] [CrossRef]
- Salt, A.N.; Mleichar, I.; Thalmann, R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 1987, 97, 984–991. [Google Scholar] [CrossRef]
- Wangemann, P.; Schacht, J. Homeostatic Mechanisms in the Cochlea. In The Cochlea; Dallos, A., Popper, N., Fay, R.R., Eds.; Springer: New York, NY, USA, 1996; pp. 130–185. [Google Scholar]
- Kofuji, P.; Ceelen, P.; Zahs, K.R.; Surbeck, L.W.; Lester, H.A.; Newman, E.A. Genetic inactivation of an inwardly rectifying potassium channel (kir4.1 Subunit) in mice: Phenotypic impact in retina. J. Neurosci. 2000, 20, 5733–5740. [Google Scholar] [CrossRef]
- Marcus, D.C.; Wu, T.; Wangemann, P.; Kofuji, P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am. J. Physiol. Cell Physiol. 2002, 282, C403–C407. [Google Scholar] [CrossRef] [PubMed]
- Rozengurt, N.; Lopez, I.; Chiu, C.-S.; Kofuji, P.; Lester, H.A.; Neusch, C. Time course of inner ear degeneration and deafness in mice lacking the Kir4.1 potassium channel subunit. Hear. Res. 2003, 177, 71–80. [Google Scholar] [CrossRef]
- Wangemann, P.; Itza, E.M.; Albrecht, B.; Wu, T.; Jabba, S.V.; Maganti, R.J.; Lee, J.H.; Everett, L.A.; Wall, S.M.; Royaux, I.E.; et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004, 2, 30. [Google Scholar] [CrossRef]
- Yang, T.; Gurrola, J.G.; Wu, H.; Chiu, S.M.; Wangemann, P.; Snyder, P.M.; Smith, R.J.H. Mutations of KCNJ10 Together with Mutations of SLC26A4 Cause Digenic Nonsyndromic Hearing Loss Associated with Enlarged Vestibular Aqueduct Syndrome. Am. J. Hum. Genet. 2009, 84, 651–657. [Google Scholar] [CrossRef]
- Pan, C.-C.; Chu, H.-Q.; Lai, Y.-B.; Sun, Y.-B.; Du, Z.-H.; Liu, Y.; Chen, J.; Tong, T.; Chen, Q.-G.; Zhou, L.-Q.; et al. Downregulation of inwardly rectifying potassium channel 5.1 expression in C57BL/6J cochlear lateral wall. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 406–409. [Google Scholar] [CrossRef]
- Golowasch, J.; Kirkwood, A.; Miller, C. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J. Exp. Biol. 1986, 124, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Miller, C. Conduction and selectivity in potassium channels. J. Membr. Biol. 1983, 71, 11–30. [Google Scholar] [CrossRef]
- Marty, A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 1981, 291, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Oberhauser, A.; Labarca, P.; Alvarez, O. Varieties of Calcium-Activated Potassium Channels. Annu. Rev. Physiol. 1989, 51, 385–399. [Google Scholar] [CrossRef]
- Lee, U.S.; Cui, J. BK channel activation: Structural and functional insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef]
- Meera, P.; Wallner, M.; Song, M.; Toro, L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. PNAS 1997, 94, 14066–14071. [Google Scholar] [CrossRef]
- Ma, Z.; Lou, X.J.; Horrigan, F.T. Role of Charged Residues in the S1–S4 Voltage Sensor of BK Channels. J. Gen. Physiol. 2006, 127, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Koval, O.M.; Fan, Y.; Rothberg, B.S. A Role for the S0 Transmembrane Segment in Voltage-dependent Gating of BK Channels. J. Gen. Physiol. 2007, 129, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.P.; Zakharov, S.I.; Liu, G.; Yang, L.; Sok, A.J.; Marx, S.O. Defining the BK channel domains required for β1-subunit modulation. PNAS 2006, 103, 5096–5101. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Meera, P.; Toro, L. Determinant for β-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: An additional transmembrane region at the N terminus. PNAS 1996, 93, 14922–14927. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Leonetti, M.D.; Pico, A.R.; Hsiung, Y.; MacKinnon, R. Structure of the Human BK Channel Ca2+ Activation Apparatus at 3.0 Å Resolution. Science 2010, 329, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Moczydlowski, E.G. BK Channel News: Full Coverage on the Calcium Bowl. J. Gen. Physiol. 2004, 123, 471–473. [Google Scholar] [CrossRef]
- Schreiber, M.; Salkoff, L. A novel calcium-sensing domain in the BK channel. Biophys. J. 1997, 73, 1355–1363. [Google Scholar] [CrossRef]
- Shi, J.; Krishnamoorthy, G.; Yang, Y.; Hu, L.; Chaturvedi, N.; Harilal, D.; Qin, J.; Cui, J. Mechanism of magnesium activation of calcium-activated potassium channels. Nature 2002, 418, 876–880. [Google Scholar] [CrossRef]
- Xia, X.-M.; Zeng, X.; Lingle, C.J. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef]
- Yang, H.; Shi, J.; Zhang, G.; Yang, J.; Delaloye, K.; Cui, J. Activation of Slo1 BK channels by Mg2+ coordinated between the voltage sensor and RCK1 domains. Nat. Struct. Mol. Biol. 2008, 15, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lee, A.; Chen, J.; Cadene, M.; Chait, B.T.; MacKinnon, R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 2002, 417, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Cadene, M.; Chait, B.T.; MacKinnon, R. Crystal structure of a Kir3. 1-prokaryotic Kir channel chimera. EMBO J. 2007, 26, 4005–4015. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Rivard, A.F.; Bächinger, H.P.; Adelman, J.P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 2001, 410, 1120–1124. [Google Scholar] [CrossRef]
- Zagotta, W.N.; Siegelbaum, S.A. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 1996, 19, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Zhou, R.; Xing, G. Possible role of potassium channel, big K in etiology of Schizophrenia. Med. Hypotheses 2006, 67, 41–43. [Google Scholar] [CrossRef]
- Laumonnier, F.; Roger, S.; Guérin, P.; Molinari, F.; M’rad, R.; Cahard, D.; Belhadj, A.; Halayem, M.; Persico, A.M.; Elia, M. Association of a functional deficit of the BK Ca channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am. J. Psychiatry 2006, 163, 1622–1629. [Google Scholar] [CrossRef]
- Lorenz, S.; Heils, A.; Kasper, J.M.; Sander, T. Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2007, 144, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Chen, Q.H.; Vilaythong, A.; Toney, G.M.; Noebels, J.L.; Aldrich, R.W. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 2005, 8, 1752–1759. [Google Scholar] [CrossRef]
- Pyott, S.J.; Duncan, R.K. Chapter Ten—BK Channels in the Vertebrate Inner Ear, in International Review of Neurobiology; Contet, C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 369–399. [Google Scholar]
- Kros, C.; Crawford, A. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J. Physiol. 1990, 421, 263–291. [Google Scholar] [CrossRef]
- Rohmann, K.N.; Wersinger, E.; Braude, J.P.; Pyott, S.J.; Fuchs, P.A. Activation of BK and SK Channels by Efferent Synapses on Outer Hair Cells in High-Frequency Regions of the Rodent Cochlea. J. Neurosci. 2015, 35, 1821–1830. [Google Scholar] [CrossRef]
- Kros, C.J.; Ruppersberg, J.P.; Rüsch, A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 1998, 394, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Pyott, S.J.; Meredith, A.L.; Fodor, A.A.; Vázquez, A.E.; Yamoah, E.N.; Aldrich, R.W. Cochlear Function in Mice Lacking the BK Channel α, β1, or β4 Subunits. J. Biol. Chem. 2007, 282, 3312–3324. [Google Scholar] [CrossRef] [PubMed]
- Rüttiger, L.; Sausbier, M.; Zimmermann, U.; Winter, H.; Braig, C.; Engel, J.; Knirsch, M.; Arntz, C.; Langer, P.; Hirt, B.; et al. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc. Natl. Acad. Sci. USA 2004, 101, 12922–12927. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S.; Sausbier, M.; Rüttiger, L.; Brandt, N.; Moeller, C.K.; Kindler, J.; Sausbier, U.; Zimmermann, U.; van Straaten, H.; Neuhuber, W.; et al. Critical role for cochlear hair cell BK channels for coding the temporal structure and dynamic range of auditory information for central auditory processing. FASEB J. 2012, 26, 3834–3843. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Jegla, T.J.; Wickenden, A.; Liu, Y.; Aldrich, R.W. Cloning and Functional Characterization of Novel Large Conductance Calcium-activated Potassium Channel β Subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 2000, 275, 6453–6461. [Google Scholar] [CrossRef] [PubMed]
- Uebele, V.N.; Lagrutta, A.; Wade, T.; Figueroa, D.J.; Liu, Y.; McKenna, E.; Austin, C.P.; Bennett, P.B.; Swanson, R. Cloning and Functional Expression of Two Families of β-Subunits of the Large Conductance Calcium-activated K+ Channel. J. Biol. Chem. 2000, 275, 23211–23218. [Google Scholar] [CrossRef]
- Wallner, M.; Meera, P.; Toro, L. Molecular basis of fast inactivation in voltage and Ca2activated K+ channels: A transmembrane β-subunit homolog. PNAS 1999, 96, 4137–4142. [Google Scholar] [CrossRef]
- Xia, X.-M.; Ding, J.P.; Lingle, C.J. Molecular Basis for the Inactivation of Ca2 and Voltage-Dependent BK Channels in Adrenal Chromaffin Cells and Rat Insulinoma Tumor Cells. J. Neurosci. 1999, 19, 5255–5264. [Google Scholar] [CrossRef]
- Scott, L.L.; Brecht, E.J.; Philpo, A.; Iyer, S.; Wu, N.S.; Mihic, S.J.; Aldrich, R.W.; Pierce, J.; Walton, J.P. A novel BK channel-targeted peptide suppresses sound evoked activity in the mouse inferior colliculus. Sci. Rep. 2017, 7, 42433. [Google Scholar] [CrossRef]
- Noh, W.; Pak, S.; Choi, G.; Yang, S.; Yang, S. Transient Potassium Channels: Therapeutic Targets for Brain Disorders. Front. Cell. Neurosci. Cell. Neurophysiol. 2019, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Grizel, A.V.; Glukhov, G.S.; Sokolova, O.S. Mechanisms of activation of voltage-gated potassium channels. Acta Nat. 2014, 6, 10–26. [Google Scholar] [CrossRef]
- Sokolova, O. Structure of cation channels, revealed by single particle electron microscopy. FEBS Lett. 2004, 564, 251–256. [Google Scholar] [CrossRef][Green Version]
- Coetzee, W.A.; Amarillo, Y.; Chiu, J.; Chow, A.; Lau, D.; McCormack, T.; Moreno, H.; Nadal, M.S.; Ozaita, A.; Pountney, D.; et al. Molecular diversity of K+ channels. Ann. N. Y. Acad. Sci. 1999, 868, 233–285. [Google Scholar] [CrossRef]
- Covarrubias, M.; Bhattacharji, A.; De Santiago-Castillo, J.A.; Dougherty, K.; Kaulin, Y.A.; Na-Phuket, T.R.; Wang, G. The neuronal Kv4 channel complex. Neurochem. Res. 2008, 33, 1558–1567. [Google Scholar] [CrossRef]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.-P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimmer, J.S.; et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef]
- Gebauer, M.; Isbrandt, D.; Sauter, K.; Callsen, B.; Nolting, A.; Pongs, O.; Bähring, R. N-type Inactivation Features of Kv4.2 Channel Gating. Biophys. J. 2004, 86, 210–223. [Google Scholar] [CrossRef]
- Hoffman, D.A.; Johnston, D. Downregulation of Transient K+ Channels in Dendrites of Hippocampal CA1 Pyramidal Neurons by Activation of PKA and PKC. J. Neurosci. 1998, 18, 3521–3528. [Google Scholar] [CrossRef]
- Brew, H.M.; Forsythe, I.D. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J. Neurosci. 1995, 15, 8011–8022. [Google Scholar] [CrossRef]
- Brew, H.M.; Forsythe, I.D. Systematic variation of potassium current amplitudes across the tonotopic axis of the rat medial nucleus of the trapezoid body. Hear. Res. 2005, 206, 116–132. [Google Scholar] [CrossRef]
- Grigg, J.J.; Brew, H.M.; Tempel, B.L. Differential expression of voltage-gated potassium channel genes in auditory nuclei of the mouse brainstem. Hear. Res. 2000, 140, 77–90. [Google Scholar] [CrossRef]
- Rusznák, Z.; Bakondi, G.; Pocsai, K.; Pór, A.; Kosztka, L.; Pál, B.; Nagy, D.; Szucs, G. Voltage-gated potassium channel (Kv) subunits expressed in the rat cochlear nucleus. J. Histochem. Cytochem. 2008, 56, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Leao, R.N.; Sun, H.; Svahn, K.; Berntson, A.; Youssoufian, M.; Paolini, A.G.; Fyffe, R.E.; Walmsley, B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J. Physiol. 2006, 571, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kaczmarek, L.K.; Perney, T.M. Localization of two high-threshold potassium channel subunits in the rat central auditory system. J. Comp. Neurol. 2001, 437, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Von Hehn, C.A.A.; Bhattacharjee, A.; Kaczmarek, L.K. Loss of Kv3.1 Tonotopicity and Alterations in cAMP Response Element-Binding Protein Signaling in Central Auditory Neurons of Hearing Impaired Mice. J. Neurosci. 2004, 24, 1936–1940. [Google Scholar] [CrossRef][Green Version]
- Brew, H.M.; Hallows, J.L.; Tempel, B.L. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J. Physiol. 2003, 548, 1–20. [Google Scholar] [CrossRef]
- Lu, Y.; Monsivais, P.; Tempel, B.L.; Rubel, E.W. Activity-dependent regulation of the potassium channel subunits Kv1.1 and Kv3.1. J. Comp. Neurol. 2004, 470, 93–106. [Google Scholar] [CrossRef]
- Macica, C.M.; von Hehn, C.A.; Wang, L.Y.; Ho, C.S.; Yokoyama, S.; Joho, R.H.; Kaczmarek, L.K. Modulation of the kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. J. Neurosci. 2003, 23, 1133–1141. [Google Scholar] [CrossRef]
- Adamson, C.L.; Reid, M.A.; Mo, Z.L.; Bowne-English, J.; Davis, R.L. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J. Comp. Neurol. 2002, 447, 331–350. [Google Scholar] [CrossRef]
- Dodson, D.; Barker, M.C.; Forsythe, I.D. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J. Neurosci. 2002, 22, 6953–6961. [Google Scholar] [CrossRef] [PubMed]
- Poveda, C.M.; Valero, M.L.; Pernia, M.; Alvarado, J.C.; Ryugo, D.K.; Merchan, M.A.; Juiz, J.M. Expression and Localization of Kv1.1 and Kv3.1b Potassium Channels in the Cochlear Nucleus and Inferior Colliculus after Long-Term Auditory Deafferentation. Brain Sci. 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Zettel, M.L.; Zhu, X.; O’Neill, W.E.; Frisina, R.D. Age-related Decline in Kv3.1b Expression in the Mouse Auditory Brainstem Correlates with Functional Deficits in the Medial Olivocochlear Efferent System. J. Assoc. Res. Otolaryngol. 2007, 8, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.R.; Pilati, N.; Balaram, P.; Large, C.H.; Kaczmarek, L.K.; Polley, D.B. Pharmacological modulation of Kv3.1 mitigates auditory midbrain temporal processing deficits following auditory nerve damage. Sci. Rep. 2017, 7, 17496. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.K.; Lee, S.Y.; Kim, D.; Joo, K.M.; Cha, C.I.; Yang, H.S.; Lee, W.B.; Chung, Y.H. Age-related changes in the distribution of Kv1.1 and Kv3.1 in rat cochlear nuclei. Neurol. Res. 2005, 27, 436–440. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazard, P.; Frisina, R.D.; Acosta, A.A.; Dasgupta, S.; Bauer, M.A.; Zhu, X.; Ding, B. Roles of Key Ion Channels and Transport Proteins in Age-Related Hearing Loss. Int. J. Mol. Sci. 2021, 22, 6158. https://doi.org/10.3390/ijms22116158
Bazard P, Frisina RD, Acosta AA, Dasgupta S, Bauer MA, Zhu X, Ding B. Roles of Key Ion Channels and Transport Proteins in Age-Related Hearing Loss. International Journal of Molecular Sciences. 2021; 22(11):6158. https://doi.org/10.3390/ijms22116158
Chicago/Turabian StyleBazard, Parveen, Robert D. Frisina, Alejandro A. Acosta, Sneha Dasgupta, Mark A. Bauer, Xiaoxia Zhu, and Bo Ding. 2021. "Roles of Key Ion Channels and Transport Proteins in Age-Related Hearing Loss" International Journal of Molecular Sciences 22, no. 11: 6158. https://doi.org/10.3390/ijms22116158
APA StyleBazard, P., Frisina, R. D., Acosta, A. A., Dasgupta, S., Bauer, M. A., Zhu, X., & Ding, B. (2021). Roles of Key Ion Channels and Transport Proteins in Age-Related Hearing Loss. International Journal of Molecular Sciences, 22(11), 6158. https://doi.org/10.3390/ijms22116158





