Proofing Direct-Seeded Rice with Better Root Plasticity and Architecture
Abstract
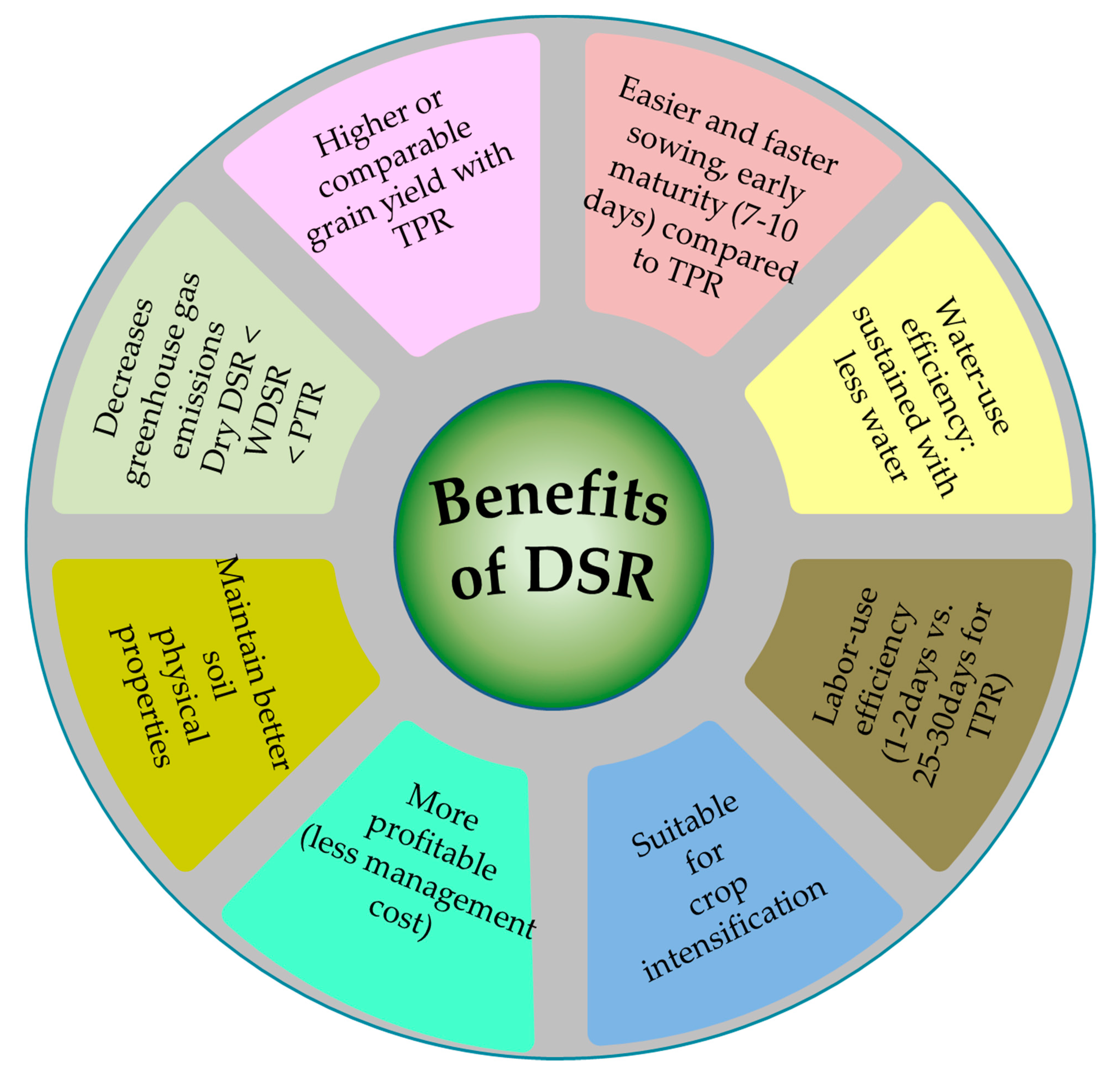
1. Introduction
2. Insights into Rice Root System Architecture
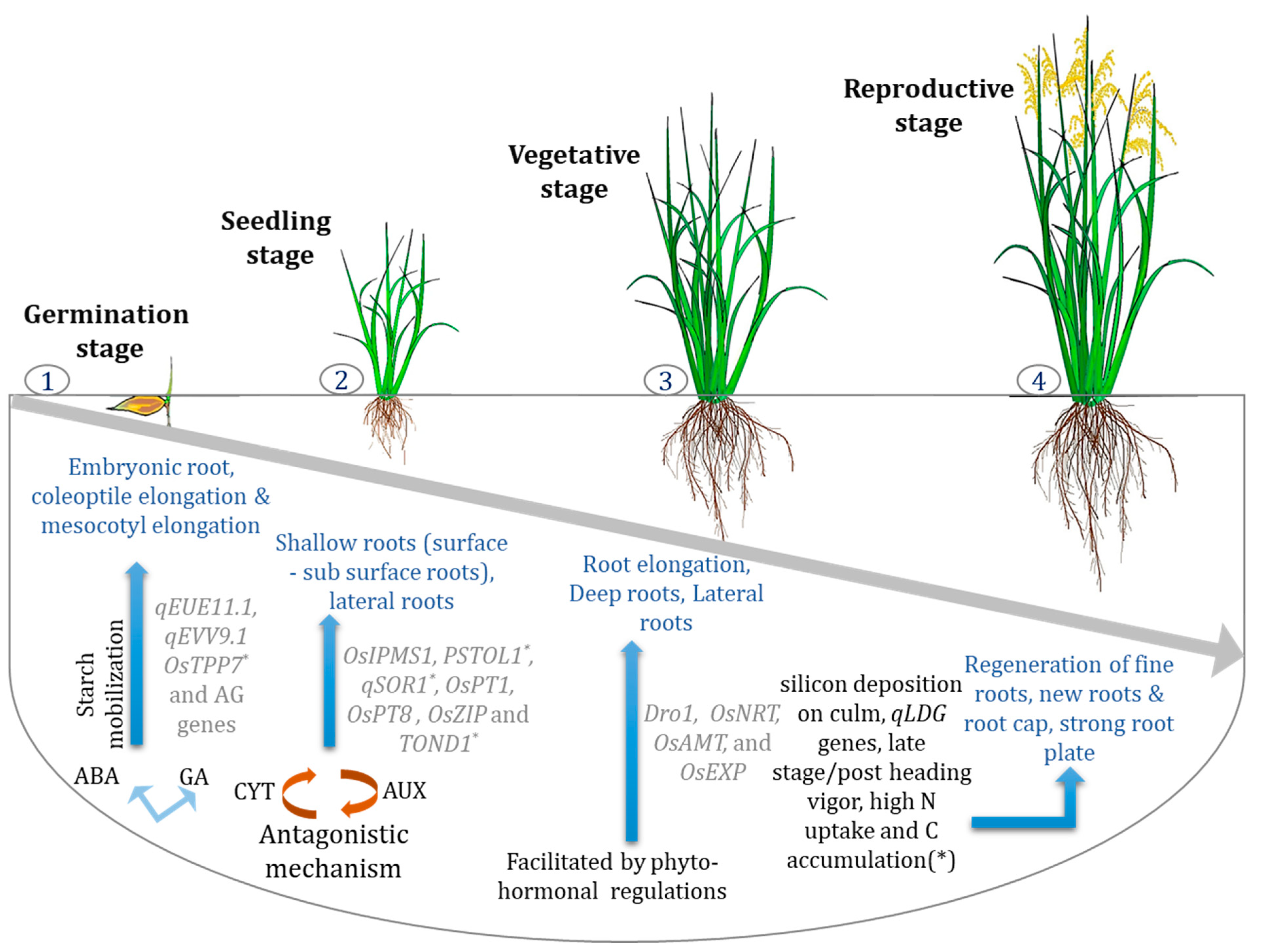
3. RSA: Infant to Early Vegetative Stage and Associated Traits
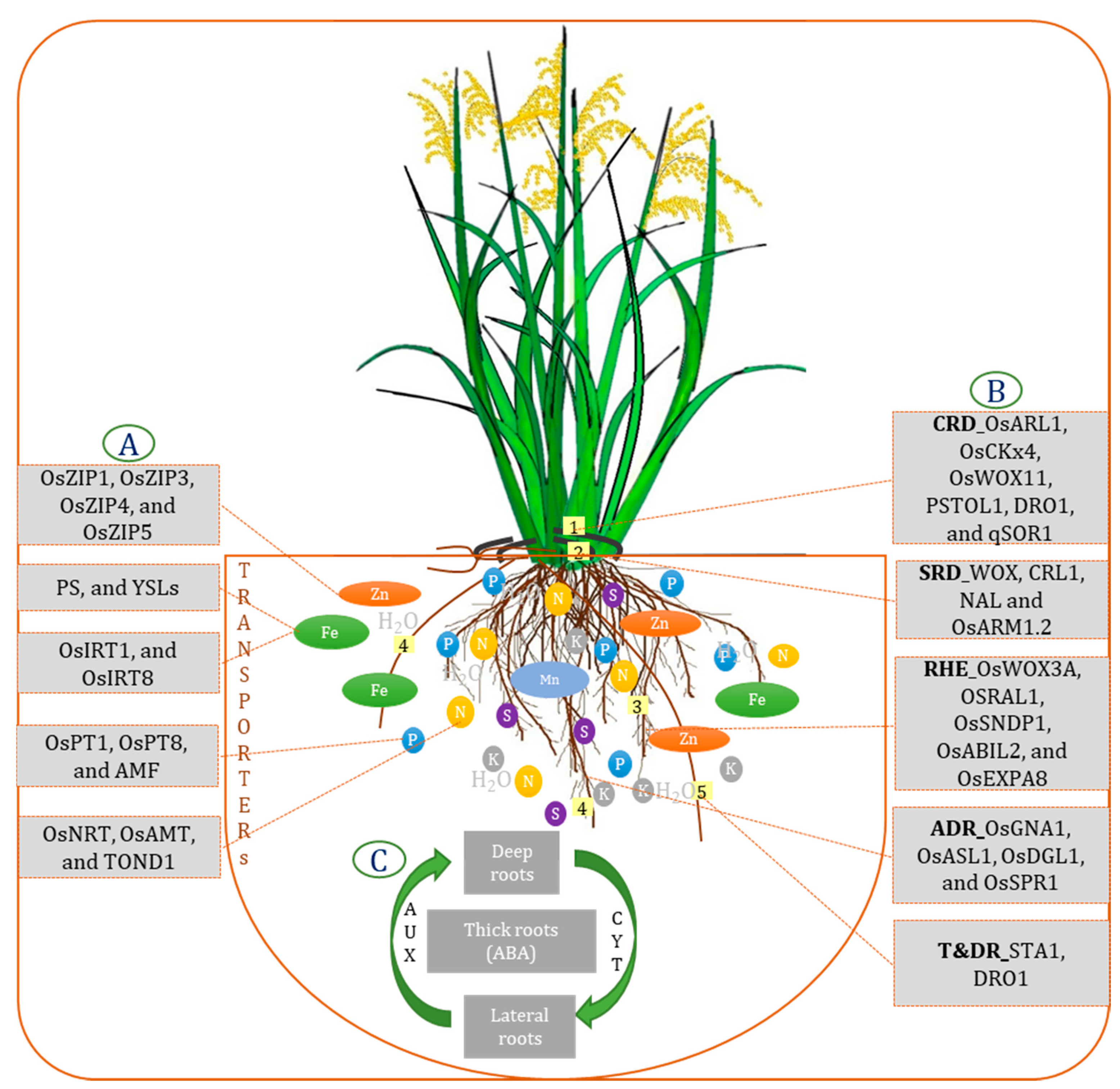
3.1. Root Requirements and Nutrient Uptake
3.2. Early Uniform Emergence and Early Root Vigor
4. Stress during Germination
4.1. Anaerobic Germination and Regulation of Associated Root Traits
4.2. Limited Moisture during Germination and Regulation of Associated Root Traits under DSR
4.3. Cold Stress and Salt Stress during Germination
5. RSA: Vegetative to Reproductive Stage
6. Biotic Stress in the Form of Weeds
7. Root Growth and the Role of Phytohormones
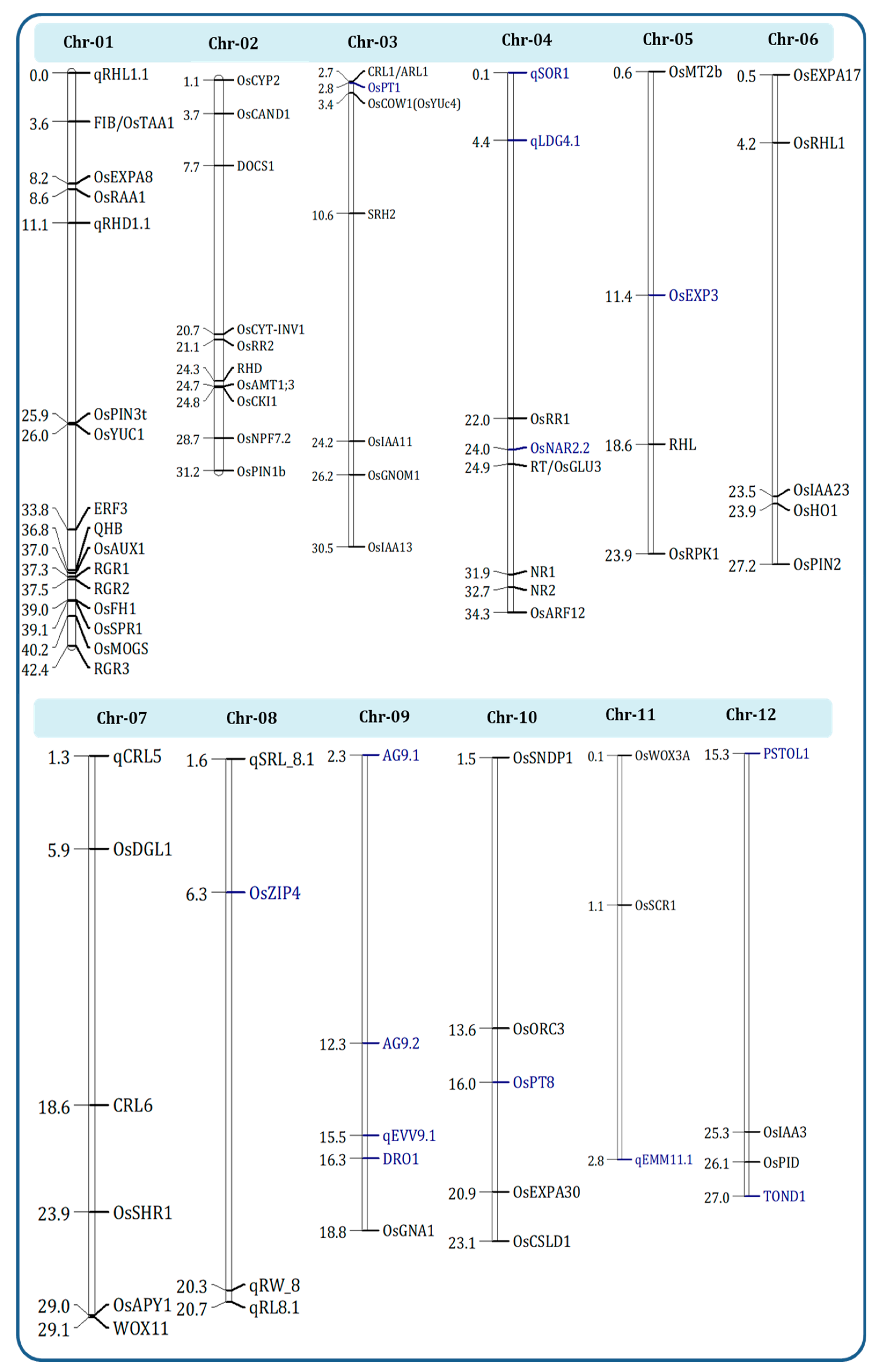
8. QTL Identification and Introgression of Root Architecture QTLs for DSR Using a Marker-Assisted Backcross Breeding Approach
9. Hybrid Development for the Direct-Seeded Rice System by Altering RSA
10. Transgenic Breeding for Root System Architecture
11. Modeling DSR with Root-Specific Traits
12. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, H.; Tewari, A.N.; Sankhyan, S.; Dubey, D.S.; Mina, U.; Singh, V.K.; Jain, N. Direct-seeded rice: Potential, performance and problems—A review. Curr. Adv. Agric. Sci. 2011, 3, 77–88. [Google Scholar]
- Tuong, T.P.; Bouman, B.A.M. Rice production in water scarce environments. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Kumar, V.; Ladha, J.K. Direct Seeding of Rice. Recent Developments and Future Research Needs. Adv. Agron. 2011, 111. [Google Scholar] [CrossRef]
- Haefele, S.M.; Kato, Y.; Singh, S. Climate ready rice: Augmenting drought tolerance with best management practices. Field Crops Res. 2016, 190. [Google Scholar] [CrossRef]
- Corton, T.M.; Bajita, J.B.; Grospe, F.S.; Pamplona, R.R.; Asis, C.A.; Wassmann, R.; Lantin, R.S.; Buendia, L.V. Methane emission from irrigated and intensively managed rice fields in Central Luzon (Philippines). Nutr. Cycl. Agroecosyst. 2000, 58. [Google Scholar] [CrossRef]
- Ohno, H.; Banayo, N.P.M.C.; Bueno, C.; Kashiwagi, J.-I.; Nakashima, T.; Iwama, K.; Corales, A.M.; Garcia, R.; Kato, Y. On-farm assessment of a new early-maturing drought-tolerant rice cultivar for dry direct seeding in rainfed lowlands. Field Crops Res. 2018, 219. [Google Scholar] [CrossRef]
- Basavalingaiah, K.; Ramesha, Y.M.; Paramesh, V.; Rajanna, G.A.; Jat, S.L.; Misra, S.D.; Gaddi, A.K.; Girisha, H.C.; Yogesh, G.S.; Raveesha, S.; et al. Energy budgeting, data envelopment analysis and greenhouse gas emission from rice production system: A case study from puddled transplanted rice and direct-seeded rice system of Karnataka, India. Sustainability 2020, 12, 6439. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, A. Direct seeded rice: Prospects, problems/constraints and researchable issues in India. Curr. Agric. Res. J. 2017, 5, 13. [Google Scholar] [CrossRef]
- Ray, S.; Vijayan, J.; Sarkar, R.K. Germination stage oxygen deficiency (GSOD): An emerging stress in the era of changing trends in climate and rice cultivation practice. Front. Plant Sci. 2016, 7, 1–4. [Google Scholar] [CrossRef]
- Mackill, D.J.; Amante, M.M.; Vergara, B.S.; Sarkarung, S. Improved Semidwarf Rice Lines with Tolerance to Submergence of Seedlings. Crop Sci. 1993, 33. [Google Scholar] [CrossRef]
- Rani, M.G.; Satyanarayana, P.V.; Ahmed, M.L.; Rani, Y.A.; Rao, V.S.; Jhansirani, P. Breeding Strategies for Lodging Resistance in Rice. Int. J. Bio-Resour. Stress Manag. 2017, 8, 895–903. [Google Scholar] [CrossRef]
- Kashiwagi, T. Identification of quantitative trait loci for resistance to bending-type lodging in rice (Oryza sativa L.). Euphytica 2014, 198. [Google Scholar] [CrossRef]
- Sagare, D.B.; Abbai, R.; Jain, A.; Jayadevappa, P.K.; Dixit, S.; Singh, A.K.; Challa, V.; Alam, S.; Singh, U.M.; Yadav, S.; et al. More and more of less and less: Is genomics-based breeding of dry direct-seeded rice (DDSR) varieties the need of hour? Plant Biotechnol. J. 2020, 18, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Varney, G.T.; Mccully, M.E.; Canny, M.J. Sites of entry of water into the symplast of maize roots. New Phytol. 1993, 125. [Google Scholar] [CrossRef]
- Chul, M.K.; Sung, H.P.; Byoung, I.J.; Su, H.P.; Soon, J.P.; Hai, L.P.; Moo, Y.E.; Dolan, L.; Han, C.D. OsCSLD1, a cellulose synthase-like D1 gene, is required for root hair morphogenesis in rice. Plant Physiol. 2007, 143. [Google Scholar] [CrossRef]
- O’Toole, J.C.; Bland, W.L. Genotypic variation in crop plant root systems. Adv. Agron. 1987, 41. [Google Scholar] [CrossRef]
- Uga, Y.; Ebana, K.; Abe, J.; Morita, S.; Okuno, K.; Yano, M. Variation in root morphology and anatomy among accessions of cultivated rice (Oryza sativa L.) with different genetic backgrounds. Breed. Sci. 2009, 59. [Google Scholar] [CrossRef]
- Courtois, B.; Ahmadi, N.; Khowaja, F.; Price, A.H.; Rami, J.F.; Frouin, J.; Hamelin, C.; Ruiz, M. Rice root genetic architecture: Meta-analysis from a drought QTL database. Rice 2009, 2, 115–128. [Google Scholar] [CrossRef]
- Rebouillat, J.; Dievart, A.; Verdeil, J.L.; Escoute, J.; Giese, G.; Breitler, J.C.; Gantet, P.; Espeout, S.; Guiderdoni, E.; Périn, C. Molecular genetics of rice root development. Rice 2009, 2. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, S. Root genetic research, an opportunity and challenge to rice improvement. Field Crops Res. 2014, 165. [Google Scholar] [CrossRef]
- Topp, C.N.; Iyer-Pascuzzi, A.S.; Anderson, J.T.; Lee, C.R.; Zurek, P.R.; Symonova, O.; Zheng, Y.; Bucksch, A.; Mileyko, Y.; Galkovskyi, T.; et al. 3D phenotyping and quantitative trait locus mapping identify core regions of the rice genome controlling root architecture. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef]
- Coudert, Y.; Périn, C.; Courtois, B.; Khong, N.G.; Gantet, P. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010, 15. [Google Scholar] [CrossRef]
- Itoh, J.I.; Nonomura, K.I.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Kitano, H.; Nagato, Y. Rice plant development: From zygote to spikelet. Plant Cell Physiol. 2005, 46. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Morita, S. Growth direction of nodal roots in rice: Its variation and contribution to root system formation. Plant Soil 1994, 165. [Google Scholar] [CrossRef]
- Gewin, V. Food: An underground revolution. Nat. News 2010, 466. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.C.; Rose, T.J.; Pariasca-Tanaka, J.; Jeong, K.; Matsuda, T.; Wissuwa, M. Phosphorus uptake commences at the earliest stages of seedling development in rice. J. Exp. Bot. 2018, 69. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G. The phosphate transporter gene ospht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156. [Google Scholar] [CrossRef]
- Sun, S.; Gu, M.; Cao, Y.; Huang, X.; Zhang, X.; Ai, P.; Zhao, J.; Fan, X.; Xu, G. A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol. 2012, 159. [Google Scholar] [CrossRef] [PubMed]
- Gamuyao, R.; Chin, J.H.; Pariasca-Tanaka, J.; Pesaresi, P.; Catausan, S.; Dalid, C.; Slamet-Loedin, I.; Tecson-Mendoza, E.M.; Wissuwa, M.; Heuer, S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 2012, 488, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.H.; Han, J.H.; Jang, S.; Song, K.; Koh, H.J.; Lee, J.H.; Yoo, S.; Chin, J.H. Early vigor of a pyramiding line containing two quantitative trait loci, phosphorus uptake 1 (Pup1) and anaerobic germination 1 (ag1) in rice (O. sativa L.). Agriculture 2020, 10, 453. [Google Scholar] [CrossRef]
- Sandhu, N.; Subedi, S.R.; Singh, V.K.; Sinha, P.; Kumar, S.; Singh, S.P.; Ghimire, S.K.; Pandey, M.; Yadaw, R.B.; Varshney, R.K.; et al. Deciphering the genetic basis of root morphology, nutrient uptake, yield, and yield-related traits in rice under dry direct-seeded cultivation systems. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Oyanagi, A.; Nakamoto, T.; Morita, S. The gravitropic response of roots and the shaping of the root system in cereal plants. Environ. Exp. Bot. 1993, 33. [Google Scholar] [CrossRef]
- Uga, Y.; Kitomi, Y.; Ishikawa, S.; Yano, M. Genetic improvement for root growth angle to enhance crop production. Breed. Sci. 2015, 65. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liang, W.; Sturrock, C.J.; Pandey, B.K.; Giri, J.; Mairhofer, S.; Wang, D.; Muller, L.; Tan, H.; York, L.M.; et al. Rice actin binding protein RMD controls crown root angle in response to external phosphate. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Uga, Y.; Hanzawa, E.; Nagai, S.; Sasaki, K.; Yano, M.; Sato, T. Identification of qSOR1, a major rice QTL involved in soil-surface rooting in paddy fields. Theor. Appl. Genet. 2012, 124. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T.; et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 117, 21242–21250. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45. [Google Scholar] [CrossRef] [PubMed]
- Uga, Y.; Yamamoto, E.; Kanno, N.; Kawai, S.; Mizubayashi, T.; Fukuoka, S. A major QTL controlling deep rooting on rice chromosome 4. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Uga, Y.; Kitomi, Y.; Yamamoto, E.; Kanno, N.; Kawai, S.; Mizubayashi, T.; Fukuoka, S. A QTL for root growth angle on rice chromosome 7 is involved in the genetic pathway of DEEPER ROOTING 1. Rice 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Kanno, N.; Kawai, S.; Mizubayashi, T.; Fukuoka, S.; Uga, Y. QTLs underlying natural variation of root growth angle among rice cultivars with the same functional allele of DEEPER ROOTING 1. Rice 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Chen, L.; Mei, H.; Wei, H.; Feng, F.; Wang, P.; Xia, H.; Li, T.; Luo, L. Quantitative trait locus mapping of deep rooting by linkage and association analysis in rice. J. Exp. Bot. 2015, 66. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Kan, C.C.; Wu, H.Y.; Yang, H.C.; Hsieh, M.H. Early molecular events associated with nitrogen deficiency in rice seedling roots. Sci. Rep. 2018, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y.; et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Fan, X.; Wei, J.; Feng, H.; Qu, H.; Xie, D.; Miller, A.J.; Xu, G. Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and long-distance transport. J. Exp. Bot. 2015, 66. [Google Scholar] [CrossRef]
- Fan, X.; Feng, H.; Tan, Y.; Xu, Y.; Miao, Q.; Xu, G. A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen. J. Integr. Plant Biol. 2016, 58. [Google Scholar] [CrossRef]
- Feng, H.; Yan, M.; Fan, X.; Li, B.; Shen, Q.; Miller, A.J.; Xu, G. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 2011, 62. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Fan, X.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 34, 1360–1372. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Tao, J.; Miller, A.J.; Fan, X.; Xu, G. Identification and functional assay of the interaction motifs in the partner protein OsNAR2.1 of the two-component system for high-affinity nitrate transport. New Phytol. 2014, 204. [Google Scholar] [CrossRef]
- Ho, C.H.; Tsay, Y.F. Nitrate, ammonium, and potassium sensing and signaling. Curr. Opin. Plant Biol. 2010, 13, 604–610. [Google Scholar] [CrossRef]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; Von Wirén, N.; Yamaya, T.; Yamaguchi, J. Distinct expression and function of three ammonium transporter genes (OsAMT1;1—1;3) in rice. Plant Cell Physiol. 2003, 44. [Google Scholar] [CrossRef]
- Suenaga, A.; Moriya, K.; Sonoda, Y.; Ikeda, A.; Von Wirén, N.; Hayakawa, T.; Yamaguchi, J.; Yamaya, T. Constitutive expression of a novel-type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol. 2003, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Z.; Wei, J.; Qu, H.; Xie, Y.; Xu, G. The OsAMT1.1 gene functions in ammonium uptake and ammonium–potassium homeostasis over low and high ammonium concentration ranges. J. Genet. Genom. 2016, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, L.; Zhu, Z.; Yuan, L.; Xie, D.; Sun, C. TOND1 confers tolerance to nitrogen deficiency in rice. Plant J. 2015, 81, 367–376. [Google Scholar] [CrossRef]
- Puig, J.; Pauluzzi, G.; Guiderdoni, E.; Gantet, P. Regulation of shoot and root development through mutual signaling. Mol. Plant 2012, 5, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62. [Google Scholar] [CrossRef]
- Joshi, E.; Kumar, D.; Lal, B.; Nepalia, V.; Gautam, P.; Vyas, A. Management of direct seeded rice for enhanced resource—Use efficiency. Plant Knowl. J. 2013, 2. [Google Scholar] [CrossRef]
- Shojima, S.; Nishizawa, N.-K.; Fushiya, S.; Nozoe, S.; Irifune, T.; Mori, S. Biosynthesis of Phytosiderophores. Plant Physiol. 1990, 93. [Google Scholar] [CrossRef] [PubMed]
- Nozoye, T.; Nagasaka, S.; Kobayashi, T.; Takahashi, M.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem. 2011, 286. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nishizawa, N.; Hayashi, H.; Chino, M.; Yoshimura, E.; Ishihara, J. Why are young rice plants highly susceptible to iron deficiency? Plant Soil 1991, 130. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 2006, 45. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wissuwa, M. Rapid crown root development confers tolerance to Zinc deficiency in rice. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Schaaf, G.; Ludewig, U.; Erenoglu, B.E.; Mori, S.; Kitahara, T.; Von Wirén, N. ZmYS1 Functions as a Proton-coupled Symporter for Phytosiderophore-and Nicotianamine-chelated Metals. J. Biol. Chem. 2004, 279. [Google Scholar] [CrossRef] [PubMed]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta Biomembr. 2000, 1465. [Google Scholar] [CrossRef]
- Johnson, N.C.; Gehring, C.A. Mycorrhizas: Symbiotic Mediators of Rhizosphere and Ecosystem Processes. In The Rhizosphere; Academic Press: Cambridge, MA, USA, 2007; ISBN 9780120887750. [Google Scholar]
- Smith, S.; Read, D. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123705266. [Google Scholar]
- Gao, X.; Kuyper, T.W.; Zou, C.; Zhang, F.; Hoffland, E. Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil 2007, 290, 283–291. [Google Scholar] [CrossRef]
- Dixit, S.; Grondin, A.; Lee, C.R.; Henry, A.; Olds, T.M.; Kumar, A. Understanding rice adaptation to varying agro-ecosystems: Trait interactions and quantitative trait loci. BMC Genet. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Torres, R.O.; Sta Cruz, M.T.; Maturan, P.C.; Jain, R.; Kumar, A.; Henry, A. Traits and QTLs for development of dry direct-seeded rainfed rice varieties. J. Exp. Bot. 2015, 66. [Google Scholar] [CrossRef]
- Dingkuhn, M.; Johnson, D.E.; Sow, A.; Audebert, A.Y. Relationships between upland rice canopy characteristics and weed competitiveness. Field Crops Res. 1999, 61. [Google Scholar] [CrossRef]
- Shipley, B. Net assimilation rate, specific leaf area and leaf mass ratio: Which is most closely correlated with relative growth rate? A meta-analysis. Funct. Ecol. 2006, 20, 565–574. [Google Scholar] [CrossRef]
- Cui, K.H.; Peng, S.B.; Xing, Y.Z.; Xu, C.G.; Yu, S.B.; Zhang, Q. Molecular dissection of seedling-vigor and associated physiological traits in rice. Theor. Appl. Genet. 2002, 105. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, T.; Ikarashi, H.; Kambayashi, M. Genetic variations in embryo and endosperm weights, seedling growth parameters and alpha-amylase activity of the germinated grains in rice (Oryza sativa L.). Ikushugaku Zasshi Jpn. J. Breed. 1986, 36. [Google Scholar] [CrossRef]
- Mahender, A.; Anandan, A.; Pradhan, S.K. Early seedling vigour, an imperative trait for direct-seeded rice: An overview on physio-morphological parameters and molecular markers. Planta 2015, 241. [Google Scholar] [CrossRef] [PubMed]
- Anandan, A.; Anumalla, M.; Pradhan, S.K.; Ali, J. Population structure, diversity and trait association analysis in rice (Oryza sativa L.) germplasm for early seedling vigor (ESV) using trait linked SSR markers. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhan, J.; Li, J.; Lu, X.; Liu, J.; Wang, Y.; Zhao, Q.; Ye, G. Genome-wide association study (GWAS) for mesocotyl elongation in rice (Oryza sativa L.) under multiple culture conditions. Genes 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Sasaki, K.; Kang, J.W.; Sato, T.; Song, W.Y.; Ahn, S.N. Mesocotyl Elongation is Essential for Seedling Emergence Under Deep-Seeding Condition in Rice. Rice 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Caton, B.P.; Cope, A.E.; Mortimer, M. Growth traits of diverse rice cultivars under severe competition: Implications for screening for competitiveness. Field Crops Res. 2003, 83. [Google Scholar] [CrossRef]
- Okami, M.; Kato, Y.; Yamagishi, J. Role of early vigor in adaptation of rice to water-saving aerobic culture: Effects of nitrogen utilization and leaf growth. Field Crops Res. 2011, 124. [Google Scholar] [CrossRef]
- Sandhu, N.; Singh, A.; Dixit, S.; Sta Cruz, M.T.; Maturan, P.C.; Jain, R.K.; Kumar, A. Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress. BMC Genet. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.G.; Li, X.Q.; Xue, Y.; Huang, Y.W.; Gao, J.; Xing, Y.Z. Comparison of quantitative trait loci controlling seedling characteristics at two seedling stages using rice recombinant inbred lines. Theor. Appl. Genet. 2004, 109. [Google Scholar] [CrossRef]
- Xie, L.; Tan, Z.; Zhou, Y.; Xu, R.; Feng, L.; Xing, Y.; Qi, X. Identification and fine mapping of quantitative trait loci for seed vigor in germination and seedling establishment in rice. J. Integr. Plant Biol. 2014, 56. [Google Scholar] [CrossRef]
- Singh, U.M.; Yadav, S.; Dixit, S.; Ramayya, P.J.; Devi, M.N.; Raman, K.A.; Kumar, A. QTL hotspots for early vigor and related traits under dry direct-seeded system in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, J.K.; Yi, Q.; Wang, Y.Z.; Zhu, Y.G.; Zhang, Z.H. Quantitative trait loci for seedling vigor in rice under field conditions. Field Crops Res. 2007, 100, 294–301. [Google Scholar] [CrossRef]
- Saengwilai, P.; Tian, X.; Lynch, J.P. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol. 2014, 166, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.X. The WUSCHEL-Related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 2009, 21. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an Auxin Response Factor in auxin signaling. Plant Cell 2005, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; He, F.F.; Ma, X.X.; Mao, C.Z.; Hodgman, C.; Lu, C.G.; Wu, P. OsCAND1 is required for crown root emergence in rice. Mol. Plant 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Pandeya, D.; Koh, H.J.; Hwang, J.Y.; Kim, G.T.; Paek, N.C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198. [Google Scholar] [CrossRef]
- Wang, H.; Taketa, S.; Miyao, A.; Hirochika, H.; Ichii, M. Isolation of a novel lateral-rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin. Plant Sci. 2006, 170. [Google Scholar] [CrossRef]
- Faiyue, B.; Vijayalakshmi, C.; Nawaz, S.; Nagato, Y.; Taketa, S.; Ichii, M.; Al-Azzawi, M.J.; Flowers, T.J. Studies on sodium bypass flow in lateral rootless mutants lrt1 and lrt2, and crown rootless mutant crl1 of rice (Oryza sativa L.). Plant Cell Environ. 2010, 33. [Google Scholar] [CrossRef]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Lim, J.H.; Paek, N.C. Rice NARROW LEAF1 Regulates Leaf and Adventitious Root Development. Plant Mol. Biol. Report. 2014, 32. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Qu, X.S.; Wan, S.; Chen, L.H.; Zhu, Y.G. Comparison of QTL controlling seedling vigour under different temperature conditions using recombinant inbred lines in rice (Oryza sativa). Ann. Bot. 2005, 95. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166. [Google Scholar] [CrossRef] [PubMed]
- Guglielminetti, L.; Yamaguchi, J.; Perata, P.; Alpi, A. Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiol. 1995, 109. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Takano, T.; Akita, S. Expression of α-expansin genes in young seedlings of rice (Oryza sativa L.). Planta 2000, 211. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.R.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, C.S.; Jeong, J.U.; Reinke, R.F.; Jeong, J.M. Marker-assisted breeding for improvement of anaerobic germination in japonica rice (Oryza sativa). Plant Breed. 2019, 138. [Google Scholar] [CrossRef]
- Armstrong, W.; Justin, S.H.F.W.; Beckett, P.M.; Lythe, S. Root adaptation to soil waterlogging. Aquat. Bot. 1991, 39. [Google Scholar] [CrossRef]
- Uga, Y.; Okuno, K.; Yano, M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 2011, 62, 2485–2494. [Google Scholar] [CrossRef]
- Waidmann, S.; Ruiz Rosquete, M.; Schöller, M.; Sarkel, E.; Lindner, H.; LaRue, T.; Petřík, I.; Dünser, K.; Martopawiro, S.; Sasidharan, R.; et al. Cytokinin functions as an asymmetric and anti-gravitropic signal in lateral roots. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Ghosal, S.; Casal, C.; Quilloy, F.A.; Septiningsih, E.M.; Mendioro, M.S.; Dixit, S. Deciphering Genetics Underlying Stable Anaerobic Germination in Rice: Phenotyping, QTL Identification, and Interaction Analysis. Rice 2019, 12. [Google Scholar] [CrossRef]
- Chung, N.J.; Paek, N.C. Photoblastism and ecophysiology of seed germination in weedy rice. Agron. J. 2003, 95, 184–190. [Google Scholar] [CrossRef]
- Jeong, J.M.; Cho, Y.C.; Jeong, J.U.; Mo, Y.J.; Kim, C.S.; Kim, W.J.; Baek, M.K.; Kim, S.M. QTL mapping and effect confirmation for anaerobic germination tolerance derived from the japonica weedy rice landrace PBR. Plant Breed. 2020, 139, 83–92. [Google Scholar] [CrossRef]
- Angaji, S.A.; Septiningsih, E.M.; Mackill, D.J.; Ismail, A.M. QTLs associated with tolerance of flooding during germination in rice (Oryza sativa L.). Euphytica 2010, 172, 159–168. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Ignacio, J.C.I.; Sendon, P.M.D.; Sanchez, D.L.; Ismail, A.M.; Mackill, D.J. QTL mapping and confirmation for tolerance of anaerobic conditions during germination derived from the rice landrace Ma-Zhan Red. Theor. Appl. Genet. 2013, 126. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Kataoka, T.; Shiratsuchi, H.; Fukushima, A.; Yamaguchi, H.; Mochida, H.; Ogiwara, H. QTLs for seedling growth of direct seeded rice under submerged and low temperature conditions. Plant Prod. Sci. 2014, 17. [Google Scholar] [CrossRef]
- Anandan, A.; Parameswaran, C.; Azharudheen, T.M.; Singh, O. Towards the development of dry direct seeded rice varieties by stacking of multiple QTLs/genes. ORYZA Int. J. Rice 2018, 55, 51–56. [Google Scholar] [CrossRef]
- Singh, A.K.; Chinnusamy, V. Enhancing rice productivity in water-stressed environments: Perspectives for genetic improvement and management. In Drought Frontiers in Rice: Crop Improvement for Increased Rainfed Production; World Scientific: Singapore, 2009; ISBN 9789814280013. [Google Scholar]
- Ahmadi, N.; Audebert, A.; Bennett, M.J.; Bishopp, A.; de Oliveira, A.C.; Courtois, B.; Diedhiou, A.; Diévart, A.; Gantet, P.; Ghesquière, A.; et al. The roots of future rice harvests. Rice 2014, 7. [Google Scholar] [CrossRef]
- O’Toole, J.C. Adaptation of rice to drought-prone environments. In Drought Resistance in Crops with Emphasis on Rice; International Rice Research Institute: Los Banos, Philippines, 1982; ISBN 9711040786. [Google Scholar]
- Fukai, S.; Cooper, M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Res. 1995, 40, 67–86. [Google Scholar] [CrossRef]
- Inukai, Y.; Sakamoto, T.; Morinaka, Y.; Miwa, M.; Kojima, M.; Tanimoto, E.; Yamamoto, H.; Sato, K.; Katayama, Y.; Matsuoka, M.; et al. ROOT GROWTH INHIBITING, a Rice Endo-1,4-β-d-Glucanase, Regulates Cell Wall Loosening and is Essential for Root Elongation. J. Plant Growth Regul. 2012, 31. [Google Scholar] [CrossRef]
- Kitomi, Y.; Itoh, J.I.; Uga, Y. Genetic mechanisms involved in the formation of root system architecture. In Rice Genomics, Genetics and Breeding; Springer: Singapore, 2018; ISBN 9789811074615. [Google Scholar]
- Shin, J.H.; Jeong, D.H.; Park, M.C.; An, G. Characterization and transcriptional expression of the α-expansin gene family in rice. Mol. Cells 2005, 20, 210–218. [Google Scholar] [PubMed]
- Zhang, J.W.; Xu, L.; Wu, Y.R.; Chen, X.A.; Liu, Y.; Zhu, S.H.; Ding, W.N.; Wu, P.; Yi, K.K. OsGLU3, a putative membrane-bound endo-1,4-beta-glucanase, is required for root cell elongation and division in rice (Oryza sativa L.). Mol. Plant 2012, 5. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, C.; Liu, R.; Han, Q.; Vandeleur, R.K.; Du, J.; Tyerman, S.; Shou, H. Constitutive overexpression of soybean plasma membrane intrinsic protein GmPIP1;6 confers salt tolerance. BMC Plant Biol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yuo, T.; Shiotani, K.; Shitsukawa, N.; Miyao, A.; Hirochika, H.; Ichii, M.; Taketa, S. Root hairless 2 (RTH2) mutant represents a loss-of-function allele of the cellulose synthase-like gene OSCSLD1 in rice (Oryza sativa L.). Breed. Sci. 2011, 61. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Xu, X.; Zhan, X.; Zhai, R.; Wu, W.; Shen, X.; Dai, G.; Cao, L.; Cheng, S. Identification of qRL7, a major quantitative trait locus associated with rice root length in hydroponic conditions. Breed. Sci. 2013, 63. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Mu, P.; Zhang, H.; Chen, C.Y.; Gao, Y.; Tian, Y.; Wen, F.; Li, Z. Mapping QTLs of root morphological traits at different growth stages in rice. Genetica 2008, 133, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.A.; Price, A.H.; Shashidhar, H.E.; Witcombe, J.R. Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor. Appl. Genet. 2006, 112, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.R.P.; Henry, A.; Yamauchi, A.; Shashidhar, H.E.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crops Res. 2011, 122. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Liu, L.; Chen, Y.; Du, Y.; Zhang, J.; Sun, H.; Zhao, Q. qRT9, a quantitative trait locus controlling root thickness and root length in upland rice. J. Exp. Bot. 2015, 66. [Google Scholar] [CrossRef]
- Brown, L.K.; George, T.S.; Thompson, J.A.; Wright, G.; Lyon, J.; Dupuy, L.; Hubbard, S.F.; White, P.J. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 2012, 110. [Google Scholar] [CrossRef]
- Ding, W.; Yu, Z.; Tong, Y.; Huang, W.; Chen, H.; Wu, P. A transcription factor with a bHLH domain regulates root hair development in rice. Cell Res. 2009, 19. [Google Scholar] [CrossRef]
- Libault, M.; Brechenmacher, L.; Cheng, J.; Xu, D.; Stacey, G. Root hair systems biology. Trends Plant Sci. 2010, 15. [Google Scholar] [CrossRef]
- Sakamoto, T.; Sakakibara, H.; Kojima, M.; Yamamoto, Y.; Nagasaki, H.; Inukai, Y.; Sato, Y.; Matsuoka, M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006, 142. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Ma, N.; Che, S.; Wang, Y.; Peng, X.; Zhang, G.; Wang, G.; Huang, J. Repression of OsEXPA3 expression leads to root system growth suppression in rice. Crop Sci. 2014, 54. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, B.; He, X.; Lv, S.; Bai, Y.; Ding, W.; Chen, M.; Cho, H.T.; Wu, P. Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011, 66. [Google Scholar] [CrossRef]
- Won, S.K.; Choi, S.B.; Kumari, S.; Cho, M.; Lee, S.H.; Cho, H.T. Root hair-specific EXPANSIN B genes have been selected for graminaceae root hairs. Mol. Cells 2010, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kim, C.M.; Xuan, Y.H.; Park, S.J.; Piao, H.L.; Je, B.I.; Liu, J.; Kim, T.H.; Kim, B.K.; Han, C.D. OsSNDP1, a Sec14-nodulin domain-containing protein, plays a critical role in root hair elongation in rice. Plant Mol. Biol. 2013, 82, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, S.; Ng, S.; Zhang, B.; Zhou, Y.; Whelan, J.; Wu, P.; Shou, H. Mutation in xyloglucan 6-xylosytransferase results in abnormal root hair development in Oryza sativa. J. Exp. Bot. 2014, 65. [Google Scholar] [CrossRef]
- Price, M.; Dilday, R.; Allen, A. Characterization of Rice (Oryza sativa L.) Roots Versus Root Pulling Resistance as Selection Indices for Draught Tolerance. J. Ark. Acad. Sci. 1989, 43, 63–65. [Google Scholar]
- Kano-Nakata, M.; Inukai, Y.; Wade, L.J.; Siopongco, J.D.; Yamauchi, A. Root development, water uptake, and shoot dry matter production under water defi cit conditions in two cssls of rice: Functional roles of root plasticity. Plant Prod. Sci. 2011, 14. [Google Scholar] [CrossRef]
- Tran, T.T.; Kano-Nakata, M.; Suralta, R.R.; Menge, D.; Mitsuya, S.; Inukai, Y.; Yamauchi, A. Root plasticity and its functional roles were triggered by water deficit but not by the resulting changes in the forms of soil N in rice. Plant Soil 2014, 386. [Google Scholar] [CrossRef]
- Suralta, R.R.; Inukai, Y.; Yamauchi, A. Dry matter production in relation to root plastic development, oxygen transport, and water uptake of rice under transient soil moisture stresses. Plant Soil 2010, 332. [Google Scholar] [CrossRef]
- Kano, M.; Inukai, Y.; Kitano, H.; Yamauchi, A. Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 2011, 342. [Google Scholar] [CrossRef]
- Kano-Nakata, M.; Gowda, V.R.P.; Henry, A.; Serraj, R.; Inukai, Y.; Fujita, D.; Kobayashi, N.; Suralta, R.R.; Yamauchi, A. Functional roles of the plasticity of root system development in biomass production and water uptake under rainfed lowland conditions. Field Crops Res. 2013, 144. [Google Scholar] [CrossRef]
- Niones, J.M.; Inukai, Y.; Suralta, R.R.; Yamauchi, A. QTL associated with lateral root plasticity in response to soil moisture fluctuation stress in rice. Plant Soil 2015, 391. [Google Scholar] [CrossRef]
- Niones, J.M.; Suralta, R.R.; Inukai, Y.; Yamauchi, A. Field evaluation on functional roles of root plastic responses on dry matter production and grain yield of rice under cycles of transient soil moisture stresses using chromosome segment substitution lines. Plant Soil 2012, 359. [Google Scholar] [CrossRef]
- Niones, J.M.; Suralta, R.R.; Inukai, Y.; Yamauchi, A. Roles of root aerenchyma development and its associated QTL in dry matter production under transient moisture stress in rice. Plant Prod. Sci. 2013, 16. [Google Scholar] [CrossRef]
- Vikram, P.; Swamy, B.P.M.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought susceptibility of modern rice varieties: An effect of linkage of drought tolerance with undesirable traits. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Wade, L.J.; Bartolome, V.; Mauleon, R.; Vasant, V.D.; Prabakar, S.M.; Chelliah, M.; Kameoka, E.; Nagendra, K.; Reddy, K.R.K.; Varma, C.M.K.; et al. Environmental response and genomic regions correlated with rice root growth and yield under drought in the oryzaSNP panel across multiple study systems. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Zhu, J.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ. 2010, 33, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Shuai, J.; Wang, P.; Shi, W.; Tao, F.; Chen, Y. Impact of chilling injury and global warming on rice yield in Heilongjiang Province. J. Geogr. Sci. 2013, 23. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Heat and chilling induced disruption of redox homeostasis and its regulation by hydrogen peroxide in germinating rice seeds (Oryza sativa L., Cultivar Ratna). Physiol. Mol. Biol. Plants 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiol. 2010, 152. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.; Murai-Hatano, M.; Ishikawa-Sakurai, J.; Hayashi, H.; Kawamura, Y.; Uemura, M. Cold stress-induced acclimation in rice is mediated by root-specific aquaporins. Plant Cell Physiol. 2012, 53. [Google Scholar] [CrossRef] [PubMed]
- Murai-Hatano, M.; Kuwagata, T.; Sakurai, J.; Nonami, H.; Ahamed, A.; Nagasuga, K.; Matsunami, T.; Fukushi, K.; Maeshima, M.; Okada, M. Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant Cell Physiol. 2008, 49. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46. [Google Scholar] [CrossRef] [PubMed]
- Buvaneshwari, S.; Riotte, J.; Sekhar, M.; Sharma, A.K.; Helliwell, R.; Kumar, M.S.M.; Braun, J.J.; Ruiz, L. Potash fertilizer promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Gopalakrishnan, T.; Kumar, L. Linking long-term changes in soil salinity to paddy land abandonment in Jaffna Peninsula, Sri Lanka. Agriculture 2021, 11, 211. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. Int. 2014, 22. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M.; Ahmad, P.; Chandna, R.; Prasad, M.N.V.; Ozturk, M. Enhancing plant productivity under salt stress: Relevance of Poly-omics. In Salt Stress in Plants: Signalling, Omics and Adaptations; Springer: New York, NY, USA, 2013; ISBN 9781461461081. [Google Scholar]
- Lin, C.C.; Kao, C.H. Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci. 2001, 160. [Google Scholar] [CrossRef]
- Saruyama, H.; Tanida, M. Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and-tolerant cultivars of rice (Oryza sativa L.). Plant Sci. 1995, 109. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25. [Google Scholar] [CrossRef]
- Horie, T.; Karahara, I.; Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 2012, 5, 1–18. [Google Scholar] [CrossRef]
- Vishal, B.; Krishnamurthy, P.; Ramamoorthy, R.; Kumar, P.P. OsTPS8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition. New Phytol. 2019, 221. [Google Scholar] [CrossRef] [PubMed]
- Hongyan, L.; Weiqin, W.; Aibin, H.; Lixiao, N. Correlation of Leaf and Root Senescence During Ripening in Dry Seeded and Transplanted Rice. Rice Sci. 2018, 25. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Q.; Wang, C.C.; Liu, Z.X.; Jiang, Y.J.; Zhai, L.Y.; Zheng, T.Q.; Xu, J.L.; Li, Z.K. Genetic dissection of seedling vigour in a diverse panel from the 3000 Rice (Oryza sativa L.) Genome Project. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Deng, J.; Feng, X.; Wang, D.; Lu, J.; Chong, H.; Shang, C.; Liu, K.; Huang, L.; Tian, X.; Zhang, Y. Root morphological traits and distribution in direct-seeded rice under dense planting with reduced nitrogen. PLoS ONE 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Qian, Q.; Bu, Q.; Li, S.; Chen, Q.; Sun, J.; Liang, W.; Zhou, Y.; Chu, C.; Li, X.; et al. Mutation of the rice narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiol. 2008, 147. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, X.; Xiong, L. Evaluation of near-isogenic lines for drought resistance QTL and fine mapping of a locus affecting flag leaf width, spikelet number, and root volume in rice. Theor. Appl. Genet. 2011, 123. [Google Scholar] [CrossRef]
- Robinson, D.; Linehan, D.J.; Caul, S. What limits nitrate uptake from soil? Plant Cell Environ. 1991, 14. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Thompson, M.V.; Holbrook, N.M. Understanding the hydraulics of porous pipes: Tradeoffs between water uptake and root length utilization. J. Plant Growth Regul. 2002, 21, 315–323. [Google Scholar] [CrossRef]
- Uga, Y.; Okuno, K.; Yano, M. QTLs underlying natural variation in stele and xylem structures of rice root. Breed. Sci. 2008, 58. [Google Scholar] [CrossRef]
- Uga, Y.; Okuno, K.; Yano, M. Fine mapping of Sta1, a quantitative trait locus determining stele transversal area, on rice chromosome 9. Mol. Breed. 2010, 26. [Google Scholar] [CrossRef]
- Ramanathan, V.; Rahman, H.; Subramanian, S.; Nallathambi, J.; Kaliyaperumal, A.; Manickam, S.; Ranganathan, C.; Raveendran, M. OsARD4 encoding an acireductone dioxygenase improves root architecture in rice by promoting development of secondary roots. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.; Liu, D.; Guo, X.; Yang, W.; Sun, J.; Li, X.; Zhan, K.; Cui, D.; Lin, J.; Zhang, A. Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J. 2013, 1. [Google Scholar] [CrossRef]
- Chuanren, D.; Bochu, W.; Pingqing, W.; Daohong, W.; Shaoxi, C. Relationship between the minute structure and the lodging resistance of rice stems. Colloids Surf. B Biointerfaces 2004, 35. [Google Scholar] [CrossRef] [PubMed]
- Terashima, K.; Ogata, T.; Akita, S. Eco-Physiological Characteristics Related with Lodging Tolerance of Rice in Direct Sowing Cultivation: II. Root growth characteristics of tolerant cultivars to root lodging. Jpn. J. Crop Sci. 1994, 63. [Google Scholar] [CrossRef]
- Yano, K.; Ookawa, T.; Aya, K.; Ochiai, Y.; Hirasawa, T.; Ebitani, T.; Takarada, T.; Yano, M.; Yamamoto, T.; Fukuoka, S.; et al. Isolation of a novel lodging resistance QTL gene involved in strigolactone signaling and its pyramiding with a QTL gene involved in another mechanism. Mol. Plant 2015, 8. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Madoka, Y.; Hirotsu, N.; Ishimaru, K. Locus prl5 improves lodging resistance of rice by delaying senescence and increasing carbohydrate reaccumulation. Plant Physiol. Biochem. 2006, 44. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Ishimaru, K. Identification and Functional Analysis of a Locus for Improvement of Lodging Resistance in Rice. Plant Physiol. 2004, 134. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.M.; Griffin, J.M.; Sylvester-Bradley, R.; Scott, R.K.; Spink, J.H.; Baker, C.J.; Clare, R.W. Controlling plant form through husbandry to minimise lodging in wheat. Field Crops Res. 2000, 67. [Google Scholar] [CrossRef]
- Piñera-Chavez, F.J.; Berry, P.M.; Foulkes, M.J.; Jesson, M.A.; Reynolds, M.P. Avoiding lodging in irrigated spring wheat. I. Stem and root structural requirements. Field Crops Res. 2016, 196, 325–336. [Google Scholar] [CrossRef]
- Baker, C.J.; Sterling, M.; Berry, P. A generalised model of crop lodging. J. Theor. Biol. 2014, 363. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Dan, D.; Yuan, Z.; Chen, Y.; Jin, J.; Yang, W.; Zhang, Z.; Li, N.; Li, S. Deciphering the Genetic Basis of Lodging Resistance in Wild Rice Oryza longistaminata. Front. Plant Sci. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Dixit, S.; Swamy, B.P.M.; Raman, A.; Kumar, S.; Singh, S.P.; Yadaw, R.B.; Singh, O.N.; Reddy, J.N.; Anandan, A.; et al. Marker Assisted Breeding to Develop Multiple Stress Tolerant Varieties for Flood and Drought Prone Areas. Rice 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Dreccer, M.F.; Condon, A.G.; Macdonald, B.; Rebetzke, G.J.; Awasi, M.A.; Borgognone, M.G.; Peake, A.; Piñera-Chavez, F.J.; Hundt, A.; Jackway, P.; et al. Genotypic variation for lodging tolerance in spring wheat: Wider and deeper root plates, a feature of low lodging, high yielding germplasm. Field Crops Res. 2020, 258. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct. Plant. Biol. 2010, 37, 147–156. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Stomata and Gas Exchange. In Water Relations of Plants and Soils; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.R.; Lynch, J.P. Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 2001, 236. [Google Scholar] [CrossRef]
- Suzuki, N.; Taketa, S.; Ichii, M. Morphological and physiological characteristics of a root-hairless mutant in rice (Oryza sativa L.). In Roots: The Dynamic Interface between Plants and the Earth; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Alder, N.N.; Sperry, J.S.; Pockman, W.T. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia 1996, 105. [Google Scholar] [CrossRef] [PubMed]
- Pockman, W.T.; Sperry, J.S. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am. J. Bot. 2000, 87. [Google Scholar] [CrossRef]
- Dingkuhn, M.; Jones, M.; Johnson, D.; Fofana, B.; Sow, A. Oryza sativa and O. glaberrima genepools for high-yielding, weed-competitive rice plant types. In Proceedings of the ACIAR PROCEEDINGS, Vientiane, Laos, 18–23 May 1997; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 1997; pp. 144–155. [Google Scholar]
- Olofsdotter, M.; Jensen, L.B.; Courtois, B. Improving crop competitive ability using allelopathy—An example from rice. Plant Breed. 2002, 121, 1–9. [Google Scholar] [CrossRef]
- Yang, X.; Kong, C. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591. [Google Scholar] [CrossRef]
- Chung, I.M.; Ham, T.H.; Cho, G.W.; Kwon, S.W.; Lee, Y.; Seo, J.; An, Y.J.; Kim, S.Y.; Kim, S.H.; Lee, J. Study of quantitative trait loci (Qtls) associated with allelopathic trait in rice. Genes 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Benková, E.; Blilou, I.; Wisniewska, J.; Hamann, T.; Ljung, K.; Woody, S.; Sandberg, G.; Scheres, B.; Jürgens, G.; et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002, 108. [Google Scholar] [CrossRef]
- Ni, J.; Wang, G.; Zhu, Z.; Zhang, H.; Wu, Y.; Wu, P. OsIAA23-mediated auxin signaling defines postembryonic maintenance of QC in rice. Plant J. 2011, 68. [Google Scholar] [CrossRef]
- Scarpella, E.; Rueb, S.; Meijer, A.H. The RADICLELESS1 gene is required for vascular pattern formation in rice. Development 2003, 130. [Google Scholar] [CrossRef] [PubMed]
- Skoog, F.; O Miller, C. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–131. [Google Scholar]
- Woo, Y.M.; Park, H.J.; Su’udi, M.; Yang, J.I.; Park, J.J.; Back, K.; Park, Y.M.; An, G. Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Mol. Biol. 2007, 65. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Hu, H.; Wang, G.H.; Li, J.; Chen, J.Y.; Wu, P. Expression of PIN genes in rice (Oryza sativa L.): Tissue specificity and regulation by hormones. Mol. Plant 2009, 2, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Takasugi, T.; Ito, Y. Identification and expression analysis of PIN genes in rice. Plant Sci. 2010, 178. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005, 46. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kyozuka, J. Characterization of OsPID, the rice ortholog of PINOID, and its possible involvement in the control of polar auxin transport. Plant Cell Physiol. 2007, 48, 540–549. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. CYTOKININ OXIDASE/DEHYDROGENASE4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol. 2014, 165. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Wang, S.; Jia, Q.; Wu, P. OsEIL1, a rice homolog of the Arabidopsis EIN3 regulates the ethylene response as a positive component. Plant Mol. Biol. 2006, 61. [Google Scholar] [CrossRef]
- Xiao, G.; Qin, H.; Zhou, J.; Quan, R.; Lu, X.; Huang, R.; Zhang, H. OsERF2 controls rice root growth and hormone responses through tuning expression of key genes involved in hormone signaling and sucrose metabolism. Plant Mol. Biol. 2016, 90. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chao, Y.Y.; Hsu, Y.Y.; Hong, C.Y.; Kao, C.H. Heme oxygenase is involved in nitric oxide-and auxin-induced lateral root formation in rice. Plant Cell Rep. 2012, 31. [Google Scholar] [CrossRef] [PubMed]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Z.; Liu, X.; Li, C.; Xiao, K.; Dong, Y.-J. Genetic Analysis and Molecular Mapping of Light-Sensitive Red-Root Mutant in Rice. Rice Sci. 2009, 16, 27–32. [Google Scholar] [CrossRef]
- Andersen, J.R.; Lübberstedt, T. Functional markers in plants. Trends Plant Sci. 2003, 8, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Pandit, E.; Sahoo, A.; Panda, R.K.; Mohanty, D.P.; Pani, D.R.; Anandan, A.; Pradhan, S.K. Survey of rice cultivars and landraces of upland ecology for Phosphorous uptake 1 (Pup1) QTL using linked and gene specific molecular markers. Oryza 2016, 53, 1–9. [Google Scholar]
- Swamy, H.K.M.; Anila, M.; Kale, R.R.; Rekha, G.; Bhadana, V.P.; Anantha, M.S.; Brajendra, P.; Balachiranjeevi, C.H.; Hajira, S.K.; Prasanna, B.L.; et al. Marker assisted improvement of low soil phosphorus tolerance in the bacterial blight resistant, fine-grain type rice variety, Improved Samba Mahsuri. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- ICAR-NRRI. Annual Report 2020; ICAR-NRRI: Odisha, India, 2021. [Google Scholar]
- Pandit, E.; Panda, R.K.; Sahoo, A.; Pani, D.R.; Pradhan, S.K. Genetic Relationship and Structure Analysis of Root Growth Angle for Improvement of Drought Avoidance in Early and Mid-Early Maturing Rice Genotypes. Rice Sci. 2020, 27, 124–132. [Google Scholar] [CrossRef]
- Arai-Sanoh, Y.; Takai, T.; Yoshinaga, S.; Nakano, H.; Kojima, M.; Sakakibara, H.; Kondo, M.; Uga, Y. Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Babu, N.N.; Krishnan, S.G.; Vinod, K.K.; Krishnamurthy, S.L.; Singh, V.K.; Singh, M.P.; Singh, R.; Ellur, R.K.; Rai, V.; Bollinedi, H.; et al. Marker aided incorporation of saltol, a major QTL associated with seedling stage salt tolerance, into Oryza sativa ‘pusa basmati 1121’. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Arul Selvi, G.S.; Kahani, F.; Hittalmani, S. Stability Analysis of Rice Root QTL-NILs and Pyramids for Root Morphology and Grain Yield. Rice Res. Open Access 2015, 3. [Google Scholar] [CrossRef]
- Kumar, K.; Meena, R.K.; Jain, S. Molecular marker analysis of selected rice lines for aerobic traits under water limited conditions. Bioscan 2017, 12, 1309–1315. [Google Scholar]
- Rani, K.; Kumar, K.; Meena, R.K.; Jain, R.K. Phenotypic and microsatellite marker analysis of selected lines of aerobic rice under water limited conditions. Ann. Agric. Res. New Ser. 2017, 38, 1–6. [Google Scholar]
- Sandhu, N.; Dixit, S.; Swamy, B.P.M.; Vikram, P.; Venkateshwarlu, C.; Catolos, M.; Kumar, A. Positive interactions of major-effect QTLs with genetic background that enhances rice yield under drought. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Rani, K.; Meena, R.K.; Mahavir; Jain, R.K.; Jain, S. Agronomic and molecular marker evaluation of selected f 4 plants of rice (Oryza sativa L.) under aerobic conditions. Agric. Res. J. 2018, 55. [Google Scholar] [CrossRef]
- Catolos, M.; Sandhu, N.; Dixit, S.; Shamsudin, N.A.A.; Naredo, M.E.B.; McNally, K.L.; Henry, A.; Diaz, M.G.; Kumar, A. Genetic loci governing grain yield and root development under variable rice cultivation conditions. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.A.A.; Swamy, B.P.M.; Ratnam, W.; Cruz, M.T.S.; Raman, A.; Kumar, A. Marker assisted pyramiding of drought yield QTLs into a popular Malaysian rice cultivar, MR219. BMC Genet. 2016, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Obara, M.; Tamura, W.; Ebitani, T.; Yano, M.; Sato, T.; Yamaya, T. Fine-mapping of qRL6.1, a major QTL for root length of rice seedlings grown under a wide range of NH4+ concentrations in hydroponic conditions. Theor. Appl. Genet. 2010, 121. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Anitha Raman, K.; Torres, R.O.; Audebert, A.; Dardou, A.; Kumar, A.; Henry, A. Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiol. 2016, 171, 2562–2576. [Google Scholar] [CrossRef] [PubMed]
- Kol, C. Genomic Designing of Climate-Smart Cereal Crops; Springer: Amsterdam, The Netherlands, 2020; ISBN 9783319933818. [Google Scholar]
- Anand, S.R.; Umesh, M.R.; Ramesha, Y.M.; Rajkumar, R.H. Evaluation of Varieties/Hybrids and Fertilizer Levels for Direct Seeded Rice (DSR) under Thungabhadra Project (TBP) Command Area of Karnataka. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 4192–4198. [Google Scholar]
- Sun, L.; Hussain, S.; Liu, H.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Implications of low sowing rate for hybrid rice varieties under dry direct-seeded rice system in Central China. Field Crops Res. 2015, 175, 87–95. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S. Performance of dry direct-seeded rice in response to genotype and seeding rate. Agron. J. 2016, 108, 257–265. [Google Scholar] [CrossRef]
- Chin, J.H.; Lu, X.; Haefele, S.M.; Gamuyao, R.; Ismail, A.; Wissuwa, M.; Heuer, S. Development and application of gene-based markers for the major rice QTL Phosphorus uptake 1. Theor. Appl. Genet. 2010, 120, 1073–1086. [Google Scholar] [CrossRef]
- George, T.; Magbanua, R.; Garrity, D.P.; Tubaña, B.S.; Quiton, J. Rapid yield loss of rice cropped successively in aerobic soil. Agron. J. 2002, 94, 981–989. [Google Scholar] [CrossRef]
- Bian, H.; Xie, Y.; Guo, F.; Han, N.; Ma, S.; Zeng, Z.; Wang, J.; Yang, Y.; Zhu, M. Distinctive expression patterns and roles of the miRNA393/TIR1 homolog module in regulating flag leaf inclination and primary and crown root growth in rice (Oryza sativa). New Phytol. 2012, 196, 149–161. [Google Scholar] [CrossRef]
- Seo, H.M.; Jung, Y.; Song, S.; Kim, Y.; Kwon, T.; Kim, D.H.; Jeung, S.J.; Yi, Y.B.; Yi, G.; Nam, M.H.; et al. Increased expression of OsPT1, a high-affinity phosphate transporter, enhances phosphate acquisition in rice. Biotechnol. Lett. 2008, 30. [Google Scholar] [CrossRef] [PubMed]
- Liscum, E.; Reed, J.W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Kaur, N.; Garg, R.; Thakur, J.K.; Tyagi, A.K.; Khurana, J.P. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa). Funct. Integr. Genom. 2006, 6, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Yu, X.; Yu, J.; He, X.; Zhang, S.; Shou, H.; Wu, P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005, 43. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-Deficient Transgenic Arabidopsis Plants Show Multiple Developmental Alterations Indicating Opposite Functions of Cytokinins in the Regulation of Shoot and Root Meristem Activity. Plant Cell 2003, 15. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, D.; Ren, Y.; Zhang, X.; Zhao, J. Characteristic and expression analysis of a metallothionein gene, OsMT2b, down-regulated by cytokinin suggests functions in root development and seed embryo germination of rice. Plant Physiol. 2008, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, T.; Wang, X.; Deng, Y.; Ma, H.; Zhang, R.; Zhao, J. OsAUX1 controls lateral root initiation in rice (Oryza sativa L.). Plant Cell Environ. 2015, 38. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Chen, H.; Jiang, J.F.; Zhao, Y.; Xu, M.L.; Xu, Y.Y.; Tan, K.H.; Xu, Z.H.; Chong, K. Overexpression of OsRAA1 causes pleiotropic phenotypes in transgenic rice plants, including altered leaf, flower, and root development and root response to gravity. Plant Physiol. 2004, 135. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, H.; Jiang, J.; Xu, Y.; Du, J.; Wang, X.; Yuan, M.; Wang, Z.; Xu, Z.; Chong, K. Rice Root Architecture Associated1 binds the proteasome subunit RPT4 and is degraded in a D-box and proteasome-dependent manner. Plant Physiol. 2008, 148. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.W.; Zhang, W.; Gray, W.M. Arabidopsis ETA2, an apparent ortholog of the human cullin-interacting protein CAND1, is required for auxin responses mediated by the SCF TIR1 ubiquitin ligase. Plant Cell 2004, 16. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Shen, Y.; Sullivan, J.A.; Rubio, V.; Xiong, Y.; Sun, T.P.; Deng, X.W. Arabidopsis CAND1, an unmodified CUL1-interacting protein, is involved in multiple developmental pathways controlled by ubiquitin/proteasome-mediated protein. Plant Cell 2004, 16, 1870–1882. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, S.; Majhi, P.K.; Anandan, A.; Mahender, A.; Veludandi, S.; Bastia, D.; Guttala, S.B.; Singh, S.K.; Saha, S.; Ali, J. Proofing Direct-Seeded Rice with Better Root Plasticity and Architecture. Int. J. Mol. Sci. 2021, 22, 6058. https://doi.org/10.3390/ijms22116058
Panda S, Majhi PK, Anandan A, Mahender A, Veludandi S, Bastia D, Guttala SB, Singh SK, Saha S, Ali J. Proofing Direct-Seeded Rice with Better Root Plasticity and Architecture. International Journal of Molecular Sciences. 2021; 22(11):6058. https://doi.org/10.3390/ijms22116058
Chicago/Turabian StylePanda, Siddharth, Prasanta Kumar Majhi, Annamalai Anandan, Anumalla Mahender, Sumanth Veludandi, Debendranath Bastia, Suresh Babu Guttala, Shravan Kumar Singh, Sanjoy Saha, and Jauhar Ali. 2021. "Proofing Direct-Seeded Rice with Better Root Plasticity and Architecture" International Journal of Molecular Sciences 22, no. 11: 6058. https://doi.org/10.3390/ijms22116058
APA StylePanda, S., Majhi, P. K., Anandan, A., Mahender, A., Veludandi, S., Bastia, D., Guttala, S. B., Singh, S. K., Saha, S., & Ali, J. (2021). Proofing Direct-Seeded Rice with Better Root Plasticity and Architecture. International Journal of Molecular Sciences, 22(11), 6058. https://doi.org/10.3390/ijms22116058







