Abstract
The TWIK-related spinal cord potassium channel (TRESK) is encoded by KCNK18, and variants in this gene have previously been associated with susceptibility to familial migraine with aura (MIM #613656). A single amino acid substitution in the same protein, p.Trp101Arg, has also been associated with intellectual disability (ID), opening the possibility that variants in this gene might be involved in different disorders. Here, we report the identification of KCNK18 biallelic missense variants (p.Tyr163Asp and p.Ser252Leu) in a family characterized by three siblings affected by mild-to-moderate ID, autism spectrum disorder (ASD) and other neurodevelopment-related features. Functional characterization of the variants alone or in combination showed impaired channel activity. Interestingly, Ser252 is an important regulatory site of TRESK, suggesting that alteration of this residue could lead to additive downstream effects. The functional relevance of these mutations and the observed co-segregation in all the affected members of the family expand the clinical variability associated with altered TRESK function and provide further insight into the relationship between altered function of this ion channel and human disease.
Keywords:
KCNK18; TRESK; K2P; intellectual disability; potassium channel; autism spectrum disorder; ASD 1. Introduction
KCNK18 encodes the TWIK-Related Spinal cord K+ channel (TRESK), a member of the two-pore domain (K2P) potassium channel family. K2P channels count 15 members, encoded by different KCNK genes [1]. At the protein level, these channels have different names according to their structural and functional similarities. They are classified into the TWIK, TREK, TASK, TALK, THIK and TRESK subfamilies [2].
The molecular architecture of the TRESK channel resembles other K2P channel subtypes except that it has a longer intracellular loop between the second and the third transmembrane domains and a shorter C-terminal tail. The intracellular loop contains fundamental modulatory sites such as phosphorylation, dephosphorylation and calcineurin binding motifs. Under resting conditions, murine TRESK is phosphorylated and maintains an inactive conformation, but, after a calcium signal, the channel is rapidly activated following dephosphorylation by the calcium-dependent phosphatase calcineurin [3,4]. To allow this process, calcineurin must bind to the channel through interaction with its non-catalytic sites, helped by the regulatory protein, 14-3-3. In humans, the process is also supported by protein kinase C (PKC) [3]. The switch between the active and resting states has been intensely investigated [4] and it is mediated by the phosphorylation of TRESK at two different regulatory regions, the 14-3-3 binding site (Serine 252, Ser252, hereafter) and three adjacent serine residues (Ser262, Ser264 and Ser267, collectively named serine cluster, hereafter). These residues are conserved in murine TRESK as Ser264, Ser274, Ser276 and Ser279.
The activated calmodulin complex stimulates the binding of calcineurin to the TRESK intracellular loop, thus promoting dephosphorylation of the two regulatory residues (Ser252 and the serine cluster) and the activation of the channel. After the decay of the calcium signal, channel activity is inhibited by the restoring of the phosphorylated state by protein kinase A, which acts at the level of Ser252, and microtubule affinity-regulating (MARK) kinases, which mediate phosphorylation at the level of the serine cluster, supported by 14-3-3 proteins [4].
Interestingly, a high basal activity and reduced response to calcium have been reported in a study using a murine TRESK mutant characterized by a constitutively dephosphorylated state of the serine cluster [4]. Remarkably, prolonged phosphorylation of this motif has been shown to cause reduced responsiveness of the channel and low basal activity [4,5], further documenting the complex modulatory process controlling TRESK function.
KCNK18 expression in the dorsal root (DRG) and trigeminal (TG) ganglia [6] led to a proposed role for this channel in a variety of pain pathways [7]; moreover, an involvement in typical migraine with aura has been suggested by different authors [8,9,10,11]. Despite the expression of KCNK18 in the basal ganglia, its mRNA is detectable also in other brain compartments, including the hypothalamus, frontal cortex, cortex, anterior cingulate cortex, hippocampus, spinal cord and substantia nigra (www.gtexportal.org (accessed on 22 April 2021)), suggesting a wider involvement of the channel in physiological brain function. Interestingly, recent studies have also reported KCNK18 variants in patients with developmental delay (DD) and intellectual disability (ID), with migraine as an associated feature in some cases [12,13]. Here, we describe the identification of a family with three siblings affected by mild to moderate ID, seizures, and autistic-like behavior with different degrees of severity, carrying biallelic KCNK18 variants, supporting a possible involvement of the gene in neurodevelopmental disorders (NDD).
2. Results
2.1. Clinical Features of the Three Affected Siblings
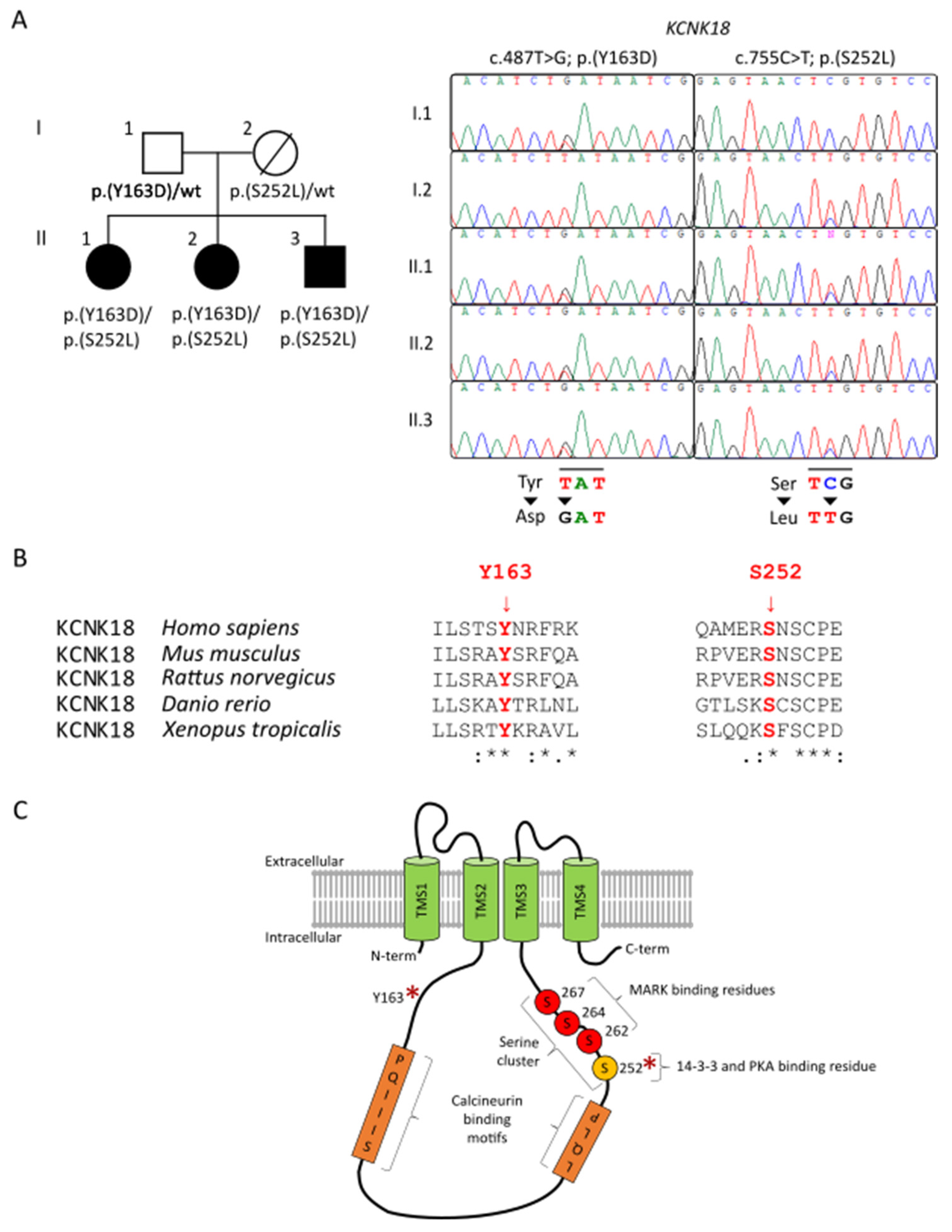
A family of European origins, with three siblings affected by an unclassified NDD, was studied (Figure 1A). The parents had no history of NDD or of any notable diseases, except for a carcinoma in the mother. The first proband was a 26-year-old female at the time of the last evaluation. Born at term after cesarean delivery, the proband showed a delay in the acquisition of developmental milestones, with the first phonemes at 18 months, walking at 24 months and sphincter control at 5 years. Mild ID was reported (total IQ—64, verbal IQ—60, performance IQ—70), with difficulties in calculation and inability to recognize the value of money. Poor language, dysarthria and attention deficit were observed. Moreover, partial temporal–spatial disorientation, poor criticism, and judgment with limitation of the possibilities of self-determination were reported. The support from a caretaker was required for everyday activity and for self-care. She suffered frequent (once a week) frontal cephalalgy. At clinical examination, the behaviour was uncooperative and restless. Even if autism spectrum disorder was not formally assessed using the Autism Diagnostic Observation Schedule (ADOS-2), nor using other behavioural tests, the patient showed an important impairment of relationships that, combined with the other symptoms, led the physician to include her in the autism spectrum.

Figure 1.
Pedigree, conservation, and graphical representation of the KCNK18 variants. (A). Pedigree of the family and Sanger sequencing chromatograms documenting co-segregation between compound heterozygosity for the two missense changes in KCNK18 and clinical traits transmitted in the family. (B). Multiple alignments of the amino acid stretches flanking the affected residues in TRESK orthologs showing conservation of both mutated residues (in red). Residues are referred to human KCNK18, NM_181840.1. (C). Schematic topology of the human K2P TRESK channel, showing location of Tyr163 (Y163) and Ser252 (S252) (red asterisks). Human TRESK is composed of 384 amino acid residues and shows the most characteristic features of K2P channels, including four transmembrane helices, two pore-forming domains, and intracellular N- and C-termini. A peculiar structural feature of TRESK (compared to other K2P channels) is the long intracellular loop between the second and third transmembrane segments (TMS), and the relatively short C-terminal tail [3]. In the image, representative domains are shown: PQIIIS and LQLP are calcineurin-binding motifs [5,17], while the serine cluster and Ser252 are indicated by the letter S and are, respectively, MARK kinases and 14-3-3 and PKA binding motifs.
The second born proband was a 19-year-old female diagnosed with a mild ID (total IQ—56, verbal IQ—47, performance IQ—76). The patient showed compromised social relations that, as with her sister, included the patient into a clinical diagnosis of autism spectrum disorder. Specific difficulties in the logical-mathematical area were reported, together with the inability to remember new concepts. The support from a second person was necessary for everyday activity. An episode of epileptic seizure was reported and supported by electroencephalography (EEG), which showed comitiality.
The last born was a 16-year-old male affected by dyslexia and difficulties in learning, but no further information were available.
The social-economic situation of the family was also taken into account, to understand if the impaired behaviour of the patients could be caused by a combination of genetic and environmental factors [14,15]; no noteworthy situation was identified, except for a worsening of the already poor social behaviour of the patients after the death of their mother.
2.2. Genomic and Structural Analyses
The patients were enrolled in the Autism Sequencing Consortium (ASC) project [16], and a WES of the whole family members performed. In the three affected members, the analysis led to the identification of compound heterozygosity for two missense changes (c.487T > G, p.Tyr163Asp, paternally inherited; c.755C > T, p.Ser252Leu, maternally inherited) (Figure 1A). Both variants affected residues that are highly conserved among orthologs (Figure 1B) and were predicted to have a disruptive impact on protein function (Table S1). Variant p.(Tyr163Asp) had not previously been reported in GnomAD database (ver 2.1.1) (www.gnomad.broadinstitute.org (accessed on 22 April 2021)), while variant p.(Ser252Leu) had an allelic frequency of 0.0001379 at the time of the analysis; both the variants were not previously reported in our in-house databases. Both affected residues are located within the intracellular domains of the channel (Figure 1C), a region that plays a key role in the conformational switching events controlling TRESK function [5]. Of note, Ser252 plays an important regulatory role in the switching process by cycling between its phosphorylated and dephosphorylated states [5]. The highly conserved nature of the two residues and their predicted location consistently supported the functional relevance of the two amino acid substitutions. WES data analysis excluded the occurrence of other functionally relevant variants compatible with known Mendelian disorders based on the expected inheritance model and clinical presentation, further supporting the relevance of the two missense variants involving KCNK18.
2.3. p.Tyr163Asp and p.Ser252Leu Do Not Alter Basal TRESK Function Genomic and Structural Analyses
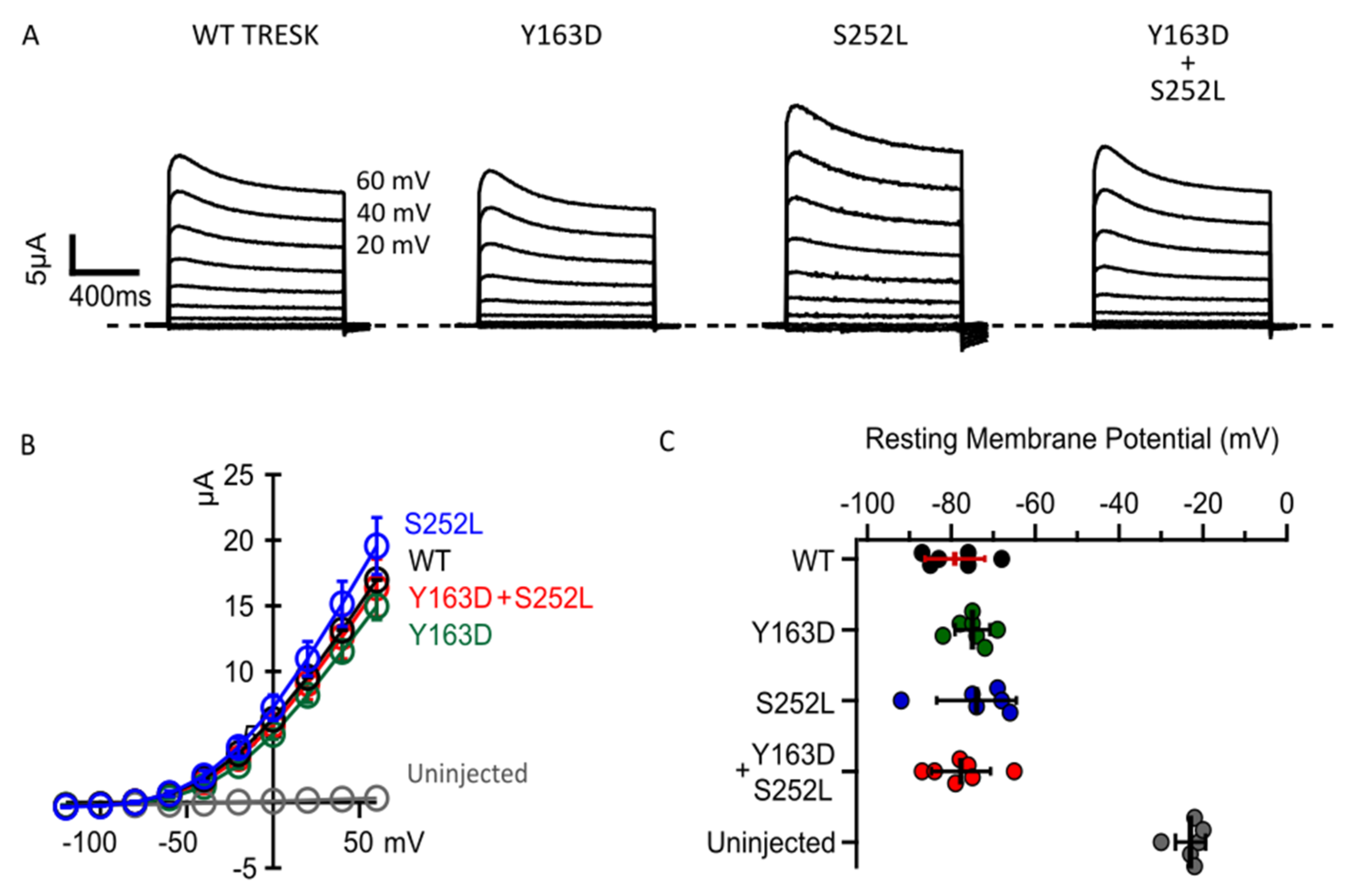
To validate the in silico predictions, we next examined whether the two missense changes in KCNK18 affect the functional properties of TRESK. Figure 2A shows representative whole-cell basal currents recorded from Xenopus oocytes injected with mRNA encoding human wild-type (WT) or the mutant TRESK channels. All channel types exhibited similar outwardly rectifying K+ current amplitudes, but the current in oocytes expressing TRESKS252L was slightly larger (Figure 2A,B). This larger basal K+ current, which is not statistically different, could be due to a reduction in the phosphorylation state of the mutant channel compared to TRESKWT. However, a robust gain of function in the basal K+ current has been observed when a serine close to the cluster was replaced with an alanine. Given that the K2P channels work as dimers, to mimic the compound heterozygous condition of affected subjects, equal amounts of the KCNK18 mutated mRNAs were co-injected into oocytes at a 1:1 ratio. Co-injection of mutant mRNAs did not substantially affect the whole-cell basal currents, which had similar amplitudes to those reported in oocytes overexpressing WT TRESK (Figure 2A,B). TRESK channels typically produce a leak K+ current that stabilizes the resting membrane potential [13]. We observed that the expression of WT TRESK or both mutants, expressed separately or together, shifted the resting cell membrane potential of the oocytes towards the K+ equilibrium potential (EK) (Figure 2C). Assuming an intracellular K+ concentration of approximately 140 mM in the oocyte, the predicted value for EK is −107 mV. A similar hyperpolarizing effect was observed upon expression of all TRESK channel types (Figure 2C). Collectively, these findings indicate that both mutations do not dramatically change the basal activity of TRESK channels, which also retain their ability to control the cell’s resting potential.
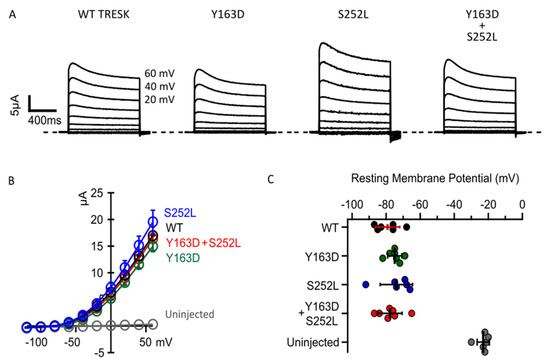
Figure 2.
p.Tyr163Asp and p.Ser252Leu do not significantly affect the basal current and ability of TRESK channels to control the cell’s resting potential. (A). Representative families of current traces for the indicated channel types. Cell membrane potential was held at −80mV. Each family of currents was evoked by voltage steps from −120 mV to 60 mV, with 20 mV increments. The recordings were performed 48 h after the injection of 1 ng of mRNA for each channel type. (B). Average IV relationships calculated from experiments as in A for the labelled channel types (color coded). The data points are mean ± standard error (0.001; n = 12). (C). Resting membrane potentials from individual oocytes un-injected (grey dots), injected with 1 ng of WT (black dots) or Y163D (green dots) or S252L (blue dots) or 0.5 ng of each Y163D and S252L (red dots) mRNA and recorded 48 h after injection. Note that the expression of all channel types similarly shifts the resting potential toward K+ reversal potential. The dots in C represent single cell recordings. The data are mean ± standard deviation.
2.4. p.Tyr163Asp and p.Ser252Leu Impair the Ability of TRESK to Properly Respond to Calcineurin Activation
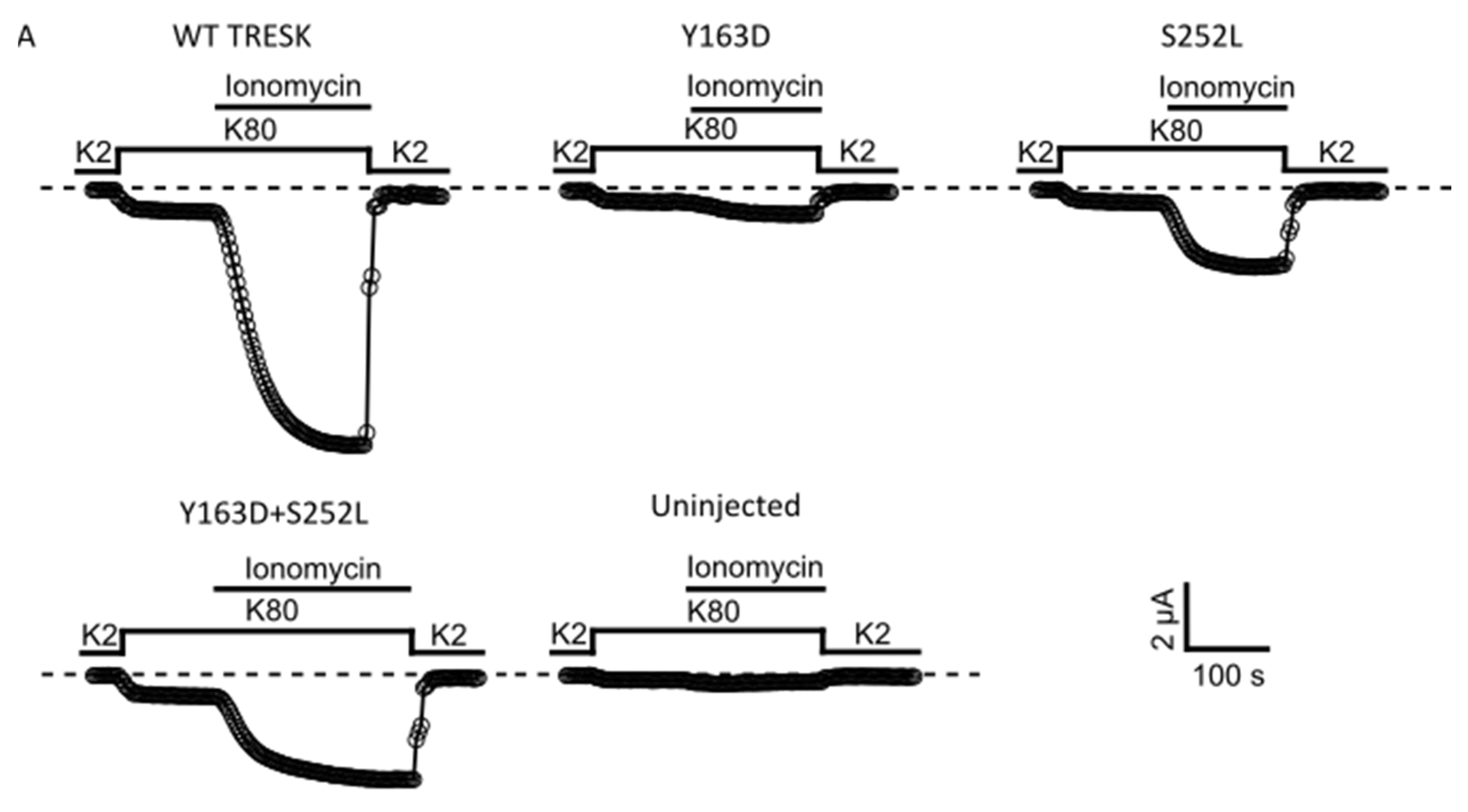
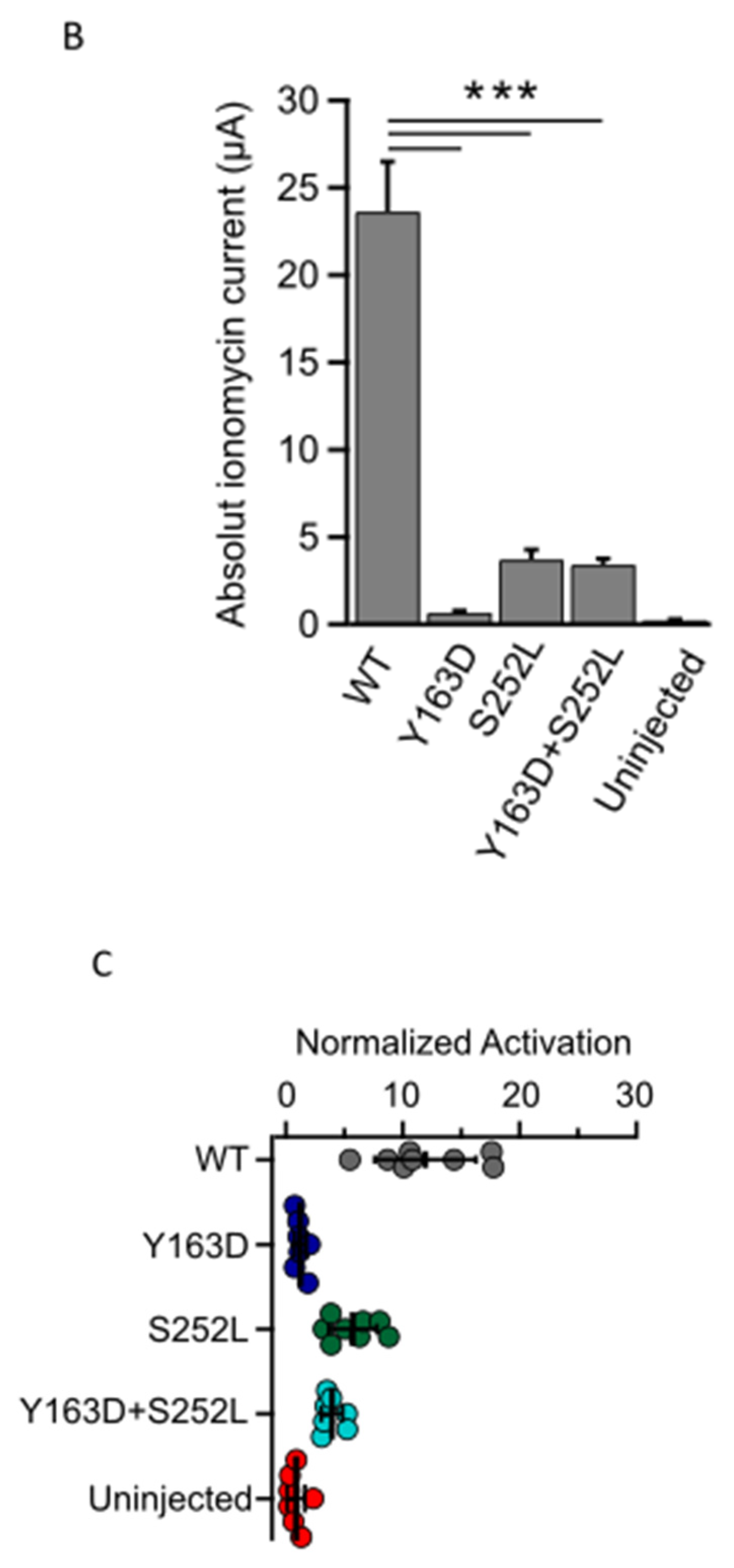
Given that the variants lie in amino acids located in regions important for the calcineurin-dependent gating of the channel, we next examined whether the mutant channels retain the ability to respond to calcineurin activation. In WT TRESK channels expressed in Xenopus oocytes, an increase in intracellular Ca2+ produced by the calcium ionophore ionomycin generally results in strong calcineurin-dependent activation of TRESK [5,18]. We therefore determined the effect of ionomycin on WT TRESK and the identified variants. As expected, 0.5 μM ionomycin induced a large, robust, and reversible activation of WT TRESK (Figure 3A). By contrast, we found that ionomycin was much less effective in the activation of homomeric and heteromeric mutant channels (Figure 3A). Indeed, the absolute values of Ca2+-activated whole-cell currents were significantly smaller than that for the WT channel (Figure 3B; WT vs. all groups p < 0.001). Moreover, we found that the extent of activation (IIono/IBasal ratios) for both homomeric and heteromeric TRESK channels was much smaller compared to that observed for the WT channel (Figure 3C). However, due to the relatively small amplitude of the unstimulated IBasal currents for the TRESKY163D and TRESKS252L mutants, these values may be overestimated. The response of un-injected control oocytes to ionomycin was insignificant, as previously reported [13,19] (Figure 3A–C) and all groups showed an obvious difference in the ionomycin-induced current when compared to un-injected oocytes (Figure 3B; WT vs. un-injected p < 0.001; Y163D vs. un-injected p = 0.02; S252L and Y163D + S252L vs. un-injected p < 0.01). The relatively small ionomycin-induced current amplitude recorded from mutants, in particular from Y163D, could be responsible for the misleading reduced level of significance when compared with that of control un-injected oocytes.
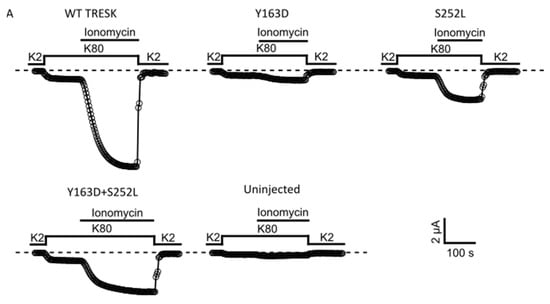
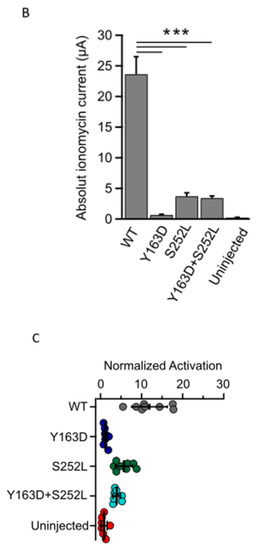
Figure 3.
KCNK18 p.Tyr163Asp and p.Ser252Leu impair the ionomycin-induced activation of TRESK channels. Representative data points showing ionomycin activation of TRESK WT, Y163D, S252L and Y163D + S252L currents as well as endogenous currents from un-injected oocytes. (A). Currents were evoked by 300 ms long voltage steps from 0 mV to −100 mV from oocytes injected with 1 ng of mRNA of the corresponding and indicated homomeric channel type and 0.5 ng for each subunit for the heteromeric channel Y163D/S252L. The sampled data represent the average of 50 ms long period of the steady-state currents recorded at −100 mV. Ionomycin activates the WT channels but has little effect on the oocytes that express the mutant channels or those that are uninjected. Note that the current decay upon reapplication of a solution containing 2 mM K+ in the recording chamber is due to the reduced K+ concentration rather than ionomycin washout. (B). Bar graph showing the mean of ionomycin activated currents (*** p < 0.001). All the groups were statistically significant compared to the uninjected group (WT vs. uninjected p < 0.001; Y163D vs. uninjected p = 0.02; S252L and Y163D + S252L vs. uninjected p < 0.01; n = 12). (C). Each point represents the steady state ionomycin activated current divided by the steady-state basal current (IIono/IBasal) recorded in the presence of 80 mM extracellular K+.
Overall, these results indicate that although the identified disease-associated KCNK18 variants do not alter the basal activity of TRESK, both markedly impair the ability of the channel to respond to calcineurin activation.
3. Discussion
TRESK background K+ channel contributes to the stabilization of the resting membrane potential of sensory neurons, particularly at the level of dorsal root and trigeminal ganglia [20,21]. Variants in the KCNK18 gene have been frequently associated with migraine [9,11,13,22]. Interestingly, patients with ID, with or without migraine, have also been reported [12,13], suggesting a contribution of dysregulated TRESK function in NDDs.
In the frame of a large genome scan project focused on patients with NDDs [16], we identified a family of European origin with three siblings affected by ID. In the oldest case (patient 1), recurrent episodes of migraine were also reported. In this family, WES allowed the identification of a new compound heterozygosity for two missense variants (Tyr163Asp and Ser252Leu) in all affected siblings. The variant occurring on residue Ser252 caught our attention, as it has been largely demonstrated that this residue is fundamental for the regulation of TRESK, being subjected to reversible phosphorylation, regulating the activation of the channel [4,23,24]. In pharmacophore-based virtual screening to reveal novel inhibitors against migraine, it has also been suggested Ser252 is one of the binding sites for Ergotamine and Lasmiditan, two drugs commonly used for migraine treatment [25].
To functionally validate the pathogenic relevance of the identified amino acid substitutions on TRESK function, electrophysiological recordings using Xenopus oocytes were performed. Slightly larger whole-cell basal K+ current amplitudes were observed in oocytes expressing TRESKS252L, which could be attributed to a reduction in the phosphorylation state of the mutant channel compared to TRESKWT. Indeed, a similar effect has been reported when the serine in position 276 (TRESKS276A) in the rat clone was replaced with an alanine [26]. On the other hand, the substitution of serine 276 with a glutamate, mimicking the dephosphorylated state, resulted in a mutant with low basal activity and reduced responsiveness. Interestingly, TRESKS276A also shows a reduced response to calcium signals. The S252 residue is part of the binding motif for 14-3-3, a family of ubiquitous adapter/regulatory proteins involved in the phosphorylation/dephosphorylation-dependent regulation of the channel. It has been shown that the 14-3-3γ isoform directly binds to the intracellular loop of TRESK and controls the kinetics of the calcium-dependent regulation of the channel [23].
Notably, 14-3-3γ plays a role in neuronal migration, morphology and brain development [27]. The gene encoding this protein (YWHAG) has been associated to developmental and epileptic encephalopathy (OMIM #717665), and autistic traits have been described in affected patients [28]. It is, therefore, intriguing to hypothesize that the S252L variant could cause an alteration in the interaction with 14-3-3γ, impairing its downstream activity and altering the same pathways involved in YWHAG-related disorders [28].
Here, we demonstrated that both the Y163D and S252L variants remarkably affect the TRESK response to intracellular Ca2+, which is able to cause calcineurin-dependent activation of the channel [5,18]. Specifically, while ionomycin induced a large and reversible activation of the WT channel, this activation was strongly reduced by the two variants, in both homomeric and heteromeric mutant channels. The exact location of both variants in the TRESK channel cannot be determined due to the lack of any 3D structural information on the regulatory domain of TRESK channels. However, in the linear sequence, Y163 is located nearby the calcineurin binding site. It could, therefore, be hypothesized that its substitution with aspartate (Y163D), a negatively charged residue, could disrupt calcineurin-channel interactions, and abolish current activation (Figure 3).
It is worth noting that the impairment of the channel activation after ionomycin induction (Figure 3) is not representative of the effect of the variants in the parents, as both the constructs were not expressed in combination with the WT one. With these experiments, we are, therefore, more likely mimicking a homozygous state of the variants instead of the heterozygous state observed in the parents. This is also in line with the higher impairment of the channel activation observed with Y163D and S252L alone compared to their combination.
The TRESK subfamily consists of only one member (KCNK18, K2P18.1) that has been cloned from the human spinal cord [29]. TRESK was shown to be expressed in rodent cerebrum, cerebellum, brain stem and, spinal cord [5]. Published evidence and data retrieved from the Allen Brain Atlas (www.portal.brain-map.org/ (accessed on 22 April 2021)) and GTEx Portal (www.gtexportal.org (accessed on 22 April 2021)) clearly show high expression of TRESK channels in human brain regions, for some of which the impairment has been associated with autism and ID [30]. How mutations in the TRESK channels identified so far [12,13] alter the functionality of these brain areas and result in autism and ID remains to be investigated. Whatever the final impact on the human brain of the mutations described here, which disrupt the calcineurin-dependent regulation of TRESK, may be, some insights could be gained by considering the effects of calcineurin inhibitors.
Indeed, Cyclosporin A and FK506 are inhibitors of the calcium/calmodulin-dependent protein phosphatase calcineurin, which abolish calcium-dependent TRESK activation. Neurological complications of Cyclosporin therapy are frequent and include dysarthria, headache, restlessness and seizures. Neurologic toxicity related to therapy with Tacrolimus (FK506), a macrolide calcineurin inhibitor, has also been reported, including restlessness, agitation, confusion, sleep problems, mental depression, dysarthria, headache and seizures. Intriguingly, dysarthria, restlessness, headache and seizures have been observed in our patients and it is tempting to speculate that these symptoms could be associated, at least in part, with Y163D- and S252L-induced disruption of calcineurin-dependent TRESK channel activation in the brain. Recently, it has been proposed that calcineurin regulation of two-pore potassium-leak channel activity (Kcnk5b) mediates activation of developmental gene transcription, which controls a broad number of developmental pathways [31]. Similarly to TRESK channels, inwardly rectifying K+ channels (Kir) control the resting potential of neurons and K+ homeostasis in the brain. Previous findings showed the importance of several Kir channel types in neurodevelopment, autism and ID [32,33,34,35,36,37,38]. Thus, growing evidence shows that K+ dependent bioelectric mechanisms can regulate not only the excitability of neurons, but also cell migration, proliferation, differentiation and gene transcription, thereby promoting coordinated developmental signaling.
In summary, our study suggests that the KCNK18 variants observed in our patients dramatically impair the ability of the TRESK channel to respond to calcineurin activation, leading to the alteration of a crucial function of the channel. Indeed, acetylcholine, glutamate or histamine activate native TRESK currents through G-protein coupled receptors [39], and this function would be nearly abolished by both Y163D and S252L variants. Collectively, our findings strengthen the notion that abnormal TRESK channel function in the central and peripheral nervous system could play crucial roles in neurologic and psychiatric diseases. Further studies on larger cohorts of patients and the characterization of transgenic animal models of the disease are, however, required to elucidate the link between migraine, autism, epilepsy and intellectual disability observed in some patients carrying TRESK variants.
4. Materials and Methods
4.1. Whole Exome Sequencing, Prioritization, and Variant Calling
DNA was extracted from total blood using the ReliaPrep Blood gDNA Miniprep kit (Promega, Madi-son, WY, USA) following the manufacturer’s protocol and quantified with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Array-CGH was performed using a 60 K whole-genome oligonucleotide microarray (Agilent Technologies, Santa Clara, CA, USA).
Patients were enrolled in the Autism Sequencing Consortium (ASC) project and their gDNA samples were sequenced at the Broad Institute on Illumina HiSeq sequencers, as previously described [16,40].
Whole exome sequencing (WES) raw data of the trio were processed and analyzed using an in-house implemented pipeline that has been previously described [41,42], which is based on the GATK Best Practices [43]. The UCSC GRCh37/hg19 version of genome assembly was used as a reference for reads alignment by means of the BWA-MEM [44] tool and the subsequent variant calling with HaplotypeCaller (GATK v3.7) [43]. We used SnpEff v.4.3 [45] and dbNSFP v.3.5 [46] tools for variants functional annotation, including Combined Annotation Dependent Depletion (CADD) v.1.3 [47], Mendelian Clinically Applicable Pathogenicity (M-CAP) v.1.0 [48] and Intervar v.0.1.6, for functional impact prediction [49]. Thereby, the analysis was narrowed to variants which affect coding sequences or splice site regions. Moreover, high-quality variants were filtered against public databases (dbSNP150 and gnomAD ver.2.0.1) so that only variants with unknown frequency or with MAF <0.1%, as well as variants occurring with frequency <1% in our population-matched database (~2000 exomes), were considered.
Further variant stratification in conformity with the American College of Medical Genetics and Genomics (ACMG) guideline [50], considering also the mode of inheritance and functional in-silico prediction of impact, allowed us to consider the final set of variants for possible associations with the phenotype. All variants are referred to GRCh37 annotation and to NM_181840.1.
Identified variants were confirmed by Sanger sequencing using standard conditions and the following primers: 5′-GGGAGATGGCAGAAGGTCTCTTTA and 5′-TTACTCCTCTCCATGGCTTGTGG; 5′-CAAACTTGGCACATGTCCTTCAC and 5′-GCATGACCCTGAAAGACAACACA.
Variants were analysed with the VarSome tool [51] as a starting point for further analysis. This allowed simultaneous evaluation of at least nine in silico predictors. Variants’ frequencies in the population were evaluated using the Genome Aggregation Database (GnomAD), browser version 2.1.1.
4.2. Constructs
Human TRESK was subcloned between the 5′ and 3′ UTR of the Xenopus β-globin gene in the oocyte expression vector, pFAW. The identified variants were introduced by site directed mutagenesis and confirmed by automated sequencing. mRNA for wild-type and mutant channels was synthesized using the T7 mMESSAGE mMACHINE kit (Ambion, Life technologies, Carlsbad, CA, USA) and mRNA concentrations were quantified by spectrophotometric analysis prior to injection.
4.3. Electrophysiological Recordings
Xenopus laevis oocytes collection and defolliculation were performed according to standard protocols which fall under the international standards of animal care, the Maltese Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals. TRESK wild-type or mutant mRNAs were microinjected into oocytes in equal quantities, unless otherwise stated. Forty-eight hours after injection, whole-cell currents were recorded at room temperature (20–22 °C) using the two-electrode voltage clamp method (Axoclamp-2B, Axon DIGIDATA 1550B, Axon Instruments, Foster City, CA, Axon). Basal K+ currents were measured in a low K+ extracellular solution (in mM: 95.4 NaCl, 2 KCl, 1.8 CaCl2, 5 HEPES pH 7.5 with NaOH). Recording electrodes (0.3÷1 MΩ) were backfilled with 3 M KCl. Currents were filtered at 100 Hz and digitized at 1 kHz for analysis. From a holding potential of −80 mV, 1-second-long voltage commands were applied from −120 mV to +60 mV, delivered in 20 mV increments. Currents were recorded using Clampex 10.7 software (Axon Instruments, Foster City, CA, USA). Ionomycin-induced TRESK currents were measured in high K+ solution (in mM: 17.4 NaCl, 80 KCl, 1.8 CaCl2, 5 HEPES, pH 7.5 with NaOH) at the end of 300 ms-long voltage steps from a holding potential of 0 mV to −100 mV. Ionomycin (free acid form) was made as a stock solution of 1 mM in DMSO and diluted in the high K+ solution to 0.5 μM on the day of the experiment. Data were analyzed with Clampfit 10.7 (Axon instruments, Foster City, CA, USA) and Igor programs.
4.4. Statistics
Data are given as mean values ± standard error of the mean (SEM), where n represents the number of oocytes. Results were reproducible in at least 2–3 different batches of oocytes. Statistical significance was determined using a student’s t-test. When error bars are not shown, they are smaller than the size of the symbol.
4.5. Online Resources
www.gtexportal.org, accessed on 22April 2021
www.gnomad.broadinstitute.org, accessed on 22 April 2021
www.portal.brain-map.org, accessed on 22 April 2021
www.varsome.com, accessed on 22 April 2021.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22116064/s1.
Author Contributions
Data curation: A.B. (Alessandro Bruselles) and L.P., A.B. (Alfredo Brusco); Conceptualization: M.P., M.C.D. and L.P.; Validation, M.C.D. and L.P.; Formal Analysis: E.N.-A., C.M., A.B. (Alessandro Bruselles), M.T., A.Z., S.D.R. and J.B.; Investigation: E.N.-A., S.J.T. and L.P.; Resources: M.P., S.J.T., M.T., A.B. (Alessandro Bruselles), J.B. and S.D.R.; Writing—Original Draft Preparation: M.C.D. and L.P., A.B. (Alfredo Brusco); Writing—Review and Editing: M.P., S.J.T., L.P., A.B. (Alfredo Brusco) and S.D.R.; Visualization: M.C.D. and E.N.-A.; Supervision: M.C.D., A.B. (Alfredo Brusco); Project Administration: M.P. and M.C.D., A.B. (Alfredo Brusco); Funding Acquisition: M.P., A.B. (Alfredo Brusco). All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding specifically appointed to the Department of Medical Sciences from the Italian Ministry for Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca—MIUR) under the programme “Dipartimenti di Eccellenza 2018–2022” Project code D15D18000410001. S.D.R. is a Fascitelli Research Scholar. WES was supported by the NIMH (MH111661). We also gratefully acknowledge the financial support of the University of Malta, Research Innovation & Development Trust (RIDT) (Grant n. I20LU08, BooKind E20LG42) and of the United Arab Emirates University (Grants n. 12M032, 31M468, 12M020). S.T was funded by the BBSRC.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Turin (protocol code 0060884 of 12 June 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are contained within this article and are available on request from the corresponding author. The WES data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We are grateful to the patients and their family for their collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gada, K.; Plant, L.D. Two-pore domain potassium channels: Emerging targets for novel analgesic drugs: IUPHAR Review 26. Br. J. Pharmacol. 2019, 176, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Andres-Enguix, I.; Shang, L.; Stansfeld, P.J.; Morahan, J.M.; Sansom, M.S.P.; Lafrenière, R.G.; Roy, B.; Griffiths, L.R.; Rouleau, G.A.; Ebers, G.; et al. Functional analysis of missense variants in the TRESK (KCNK18) K + channel. Sci. Rep. 2012, 2, 237. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, P.; Czirjak, G. Properties, regulation, pharmacology, and functions of the K2P channel, TRESK. Pflügers Arch. Eur. J. Physiol. 2014, 467, 945–958. [Google Scholar] [CrossRef]
- Czirják, G.; Enyedi, P. TRESK background K+ channel is inhibited by phosphorylation via two distinct pathways. J. Biol. Chem. 2010, 285, 14549–14557. [Google Scholar] [CrossRef] [PubMed]
- Czirják, G.; Tóth, Z.E.; Enyedi, P. The Two-pore Domain K+ Channel, TRESK, Is Activated by the Cytoplasmic Calcium Signal through Calcineurin. J. Biol. Chem. 2004, 279, 18550–18558. [Google Scholar] [CrossRef]
- Bautista, D.M.; Sigal, Y.M.; Milstein, A.D.; Garrison, J.L.; Zorn, J.A.; Tsuruda, P.R.; Nicoll, R.A.; Julius, D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci. 2008, 11, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Liu, J.; Sabbadini, M.; Au, P.; Xie, G.-X.; Yost, C.S. Regional expression of the anesthetic-activated potassium channel TRESK in the rat nervous system. Neurosci. Lett. 2009, 465, 79–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pettingill, P.; Weir, G.A.; Wei, T.; Wu, Y.; Flower, G.; Lalic, T.; Handel, A.; Duggal, G.; Chintawar, S.; Cheung, J.; et al. A causal role for TRESK loss of function in migraine mechanisms. Brain 2019, 142, 3852–3867. [Google Scholar] [CrossRef]
- Royal, P.; Andres-Bilbe, A.; Prado, P.Á.; Verkest, C.; Wdziekonski, B.; Schaub, S.; Baron, A.; Lesage, F.; Gasull, X.; Levitz, J.; et al. Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK. Neuron 2019, 101, 232–245.e6. [Google Scholar] [CrossRef]
- Lafrenière, R.G.; Cader, Z.; Poulin, J.-F.; Andres-Enguix, I.; Simoneau, M.; Gupta, N.; Boisvert, K.; Lafrenière, F.; McLaughlan, S.; Dubé, M.-P.; et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 2010, 16, 1157–1160. [Google Scholar] [CrossRef]
- Rainero, I.; Rubino, E.; Gallone, S.; Zavarise, P.; Carli, D.; Boschi, S.; Fenoglio, P.; Savi, L.; Gentile, S.; Benna, P.; et al. KCNK 18 (TRESK) genetic variants in Italian patients with migraine. Headache J. Head Face Pain 2014, 54, 1515–1522. [Google Scholar] [CrossRef]
- Han, J.Y.; Jang, J.H.; Park, J.; Lee, I.G. Targeted next-generation sequencing of Korean patients with developmental delay and/or intellectual disability. Front. Pediatr. 2018, 6, 391. [Google Scholar] [CrossRef]
- Imbrici, P.; Nematian-Ardestani, E.; Hasan, S.; Pessia, M.; Tucker, S.J.; D’Adamo, M.C. Altered functional properties of a missense variant in the TRESK K+ channel (KCNK18) associated with migraine and intellectual disability. Pflügers Arch. Eur. J. Physiol. 2020, 472, 923–930. [Google Scholar] [CrossRef]
- Schalock, R.L.; Luckasson, R.; Tassé, M.J. The contemporary view of intellectual and developmental disabilities: Implications for psychologists. Psicothema 2019, 31, 223–228. [Google Scholar] [CrossRef]
- de Giorgio, A. The roles of motor activity and environmental enrichment in intellectual disability. Somatosens. Mot. Res. 2017, 34, 34–43. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Czirják, G.; Enyedi, P. The LQLP calcineurin docking site is a major determinant of the calcium-dependent activation of human TRESK background K+ channel. J. Biol. Chem. 2014, 289, 29506–29518. [Google Scholar] [CrossRef]
- Czirják, G.; Enyedi, P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J. Biol. Chem. 2006, 281, 14677–14682. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Plant, S. Mechanism of release of Ca2+ from intracellular stores in response to ionomycin in oocytes of the frog Xenopus laevis. J. Physiol. 1992, 458, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Cadaveira-Mosquera, A.; Pérez, M.; Reboreda, A.; Rivas-Ramírez, P.; Fernández-Fernández, D.; Lamas, J.A. Expression of K2P channels in sensory and motor neurons of the autonomic nervous system. J. Mol. Neurosci. 2012, 48, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 2006, 291, C138–C146. [Google Scholar] [CrossRef]
- Maher, B.H.; Taylor, M.; Stuart, S.; Okolicsanyi, R.K.; Roy, B.; Sutherland, H.G.; Haupt, L.M.; Griffiths, L.R. Analysis of 3 common polymorphisms in the KCNK18 gene in an Australian Migraine Case-control cohort. Gene 2013, 528, 343–346. [Google Scholar] [CrossRef][Green Version]
- Czirják, G.; Vuity, D.; Enyedi, P. Phosphorylation-dependent binding of 14-3-3 proteins controls TRESK regulation. J. Biol. Chem. 2008, 283, 15672–15680. [Google Scholar] [CrossRef]
- Rahm, A.-K.; Gierten, J.; Kisselbach, J.; Staudacher, I.; Staudacher, K.; Schweizer, P.A.; Becker, R.; Katus, H.A.; Thomas, D. PKC-dependent activation of human K2P18.1 K+ channels. Br. J. Pharmacol. 2012, 166, 764–773. [Google Scholar] [CrossRef]
- Sehgal, A.; Hassan, M.; Rashid, S. Pharmacoinformatics elucidation of potential drug targets against migraine to target ion channel protein KCNK18. Drug Des. Dev. Ther. 2014, 8, 571–581. [Google Scholar] [CrossRef]
- Lengyel, M.; Dobolyi, A.; Czirják, G.; Enyedi, P. Selective and state-dependent activation of TRESK (K2P18.1) background potassium channel by cloxyquin. Br. J. Pharmacol. 2017, 174, 2102–2113. [Google Scholar] [CrossRef]
- Cornell, B.; Toyo-oka, K. 14-3-3 proteins in brain development: Neurogenesis, neuronal migration and neuromorphogenesis. Front. Mol. Neurosci. 2017, 10, 318. [Google Scholar] [CrossRef]
- Guella, I.; McKenzie, M.B.; Evans, D.M.; Buerki, S.E.; Toyota, E.B.; Van Allen, M.I.; Suri, M.; Elmslie, F.; Simon, M.E.; van Gassen, K.L.; et al. De Novo Mutations in YWHAG Cause Early-Onset Epilepsy. Am. J. Hum. Genet. 2017, 101, 300–310. [Google Scholar] [CrossRef]
- Sano, Y.; Inamura, K.; Miyake, A.; Mochizuki, S.; Kitada, C.; Yokoi, H.; Nozawa, K.; Okada, H.; Matsushime, H.; Furuichi, K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J. Biol. Chem. 2003, 278, 27406–27412. [Google Scholar] [CrossRef]
- Liu, C.; Au, J.D.; Zou, H.L.; Cotten, J.F.; Yost, C.S. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesthesia Analg. 2004, 99, 1715–1722. [Google Scholar] [CrossRef]
- Yi, C.; Spitters, T.W.; Al-Far, E.A.-D.A.; Wang, S.; Xiong, T.; Cai, S.; Yan, X.; Guan, K.; Wagner, M.; El-Armouche, A.; et al. A calcineurin-mediated scaling mechanism that controls a K+-leak channel to regulate morphogen and growth factor transcription. eLife 2021, 10. [Google Scholar] [CrossRef]
- Sun, C.; Zou, M.; Li, L.; Li, D.; Ma, Y.; Xia, W.; Wu, L.; Ren, H. Association study between inwardly rectifying potassium channels 2.1 and 4.1 and autism spectrum disorders. Life Sci. 2018, 213, 183–189. [Google Scholar] [CrossRef]
- Sicca, F.; Ambrosini, E.; Marchese, M.; Sforna, L.; Servettini, I.; Valvo, G.; Brignone, M.S.; Lanciotti, A.; Moro, F.; Grottesi, A.; et al. Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Sicca, F.; Imbrici, P.; D’Adamo, M.C.; Moro, F.; Bonatti, F.; Brovedani, P.; Grottesi, A.; Guerrini, R.; Masi, G.; Santorelli, F.M.; et al. Autism with Seizures and Intellectual Disability: Possible Causative Role of Gain-of-function of the Inwardly-Rectifying K+ Channel Kir4.1. Neurobiol. Dis. 2011, 43, 239–247. [Google Scholar] [CrossRef]
- D’Adamo, M.C.; Catacuzzeno, L.; Di Giovanni, G.; Franciolini, F.; Pessia, M. Erratum: K+ channelepsy: Progress in the neurobiology of potassium channels and epilepsy. Front. Cell. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef]
- Hasan, S.M.; Balobaid, A.; Grottesi, A.; Dabbagh, O.; Cenciarini, M.; Rawashdeh, R.; Al-Sagheir, A.; Bove, C.; Macchioni, L.; Pessia, M.; et al. Lethal digenic mutations in the K+ channels Kir4.1 (KCNJ10) and SLACK (KCNT1) associated with severe-disabling seizures and neurodevelopmental delay. J. Neurophysiol. 2017, 118, 2402–2411. [Google Scholar] [CrossRef]
- Guglielmi, L.; Servettini, I.; Caramia, M.; Catacuzzeno, L.; Franciolini, F.; D’Adamo, M.C.; Pessia, M. Update on the implication of potassium channels in autism: K+ channelautism spectrum disorder. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- D’Adamo, M.C.; Liantonio, A.; Conte, E.; Pessia, M.; Imbrici, P. Ion Channels Involvement in Neurodevelopmental Disorders. Neuroscience 2020, 440, 337–359. [Google Scholar] [CrossRef]
- Kang, D.; Kim, G.-T.; Kim, E.-J.; La, J.-H.; Lee, J.-S.; Lee, E.-S.; Park, J.-Y.; Hong, S.-G.; Han, J. Lamotrigine inhibits TRESK regulated by G-protein coupled receptor agonists. Biochem. Biophys. Res. Commun. 2008, 367, 609–615. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Flex, E.; Martinelli, S.; Van Dijck, A.; Ciolfi, A.; Cecchetti, S.; Coluzzi, E.; Pannone, L.; Andreoli, C.; Radio, F.C.; Pizzi, S.; et al. Aberrant Function of the C-Terminal Tail of HIST1H1E Accelerates Cellular Senescence and Causes Premature Aging. Am. J. Hum. Genet. 2019, 105, 493–508. [Google Scholar] [CrossRef]
- Bauer, C.K.; Calligari, P.; Radio, F.C.; Caputo, V.; Dentici, M.L.; Falah, N.; High, F.; Pantaleoni, F.; Barresi, S.; Ciolfi, A.; et al. Mutations in KCNK4 that Affect Gating Cause a Recognizable Neurodevelopmental Syndrome. Am. J. Hum. Genet. 2018, 103, 621–630. [Google Scholar] [CrossRef]
- Van Der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From fastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Li, C.; Boerwinkle, E. dbNSFP v3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Hum. Mutat. 2016, 37, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O‘Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef]
- Jagadeesh, K.A.; Wenger, A.M.; Berger, M.J.; Guturu, H.; Stenson, P.D.; Cooper, D.N.; Bernstein, J.A.; Bejerano, G. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat. Genet. 2016, 48, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, K. InterVar: Clinical Interpretation of Genetic Variants by the 2015 ACMG-AMP Guidelines. Am. J. Hum. Genet. 2017, 100, 267–280. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).