BMI-1 Expression Heterogeneity in Endometriosis-Related and Non-Endometriotic Ovarian Carcinoma
Abstract
1. Introduction
2. Results
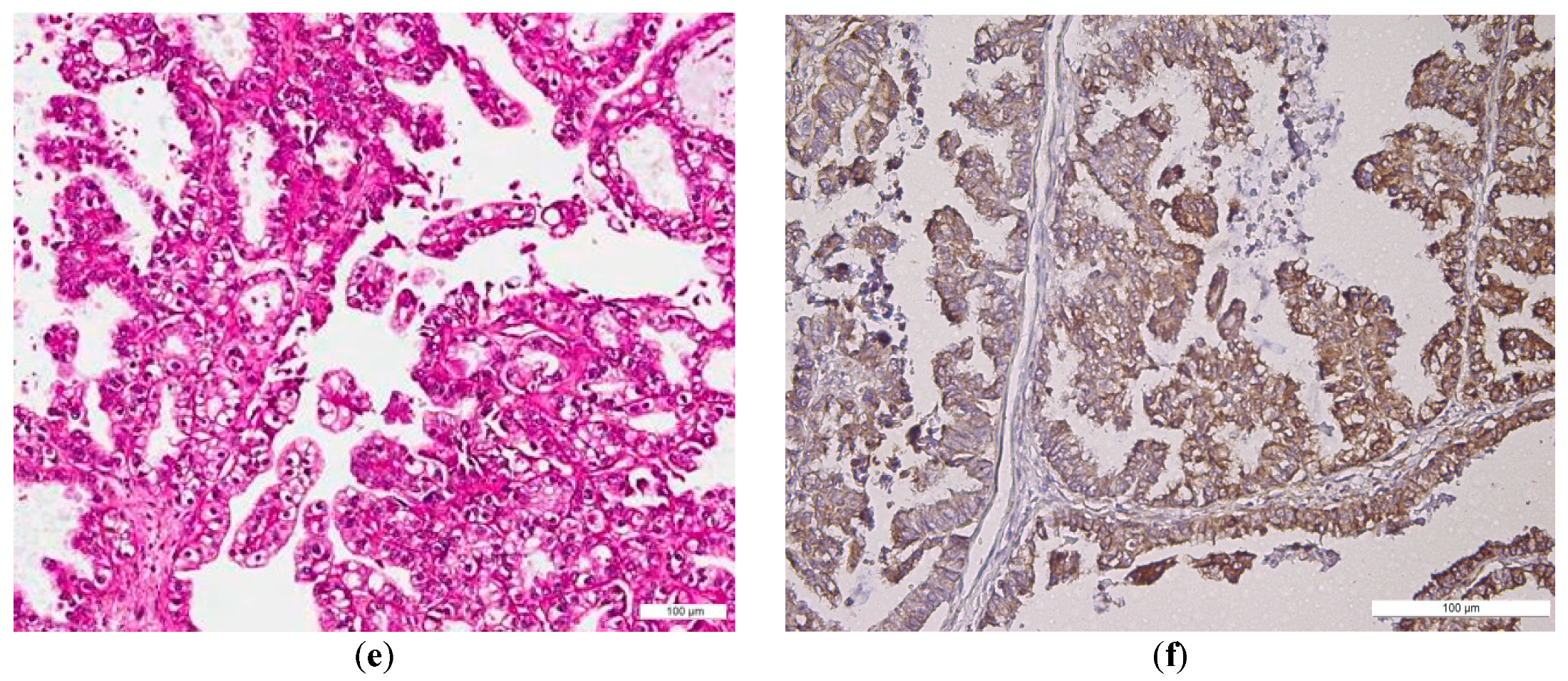
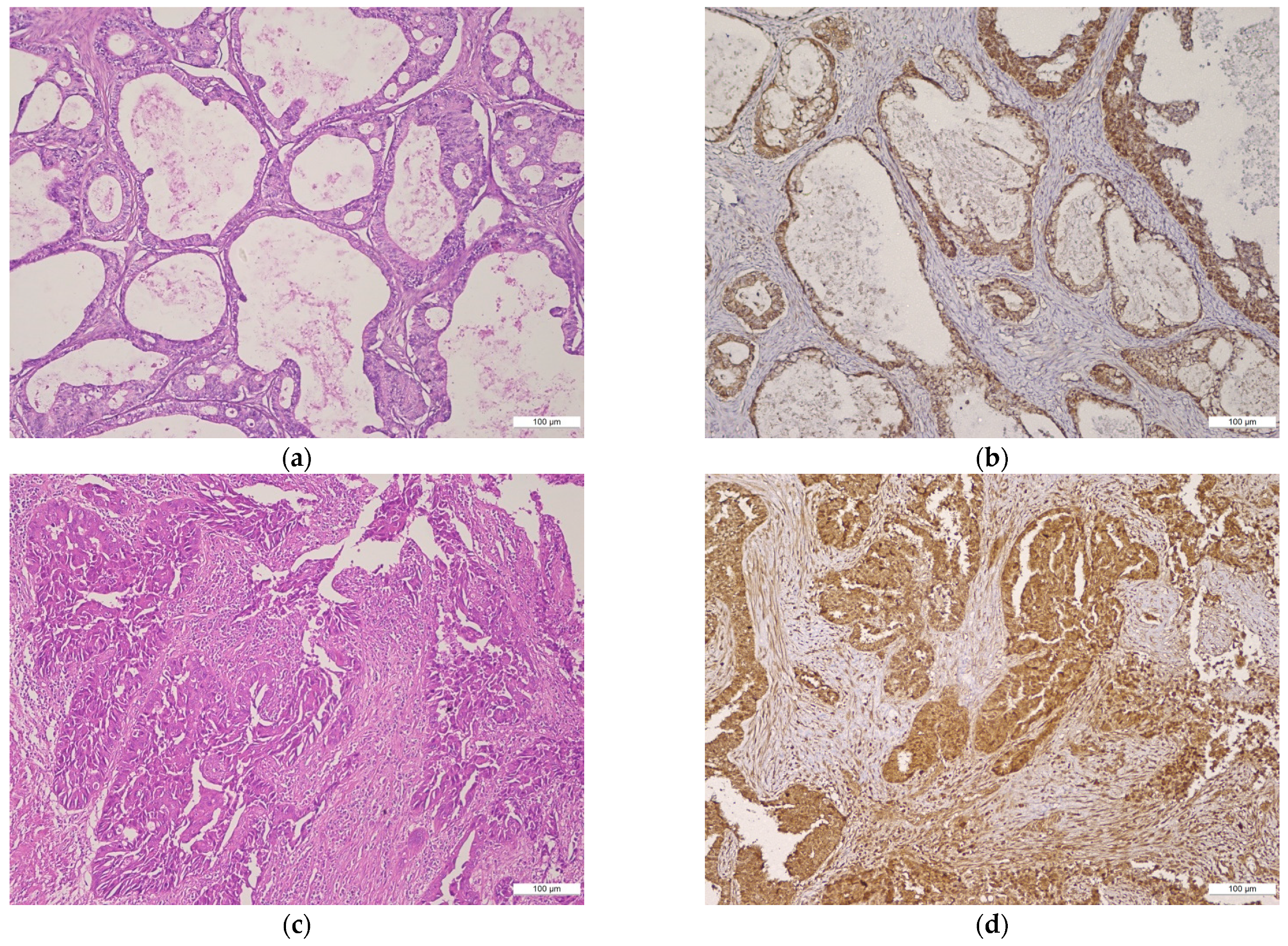
2.1. BMI-1 Expression—Qualitative Assessment
2.2. BMI Expression—Semi-Quantitative Assessment
2.3. Relationship between BMI-1 Epithelial and Stromal Expression, and Clinicopathological Parameters in EOC
2.4. Relationship between BMI-1 Epithelial and Stromal Expression and Clinicopathological Parameters in NEOC
3. Discussion
4. Materials and Methods
4.1. Patients
4.1.1. Clinicopathological and Tumor Serum Marker Profile of the Study Cohort
4.1.2. Clinicopathological and Tumor Serum Marker Profile of the EOC and NEOC Groups
4.2. Immunohistochemistry (IHC)
4.3. Semi-Quantitative Assessment
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wei, J.-J.; William, J.; Bulun, S. Endometriosis and Ovarian Cancer: A Review of Clinical, Pathologic, and Molecular Aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Ban Frangež, H.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro Omnibus, Omnes pro Uno: A Novel, Evidence-Based, Unifying Theory for the Pathogenesis of Endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Chen, C.-C.; Tsai, E.-M.; Er, T.-K. Endometriosis-Associated Ovarian Cancer: What Have We Learned so Far? Clin. Chim. Acta 2019, 493, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, A.; Augusto, K.; Portela, M.; Sucupira, L.C.; Oliveira, L.A.; Pouchaim, A.J.; Mesquita Nóbrega, L.R.; de Magalhães, T.F.; Sobreira, L.R. Endometriosis and Ovarian Cancer: An Integrative Review (Endometriosis and Ovarian Cancer). APJCP 2017, 18. [Google Scholar] [CrossRef]
- Vargas, A.N. Natural History of Ovarian Cancer. Ecancermedicalscience 2014, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Motohara, T.; Yoshida, G.J.; Katabuchi, H. The Hallmarks of Ovarian Cancer Stem Cells and Niches: Exploring Their Harmonious Interplay in Therapy Resistance. Semin. Cancer Biol. 2021, S1044579X21000997. [Google Scholar] [CrossRef]
- Pieterse, Z.; Amaya-Padilla, M.A.; Singomat, T.; Binju, M.; Madjid, B.D.; Yu, Y.; Kaur, P. Ovarian Cancer Stem Cells and Their Role in Drug Resistance. Int. J. Biochem. Cell Biol. 2019, 106, 117–126. [Google Scholar] [CrossRef]
- Muinao, T.; Deka Boruah, H.P.; Pal, M. Diagnostic and Prognostic Biomarkers in Ovarian Cancer and the Potential Roles of Cancer Stem Cells—An Updated Review. Exp. Cell Res. 2018, 362, 1–10. [Google Scholar] [CrossRef]
- Honig, A.; Weidler, C.; Häusler, S.; Krockenberger, M.; Buchholz, S.; Köster, F.; Segerer, S.E.; Dietl, J.; Engel, J.B. Overexpression of Polycomb Protein BMI-1 in Human Specimens of Breast, Ovarian, Endometrial and Cervical Cancer. Anticancer Res. 2010, 30, 1559–1564. [Google Scholar]
- Qin, Z.-K.; Yang, J.-A.; Ye, Y.-L.; Zhang, X.; Xu, L.-H.; Zhou, F.-J.; Han, H.; Liu, Z.-W.; Song, L.-B.; Zeng, M.-S. Expression of Bmi-1 Is a Prognostic Marker in Bladder Cancer. BMC Cancer 2009, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Beà, S.; Tort, F.; Pinyol, M.; Puig, X.; Hernández, L.; Hernández, S.; Fernandez, P.L.; van Lohuizen, M.; Colomer, D.; Campo, E. BMI-1 Gene Amplification and Overexpression in Hematological Malignancies Occur Mainly in Mantle Cell Lymphomas. Cancer Res. 2001, 61, 2409–2412. [Google Scholar] [PubMed]
- van Kemenade, F.J.; Raaphorst, F.M.; Blokzijl, T.; Fieret, E.; Hamer, K.M.; Satijn, D.P.; Otte, A.P.; Meijer, C.J. Coexpression of BMI-1 and EZH2 Polycomb-Group Proteins Is Associated with Cycling Cells and Degree of Malignancy in B-Cell Non-Hodgkin Lymphoma. Blood 2001, 97, 3896–3901. [Google Scholar] [CrossRef] [PubMed]
- Raaphorst, F.M. Deregulated Expression of Polycomb-Group Oncogenes in Human Malignant Lymphomas and Epithelial Tumors. Hum. Mol. Genet. 2005, 14 (Suppl. 1), R93–R100. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Hu, B.; Li, T.; Ma, J.; Alam, G.; Gunning, W.T.; Ding, H.-F. Bmi-1 Is Essential for the Tumorigenicity of Neuroblastoma Cells. Am. J. Pathol. 2007, 170, 1370–1378. [Google Scholar] [CrossRef]
- Crea, F.; Duhagon Serrat, M.A.; Hurt, E.M.; Thomas, S.B.; Danesi, R.; Farrar, W.L. BMI1 Silencing Enhances Docetaxel Activity and Impairs Antioxidant Response in Prostate Cancer. Int. J. Cancer 2011, 128, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.F.; Albinger-Hegyi, A.; Soltermann, A.; Roessle, M.; Graf, N.; Haerle, S.K.; Holzmann, D.; Moch, H.; Hegyi, I. Expression Patterns of Bmi-1 and P16 Significantly Correlate with Overall, Disease-Specific, and Recurrence-Free Survival in Oropharyngeal Squamous Cell Carcinoma. Cancer 2011, 117, 4659–4670. [Google Scholar] [CrossRef]
- Song, L.-B.; Zeng, M.-S.; Liao, W.-T.; Zhang, L.; Mo, H.-Y.; Liu, W.-L.; Shao, J.-Y.; Wu, Q.-L.; Li, M.-Z.; Xia, Y.-F.; et al. Bmi-1 Is a Novel Molecular Marker of Nasopharyngeal Carcinoma Progression and Immortalizes Primary Human Nasopharyngeal Epithelial Cells. Cancer Res. 2006, 66, 6225–6232. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, S.Y.; Jeong, S.-H.; Kim, S.Y.; Moon, S.K.; Joo, J.H.; Lee, Y.; Choe, I.S.; Kim, J.W. Overexpression of Bmi-1 Oncoprotein Correlates with Axillary Lymph Node Metastases in Invasive Ductal Breast Cancer. Breast 2004, 13, 383–388. [Google Scholar] [CrossRef]
- Guo, B.-H.; Feng, Y.; Zhang, R.; Xu, L.-H.; Li, M.-Z.; Kung, H.-F.; Song, L.-B.; Zeng, M.-S. Bmi-1 Promotes Invasion and Metastasis, and Its Elevated Expression Is Correlated with an Advanced Stage of Breast Cancer. Mol. Cancer 2011, 10, 10. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Li, J.; Guo, W.-J. Expression and Clinicopathological Significance of Mel-18 and Bmi-1 MRNA in Gastric Carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 143. [Google Scholar] [CrossRef]
- Proctor, E.; Waghray, M.; Lee, C.J.; Heidt, D.G.; Yalamanchili, M.; Li, C.; Bednar, F.; Simeone, D.M. Bmi1 Enhances Tumorigenicity and Cancer Stem Cell Function in Pancreatic Adenocarcinoma. PLoS ONE 2013, 8, e55820. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Tsujimura, T.; Tao, L.; Kamikonya, N.; Fujiwara, Y. The Oncoprotein and Stem Cell Renewal Factor BMI1 Associates with Poor Clinical Outcome in Oesophageal Cancer Patients Undergoing Preoperative Chemoradiotherapy. BMC Cancer 2012, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Vonlanthen, S.; Heighway, J.; Altermatt, H.J.; Gugger, M.; Kappeler, A.; Borner, M.M.; van Lohuizen, M.; Betticher, D.C. The Bmi-1 Oncoprotein Is Differentially Expressed in Non-Small Cell Lung Cancer and Correlates with INK4A-ARF Locus Expression. Br. J. Cancer 2001, 84, 1372–1376. [Google Scholar] [CrossRef]
- Breuer, R.H.J.; Snijders, P.J.F.; Sutedja, G.T.; Sewalt, R.G.A.B.; Otte, A.P.; Postmus, P.E.; Meijer, C.J.L.M.; Raaphorst, F.M.; Smit, E.F. Expression of the P16(INK4a) Gene Product, Methylation of the P16(INK4a) Promoter Region and Expression of the Polycomb-Group Gene BMI-1 in Squamous Cell Lung Carcinoma and Premalignant Endobronchial Lesions. Lung Cancer 2005, 48, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Elkashty, O.A.; Ashry, R.; Tran, S.D. Head and Neck Cancer Management and Cancer Stem Cells Implication. Saudi Dent. J. 2019, 31, 395–416. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, I.M.; Puntervoll, H.E.; Otte, A.P.; Akslen, L.A. Loss of BMI-1 Expression Is Associated with Clinical Progress of Malignant Melanoma. Mod. Pathol. 2008, 21, 583–590. [Google Scholar] [CrossRef]
- Sedassari, B.T.; Rodrigues, M.F.S.D.; Mariano, F.V.; Altemani, A.; Nunes, F.D.; Sousa, S. The Stem Cell Marker Bmi-1 Is Sensitive in Identifying Early Lesions of Carcinoma Ex Pleomorphic Adenoma. Medicine 2015, 94, e1035. [Google Scholar] [CrossRef]
- Mihara, K.; Chowdhury, M.; Nakaju, N.; Hidani, S.; Ihara, A.; Hyodo, H.; Yasunaga, S.; Takihara, Y.; Kimura, A. Bmi-1 Is Useful as a Novel Molecular Marker for Predicting Progression of Myelodysplastic Syndrome and Patient Prognosis. Blood 2006, 107, 305–308. [Google Scholar] [CrossRef]
- Jiao, K.; Jiang, W.; Zhao, C.; Su, D.; Zhang, H. Bmi-1 in Gallbladder Carcinoma: Clinicopathology and Mechanism of Regulation of Human Gallbladder Carcinoma Proliferation. Oncol. Lett. 2019, 18, 1365–1371. [Google Scholar] [CrossRef]
- Douglas, D.; Hsu, J.H.-R.; Hung, L.; Cooper, A.; Abdueva, D.; van Doorninck, J.; Peng, G.; Shimada, H.; Triche, T.J.; Lawlor, E.R. BMI-1 Promotes Ewing Sarcoma Tumorigenicity Independent of CDKN2A Repression. Cancer Res. 2008, 68, 6507–6515. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.-C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.-M.; Nephew, K.P. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Bhattacharyya, S.; Szabolcs, A.; Rodriguez-Aguayo, C.; Jennings, N.B.; Lopez-Berestein, G.; Mukherjee, P.; Sood, A.K.; Bhattacharya, R. Enhancing Chemotherapy Response with Bmi-1 Silencing in Ovarian Cancer. PLoS ONE 2011, 6, e17918. [Google Scholar] [CrossRef] [PubMed]
- Vathipadiekal, V.; Saxena, D.; Mok, S.C.; Hauschka, P.V.; Ozbun, L.; Birrer, M.J. Identification of a Potential Ovarian Cancer Stem Cell Gene Expression Profile from Advanced Stage Papillary Serous Ovarian Cancer. PLoS ONE 2012, 7, e29079. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, F.B.; Sui, G.J.; Jin, X.M. Bmi-1 SiRNA Inhibited Ovarian Cancer Cell Line Growth and Decreased Telomerase Activity. Br. J. Biomed. Sci. 2012, 69, 62–66. [Google Scholar] [CrossRef] [PubMed]
- He, Q.-Z.; Luo, X.-Z.; Wang, K.; Zhou, Q.; Ao, H.; Yang, Y.; Li, S.-X.; Li, Y.; Zhu, H.-T.; Duan, T. Isolation and Characterization of Cancer Stem Cells from High-Grade Serous Ovarian Carcinomas. Cell Physiol. Biochem. 2014, 33, 173–184. [Google Scholar] [CrossRef]
- Dey, A.; Xiong, X.; Crim, A.; Dwivedi, S.K.D.; Mustafi, S.B.; Mukherjee, P.; Cao, L.; Sydorenko, N.; Baiazitov, R.; Moon, Y.-C.; et al. Evaluating the Mechanism and Therapeutic Potential of PTC-028, a Novel Inhibitor of BMI-1 Function in Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Shishido, A.; Mori, S.; Yokoyama, Y.; Hamada, Y.; Minami, K.; Qian, Y.; Wang, J.; Hirose, H.; Wu, X.; Kawaguchi, N.; et al. Mesothelial Cells Facilitate Cancer Stem-like Properties in Spheroids of Ovarian Cancer Cells. Oncol. Rep. 2018, 40, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-F.; He, W.-P.; Cai, M.-Y.; He, L.-R.; Luo, J.-H.; Deng, H.-X.; Guan, X.-Y.; Zeng, M.-S.; Zeng, Y.-X.; Xie, D. Intensive Expression of Bmi-1 Is a New Independent Predictor of Poor Outcome in Patients with Ovarian Carcinoma. BMC Cancer 2010, 10, 133. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Nicoloso, M.; Arvizo, R.; Wang, E.; Cortez, A.; Rossi, S.; Calin, G.A.; Mukherjee, P. MiR-15a and MiR-16 Control Bmi-1 Expression in Ovarian Cancer. Cancer Res. 2009, 69, 9090–9095. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Sun, B.-L.; Tian, S.-X.; Li, L.; Zhao, Y.-C.; Shi, P.-P. MicroRNA-132 Reverses Cisplatin Resistance and Metastasis in Ovarian Cancer by the Targeted Regulation on Bmi-1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3635–3644. [Google Scholar] [CrossRef] [PubMed]
- Abd El hafez, A.; El-Hadaad, H.A. Immunohistochemical Expression and Prognostic Relevance of Bmi-1, a Stem Cell Factor, in Epithelial Ovarian Cancer. Ann. Diagn. Pathol. 2014, 18, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Iseki, C.; Kikuchi, M.; Miyakawa, K.; Yoshizaki, M.; Yoshioka, H.; Watanabe, J. Bmi-1 Immunohistochemical Expression in Endometrial Carcinoma Is Correlated with Prognostic Activity. Medicina 2020, 56, 72. [Google Scholar] [CrossRef]
- Silva, J.; García, V.; García, J.M.; Peña, C.; Domínguez, G.; Díaz, R.; Lorenzo, Y.; Hurtado, A.; Sánchez, A.; Bonilla, F. Circulating Bmi-1 MRNA as a Possible Prognostic Factor for Advanced Breast Cancer Patients. Breast Cancer Res. 2007, 9, R55. [Google Scholar] [CrossRef]
- Choi, Y.J.; Choi, Y.L.; Cho, E.Y.; Shin, Y.K.; Sung, K.W.; Hwang, Y.K.; Lee, S.J.; Kong, G.; Lee, J.E.; Kim, J.S.; et al. Expression of Bmi-1 Protein in Tumor Tissues Is Associated with Favorable Prognosis in Breast Cancer Patients. Breast Cancer Res. Treat. 2009, 113, 83–93. [Google Scholar] [CrossRef]
- Zhang, F.; Sui, L.; Xin, T. Correlations of BMI-1 Expression and Telomerase Activity in Ovarian Cancer Tissues. Exp. Oncol. 2008, 30, 70–74. [Google Scholar]
- Zhang, F.B.; Sui, L.H.; Xin, T. Correlation of Bmi-1 Expression and Telomerase Activity in Human Ovarian Cancer. Br. J. Biomed. Sci. 2008, 65, 172–177. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Wang, Y.; Gao, X.; Wang, W.; Liu, H.; He, H.; Liang, Y.; Pan, K.; Wu, H.; et al. Relationship of Tumor Marker CA125 and Ovarian Tumor Stem Cells: Preliminary Identification. J. Ovarian Res. 2015, 8, 19. [Google Scholar] [CrossRef]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian Carcinoma Subtypes Are Different Diseases: Implications for Biomarker Studies. PLoS Med. 2008, 5, e232. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, I.M.; Halvorsen, O.J.; Collett, K.; Stefansson, I.M.; Straume, O.; Haukaas, S.A.; Salvesen, H.B.; Otte, A.P.; Akslen, L.A. EZH2 Expression Is Associated with High Proliferation Rate and Aggressive Tumor Subgroups in Cutaneous Melanoma and Cancers of the Endometrium, Prostate, and Breast. J. Clin. Oncol. 2006, 24, 268–273. [Google Scholar] [CrossRef] [PubMed]


| BMI-1 | EOC | NEOC | ||||
|---|---|---|---|---|---|---|
| High Score/ Positive Reaction | Low Score/ Negative Reaction | p Value | High Score/ Positive Reaction | Low Score/ Negative Reaction | p Value | |
| Epithelial tumor cells | 5 (26.31%) | 14 (73.68%) | 0.04 | 26 (92.85%) | 2 (7.14%) | 0.001 |
| Stromal cells | 11 (57.89%) | 8 (42.10%) | 23 (82.14%) | 5 (17.85%) | ||
| Clinicopathological Characteristics | # | Tumor Cells BMI-1 | p Value | Stromal BMI-1 | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Score | High Score | Negative Reaction | Positive Reaction | ||||||||
| # | % | # | % | # | % | # | % | ||||
| Age | |||||||||||
| <55 age | 8 | 5 | 62.5 | 3 | 37.5 | 0.34 | 4 | 50 | 4 | 50 | 0.55 |
| ≥55 age | 11 | 9 | 81.82 | 2 | 18.18 | 4 | 36.36 | 7 | 63.64 | ||
| Tumor stage | |||||||||||
| 1 | 4 | 4 | 100 | 0 | 0 | 0.48 | 1 | 25 | 3 | 75 | 0.55 |
| 2 | 6 | 4 | 66.66 | 2 | 33.33 | 3 | 50 | 3 | 50 | ||
| 3 | 8 | 5 | 62.50 | 3 | 37.50 | 3 | 37.50 | 5 | 62.50 | ||
| 4 | 1 | 1 | 100 | 0 | 0 | 1 | 100 | 0 | 0 | ||
| Tumor grade | |||||||||||
| I/II | 7 | 7 | 100 | 0 | 0 | 0.04 | 4 | 57.14 | 3 | 42.85 | 0.31 |
| III | 12 | 7 | 58.33 | 5 | 41.66 | 4 | 33.33 | 8 | 66.66 | ||
| Histological subtype | |||||||||||
| LGSC | 0 | 0 | 0 | 0 | 0 | 0.78 | 0 | 0 | 0 | 0 | 0.93 |
| LGEC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| COC | 4 | 3 | 75 | 1 | 25 | 1 | 25 | 3 | 75 | ||
| MOC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| HGSC | 3 | 1 | 33.33 | 2 | 66.66 | 1 | 33.33 | 2 | 66.66 | ||
| HGEC | 8 | 6 | 75 | 2 | 25 | 5 | 62.50 | 3 | 37.50 | ||
| Undifferentiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Mixed | 4 | 4 | 100 | 0 | 0 | 1 | 25 | 3 | 75 | ||
| Type | |||||||||||
| Type I | 4 | 3 | 75 | 1 | 25 | 0.94 | 1 | 25 | 3 | 75 | 0.57 |
| Type II | 15 | 11 | 73.33 | 4 | 26.67 | 7 | 46.67 | 8 | 53.33 | ||
| Residual disease | |||||||||||
| NED/<1 cm | 6 | 5 | 83.33 | 1 | 16.67 | 0.51 | 2 | 33.33 | 4 | 66.67 | 0.59 |
| ≥1 cm | 13 | 9 | 69.23 | 4 | 30.77 | 6 | 46.15 | 7 | 53.85 | ||
| CA 125—median value | |||||||||||
| <1201.5 U/mL | 10 | 8 | 80 | 2 | 20 | 0.51 | 2 | 20 | 8 | 80 | 0.03 |
| ≥1201.5 U/mL | 9 | 6 | 66.67 | 3 | 33.33 | 6 | 66.67 | 3 | 33.33 | ||
| Clinicopathological Characteristics | # | Tumor Cells BMI-1 | p Value | Stromal BMI-1 | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Score | High Score | Negative Reaction | Positive Reaction | ||||||||
| # | % | # | % | # | % | # | % | ||||
| Age | |||||||||||
| <55 age | 14 | 1 | 7.14 | 13 | 92.86 | 0.30 | 2 | 14.29 | 12 | 85.71 | 0.62 |
| ≥55 age | 14 | 1 | 7.14 | 13 | 92.86 | 3 | 21.43 | 11 | 78.57 | ||
| Tumor stage | |||||||||||
| 1 | 13 | 1 | 7.69 | 12 | 92.30 | 0.91 | 3 | 23.07 | 10 | 76.92 | 0.71 |
| 2 | 5 | 0 | 0 | 5 | 100 | 0 | 0 | 5 | 100 | ||
| 3 | 10 | 1 | 10 | 9 | 90 | 2 | 20 | 8 | 80 | ||
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Tumor grade | |||||||||||
| I/II | 15 | 0 | 0 | 15 | 100 | 0.11 | 3 | 20 | 12 | 80 | 0.75 |
| III | 13 | 2 | 15.38 | 11 | 84.61 | 2 | 15.38 | 11 | 84.61 | ||
| Histological subtype | |||||||||||
| LGSC | 4 | 0 | 0 | 4 | 100 | 0.002 | 0 | 0 | 4 | 100 | 0.04 |
| LGEC | 5 | 0 | 0 | 5 | 100 | 3 | 60 | 2 | 40 | ||
| COC | 5 | 0 | 0 | 5 | 100 | 0 | 0 | 5 | 100 | ||
| MOC | 5 | 1 | 20 | 4 | 80 | 1 | 20 | 4 | 80 | ||
| HGSC | 5 | 0 | 0 | 5 | 100 | 0 | 0 | 5 | 100 | ||
| HGEC | 3 | 0 | 0 | 3 | 100 | 0 | 0 | 3 | 100 | ||
| Undifferentiated | 1 | 1 | 100 | 0 | 0 | 1 | 100 | 0 | 0 | ||
| Mixed | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Type | |||||||||||
| Type I | 19 | 1 | 5.26 | 18 | 94.74 | 0.57 | 4 | 21.05 | 15 | 78.95 | 0.43 |
| Type II | 9 | 1 | 11.11 | 8 | 88.89 | 0 | 0 | 9 | 100 | ||
| Residual disease | |||||||||||
| NED/<1 cm | 16 | 1 | 6.25 | 15 | 93.75 | 0.83 | 2 | 12.5 | 14 | 87.5 | 0.72 |
| ≥1 cm | 12 | 1 | 8.33 | 11 | 91.67 | 1 | 8.33 | 11 | 91.67 | ||
| CA 125—median value | |||||||||||
| <101 | 14 | 1 | 7.14 | 13 | 92.86 | 1 | 4 | 28.57 | 10 | 71.43 | 0.13 |
| ≥101 | 14 | 1 | 7.14 | 13 | 92.30 | 1 | 7.14 | 13 | 92.86 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozneanu, L.; Balan, R.A.; Păvăleanu, I.; Giuşcă, S.E.; Căruntu, I.-D.; Amalinei, C. BMI-1 Expression Heterogeneity in Endometriosis-Related and Non-Endometriotic Ovarian Carcinoma. Int. J. Mol. Sci. 2021, 22, 6082. https://doi.org/10.3390/ijms22116082
Lozneanu L, Balan RA, Păvăleanu I, Giuşcă SE, Căruntu I-D, Amalinei C. BMI-1 Expression Heterogeneity in Endometriosis-Related and Non-Endometriotic Ovarian Carcinoma. International Journal of Molecular Sciences. 2021; 22(11):6082. https://doi.org/10.3390/ijms22116082
Chicago/Turabian StyleLozneanu, Ludmila, Raluca Anca Balan, Ioana Păvăleanu, Simona Eliza Giuşcă, Irina-Draga Căruntu, and Cornelia Amalinei. 2021. "BMI-1 Expression Heterogeneity in Endometriosis-Related and Non-Endometriotic Ovarian Carcinoma" International Journal of Molecular Sciences 22, no. 11: 6082. https://doi.org/10.3390/ijms22116082
APA StyleLozneanu, L., Balan, R. A., Păvăleanu, I., Giuşcă, S. E., Căruntu, I.-D., & Amalinei, C. (2021). BMI-1 Expression Heterogeneity in Endometriosis-Related and Non-Endometriotic Ovarian Carcinoma. International Journal of Molecular Sciences, 22(11), 6082. https://doi.org/10.3390/ijms22116082






