Novel Approach to Tooth Chemistry. Quantification of the Dental-Enamel Junction
Abstract
1. Introduction
2. Results and Discussion
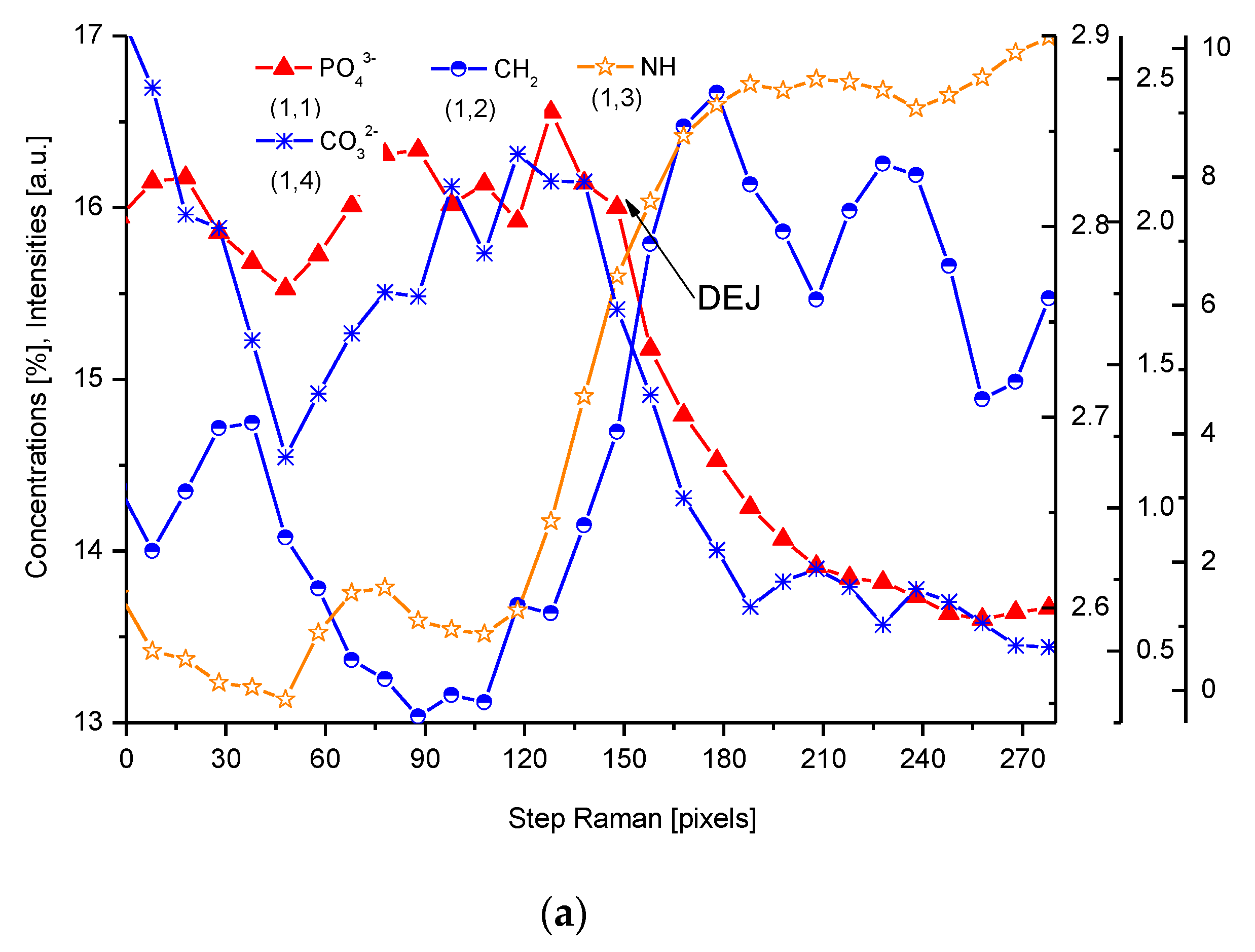
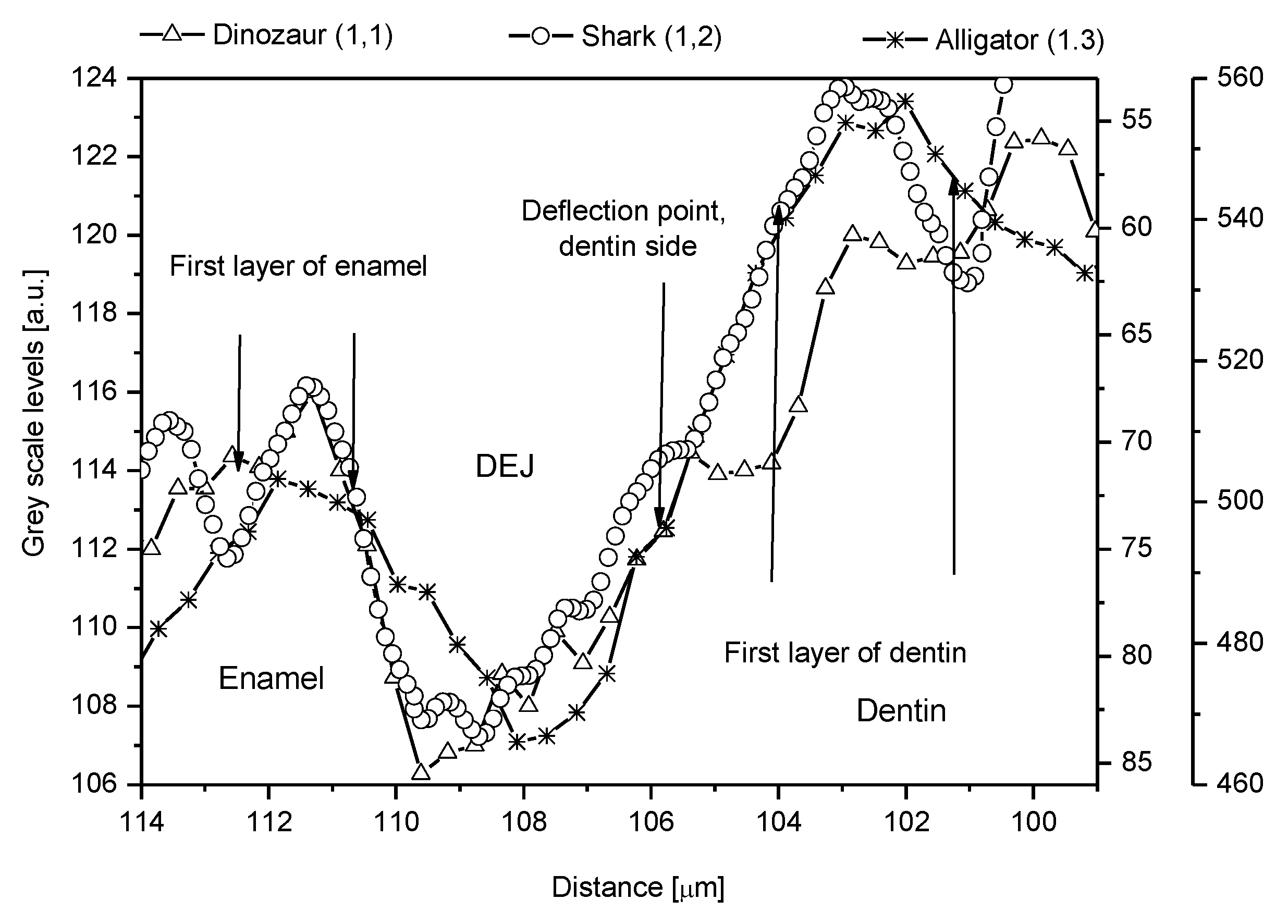
2.1. Cross-Sectional Outline of the DEJ
2.2. Discussion of DEJ Model
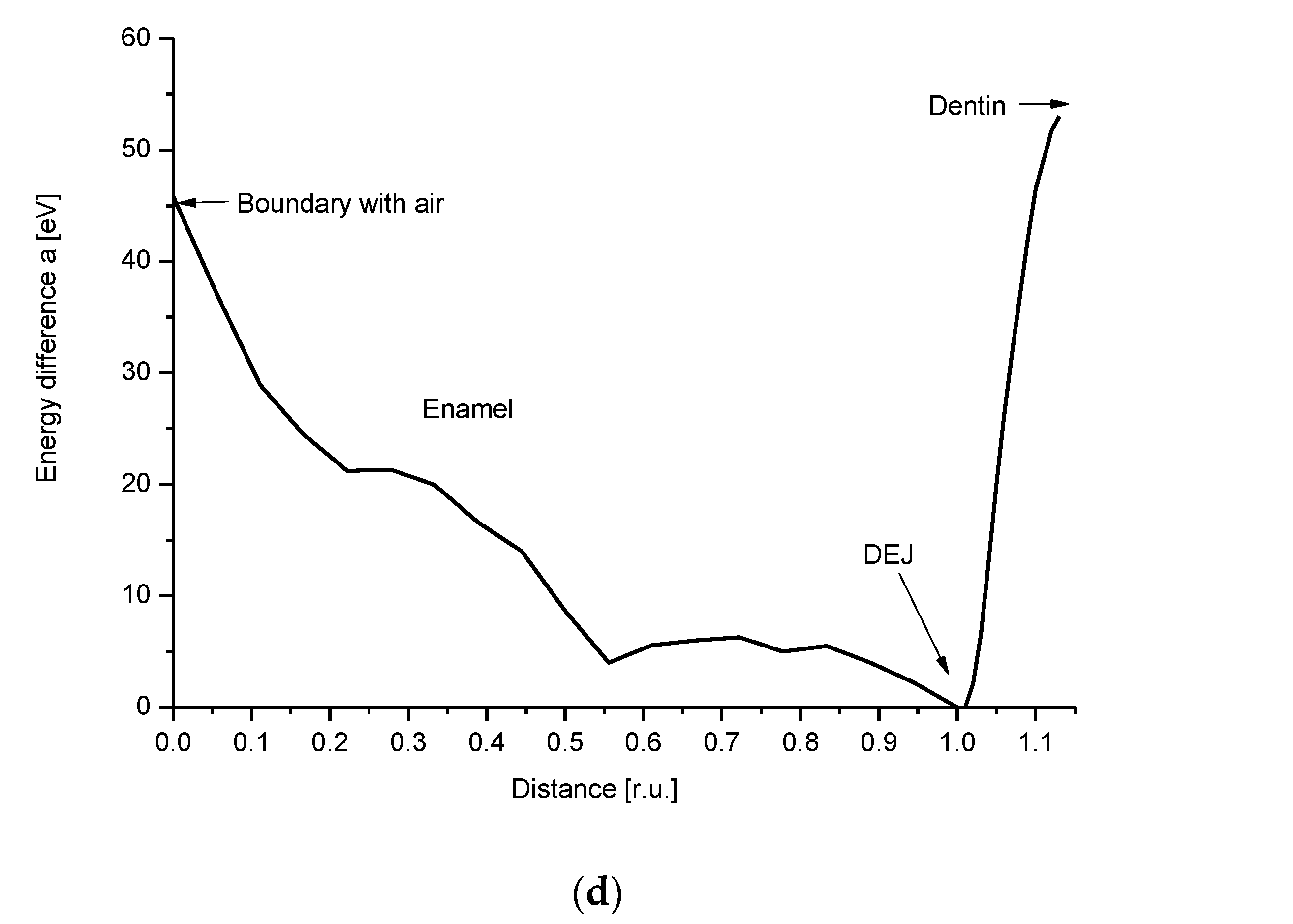
2.3. Energetic Relationships
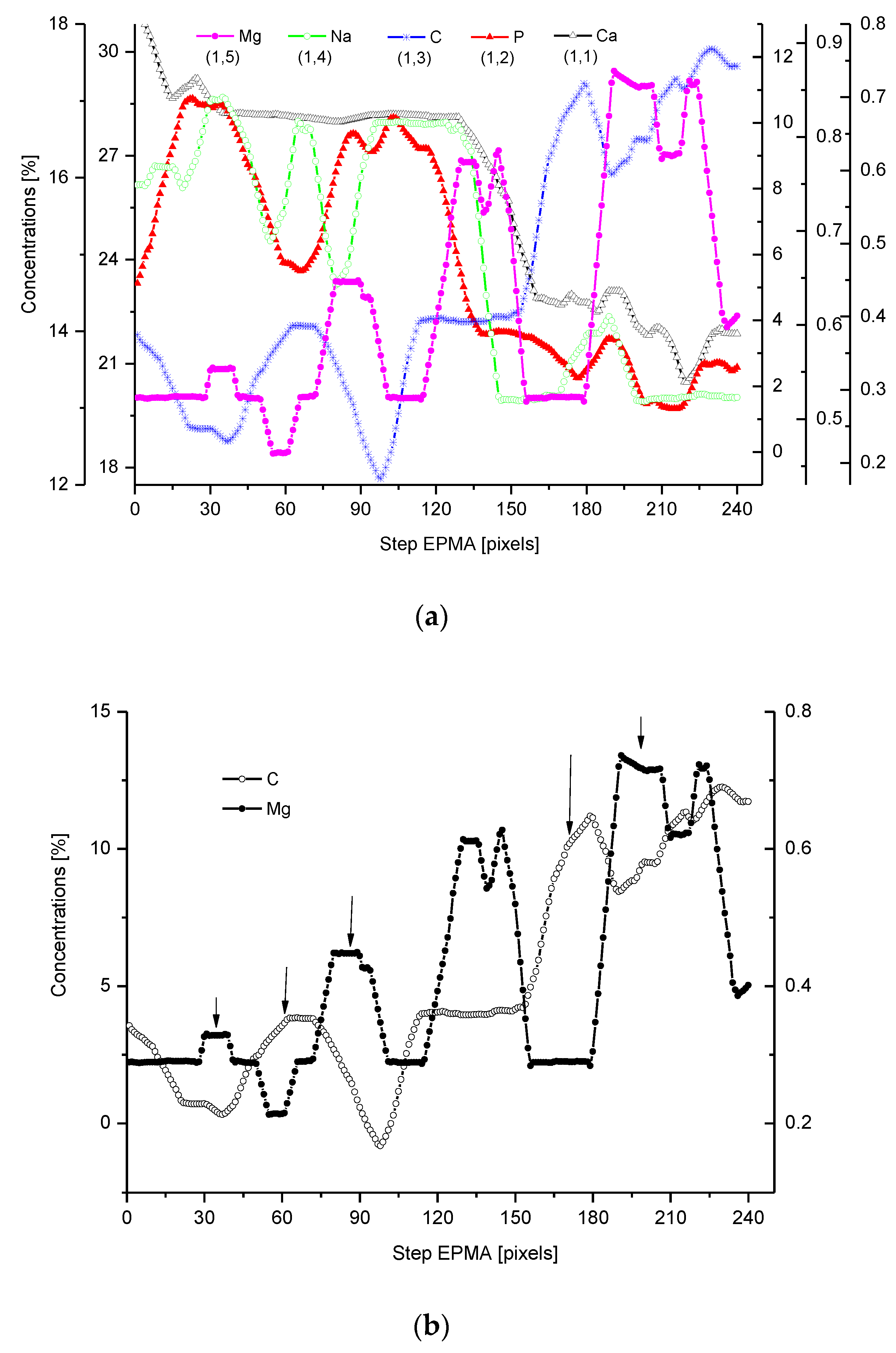
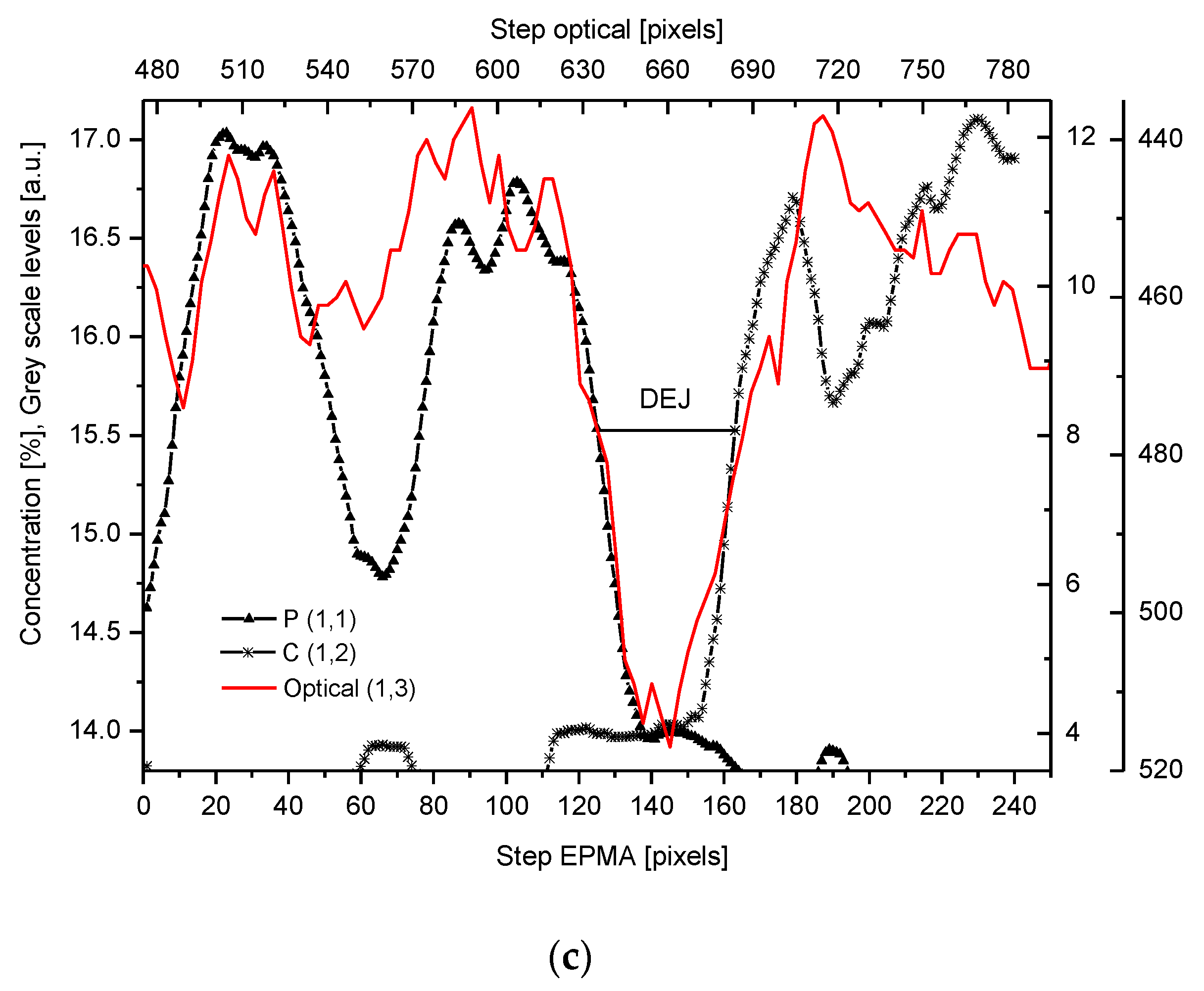
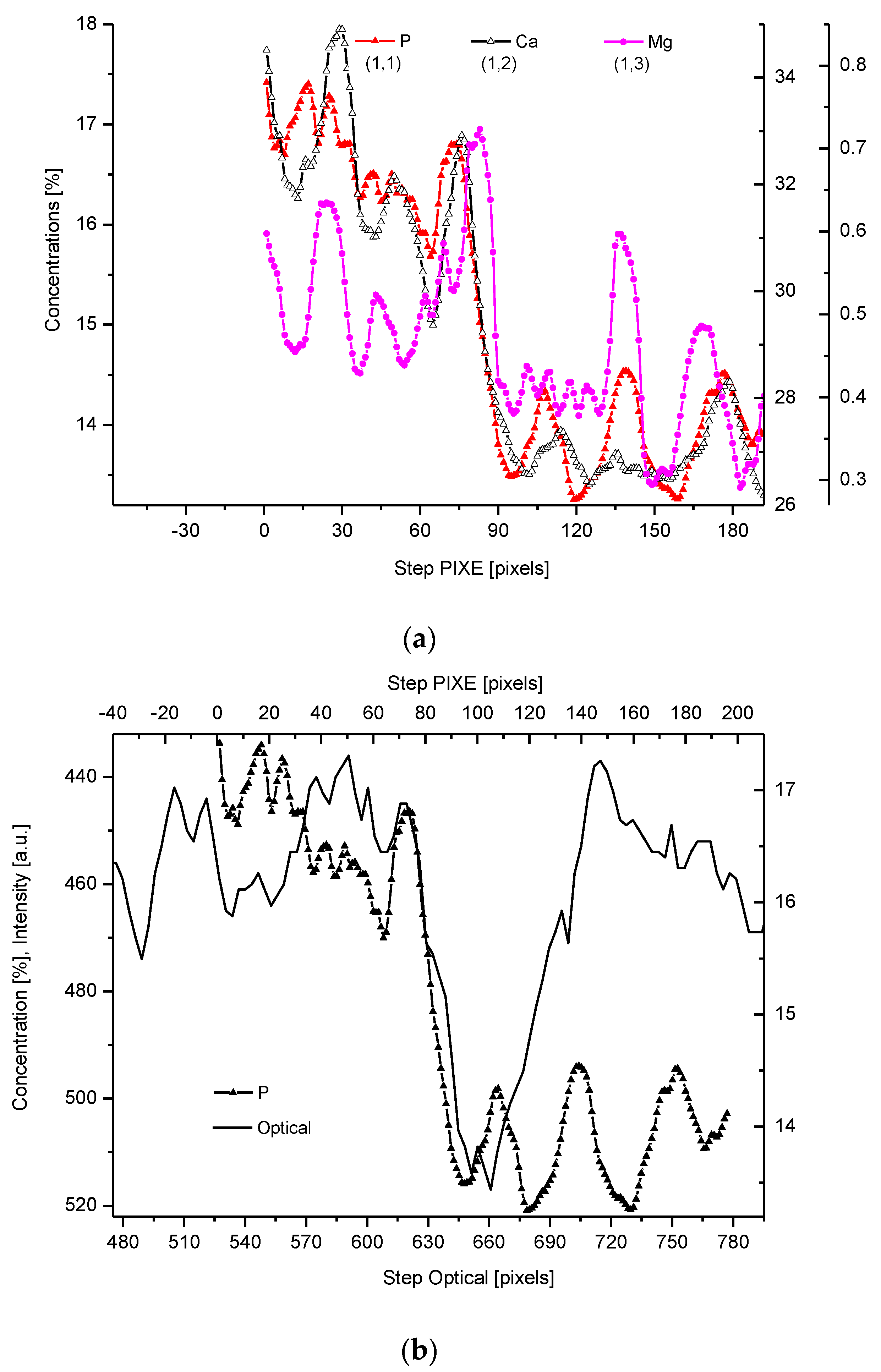
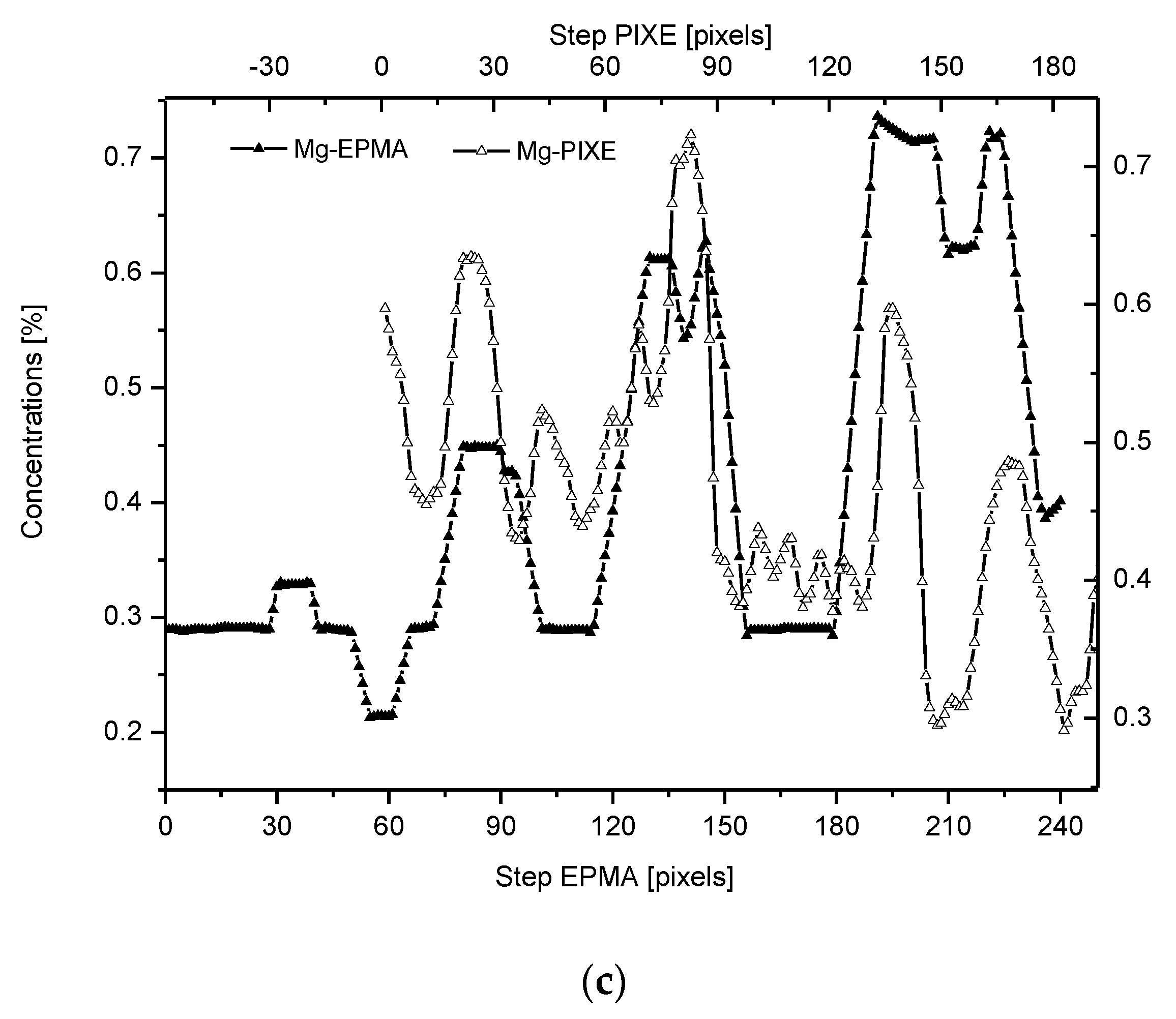
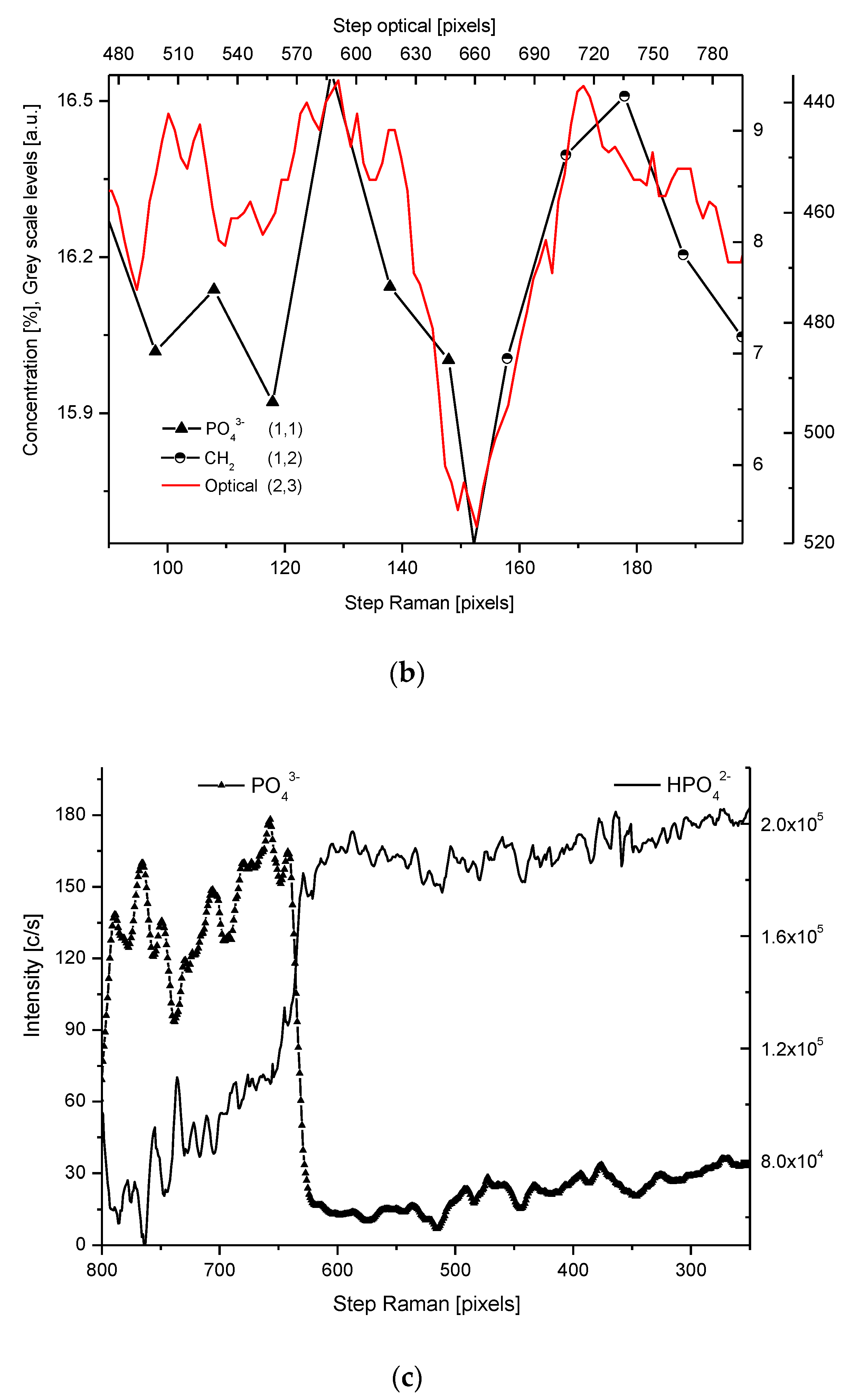
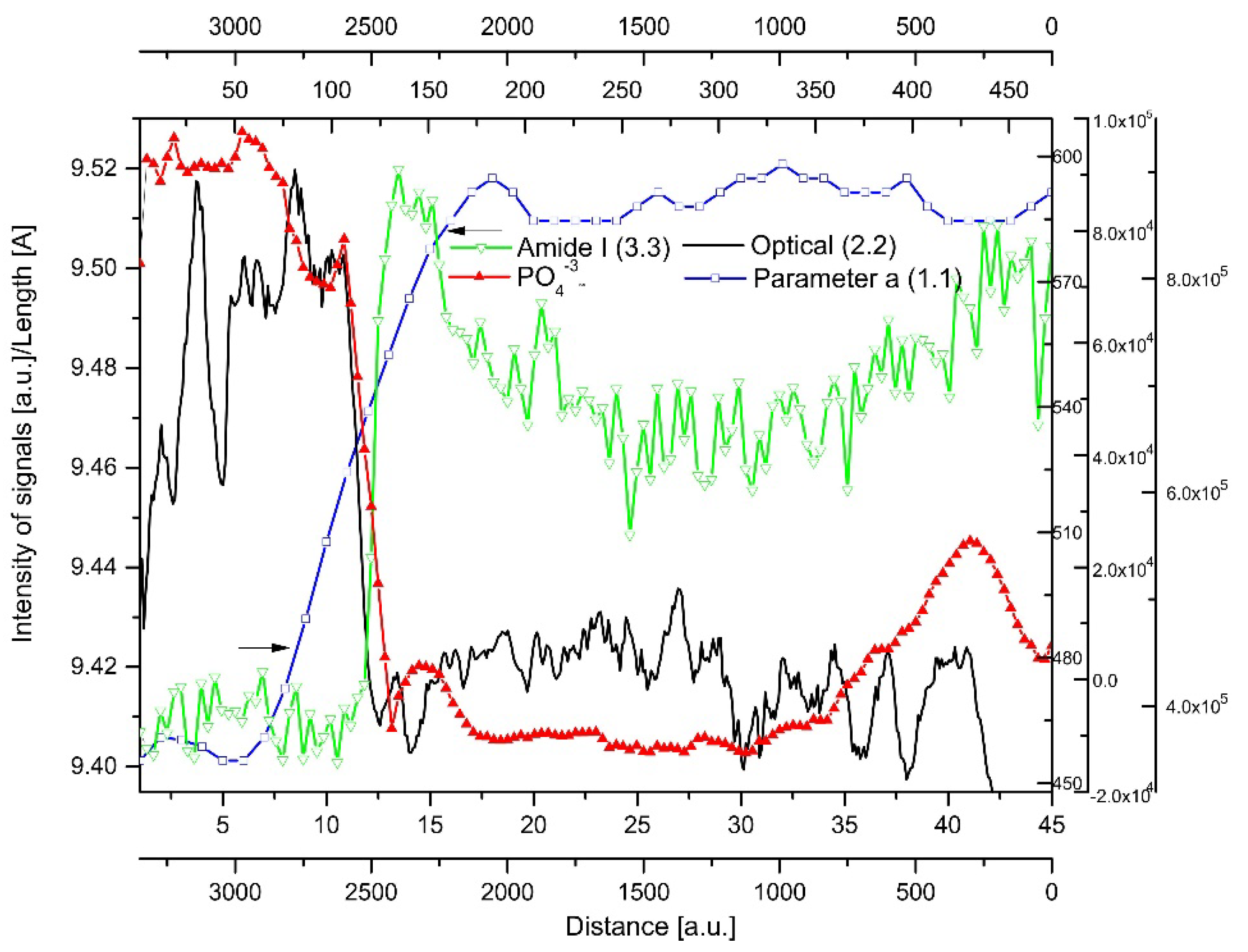
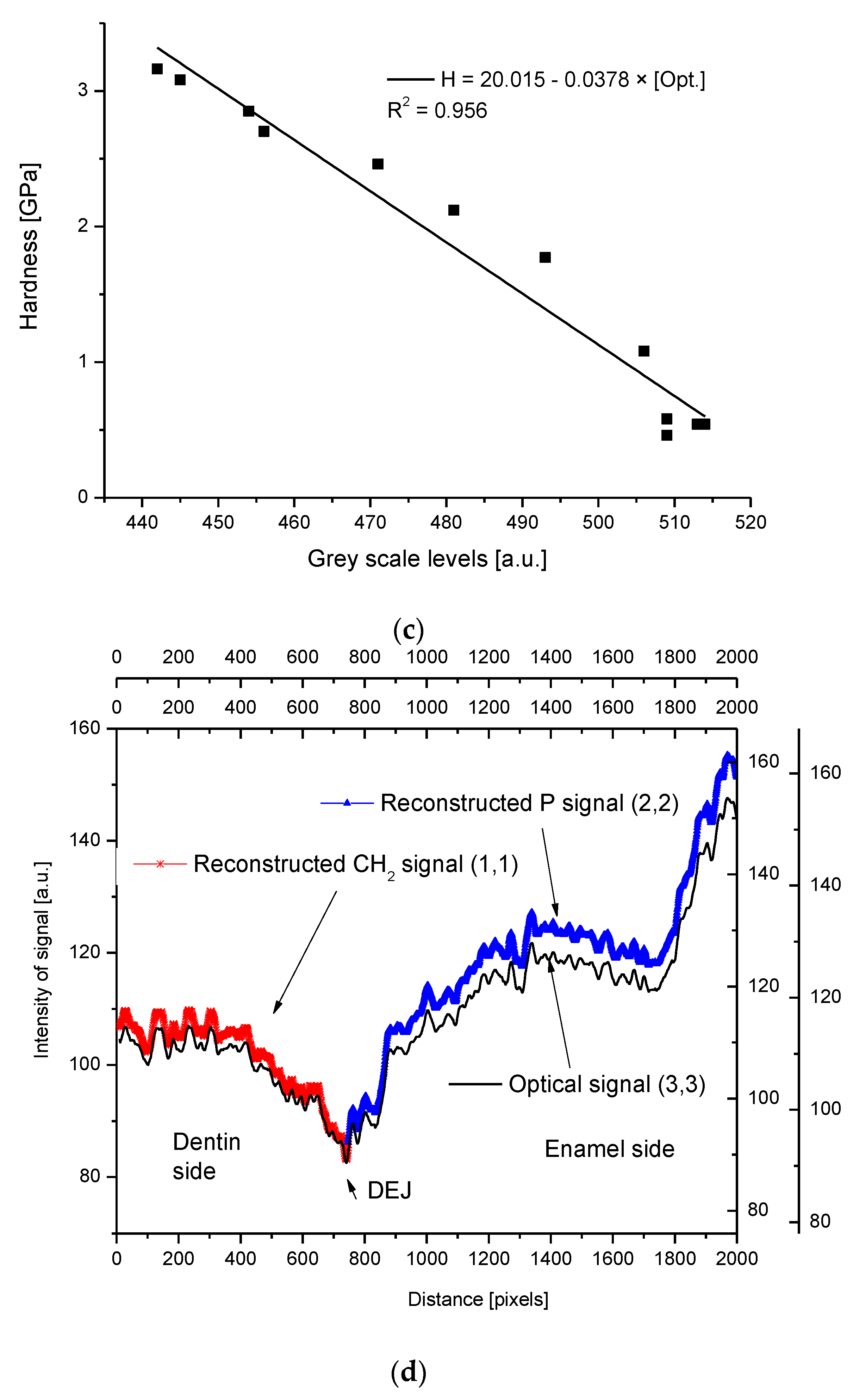
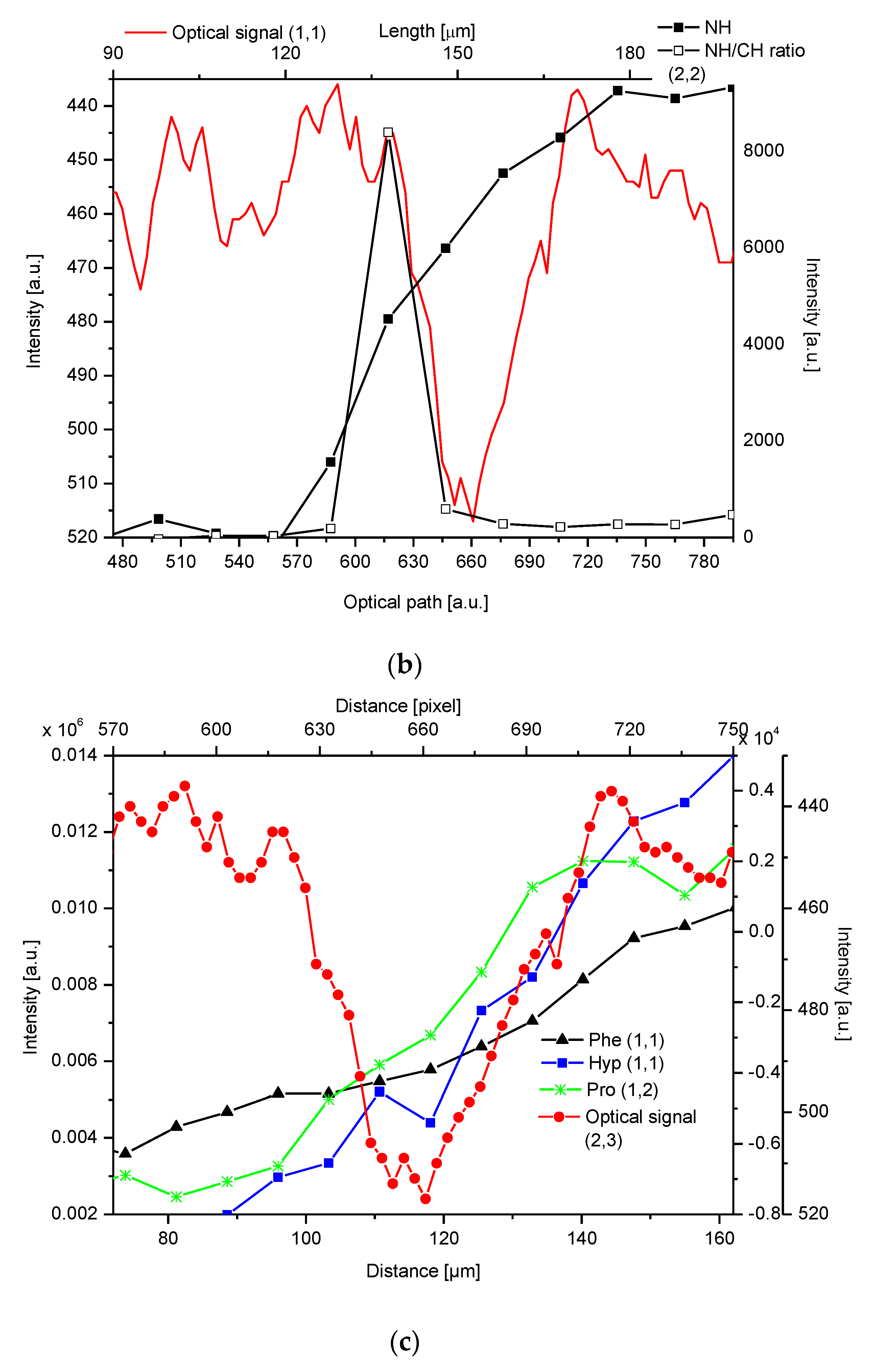
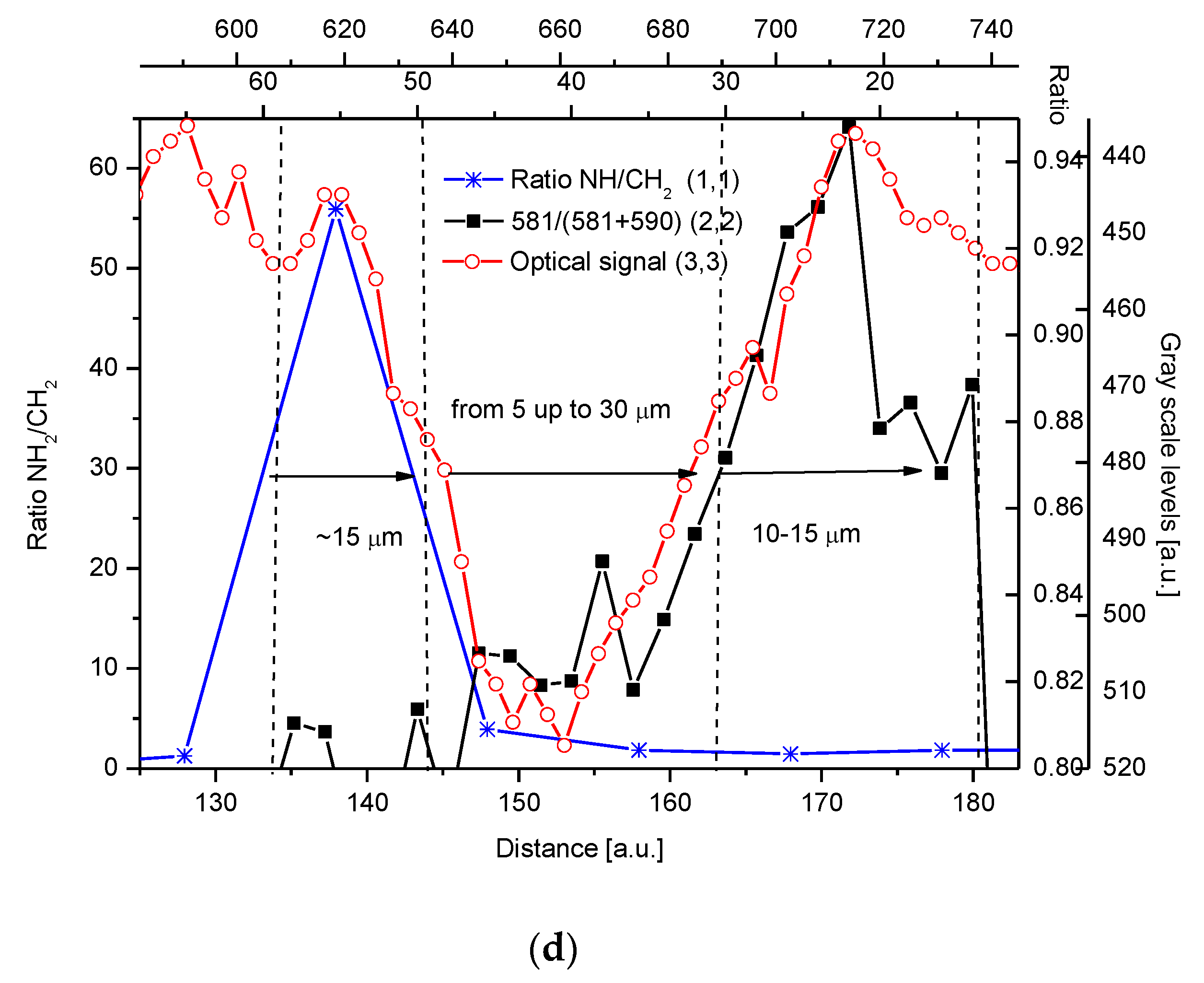
2.4. Correlations
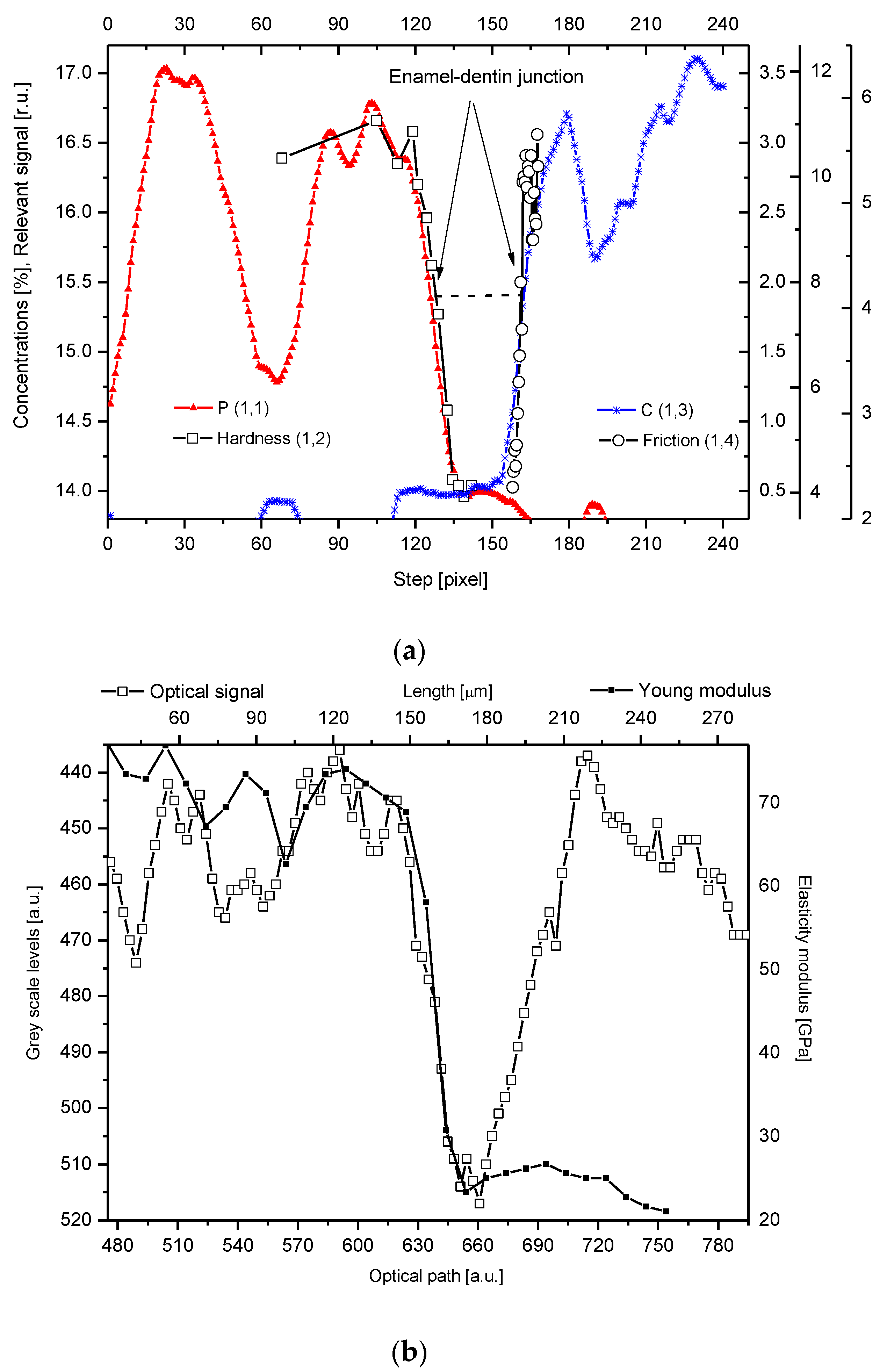
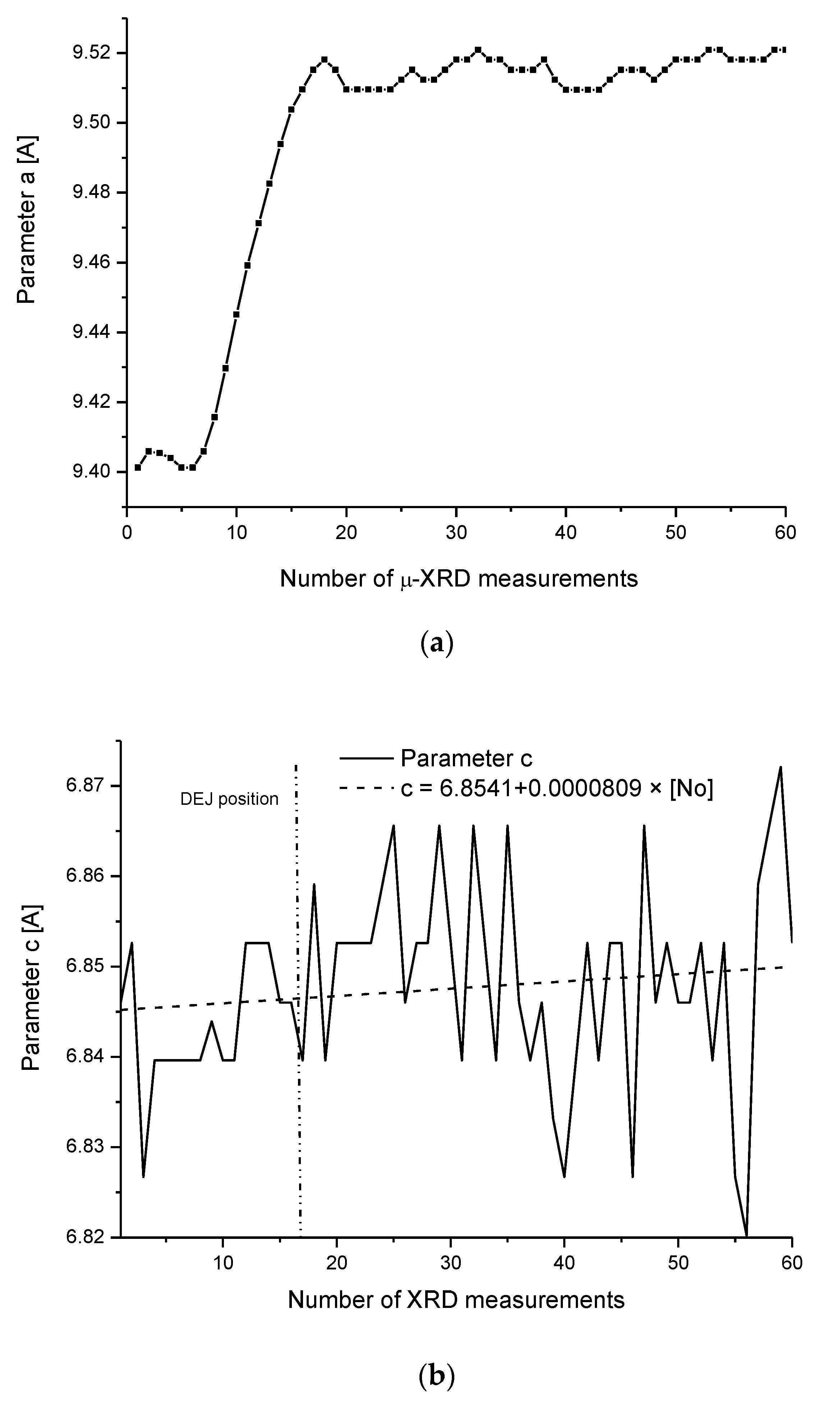
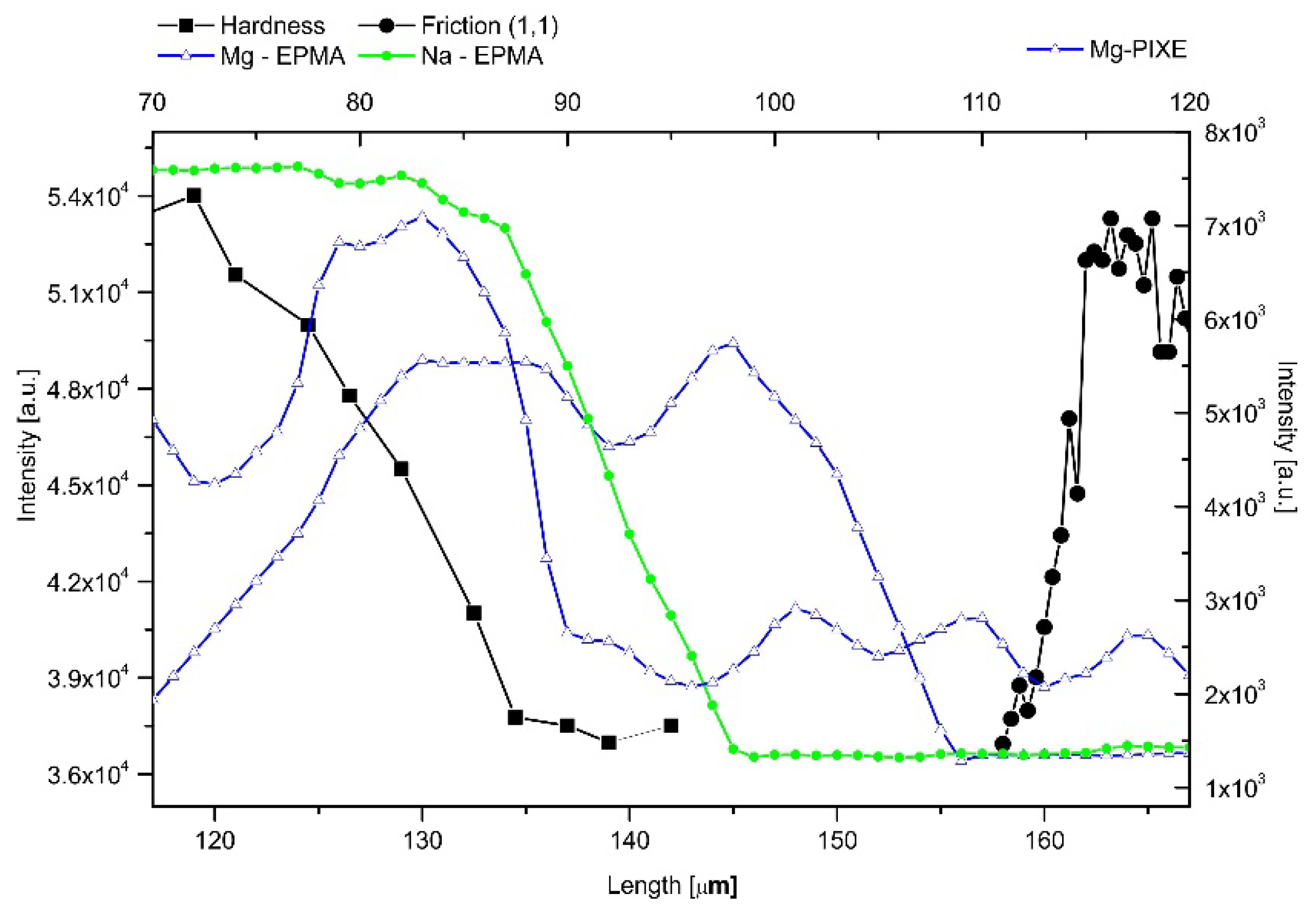
2.5. Internal Structure of the DEJ and Adhering Zones
2.6. General Remarks
3. Materials and Methods
3.1. Materials
3.2. EPMA
3.3. µ-PIXE
3.4. Micro-Raman
3.5. Micro-XRD
3.6. Optical Microscopy
3.7. External Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Rani, K.; Roychoudhury, A.; Chawla, A.; Nikolajeff, F.; Kumar, S. Novel insights into regulation of human teeth biomineralization: Deciphering the role of post-translational modifications in a tooth protein extract. Int. J. Mol. Sci. 2019, 20, 4035. [Google Scholar] [CrossRef]
- Gil-Bona, A.; Bidlack, F.B. Tooth enamel and its dynamic protein matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef]
- Arsenault, A.L.; Robinson, B.W. The dentino-enamel junction: A structural and microanalytical study of early mineralization. Calcif. Tissue Int. 1989, 45, 111–121. [Google Scholar] [CrossRef]
- Fang, P.-A.; Lam, R.S.K.; Beniash, E. Relationships between dentin and enamel mineral at the dentino-enamel boundary: Electron tomography and high-resolution transmission electron microscopy study. Eur. J. Oral Sci. 2011, 119, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Douglas, W.H. Structure-property relations and crack resistance at the bovine dentin-enamel junction. J. Dent. Res. 1994, 73, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Imbeni, V.; Kruzic, J.J.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O. The dentin-enamel junction and the fracture of human teeth. Nat. Mater. 2005, 4, 229–232. [Google Scholar] [CrossRef]
- Hoppe, K.A.; Stover, S.; Pascoe, J.; Amundson, R. Tooth enamel biomineralization in extant horses: Implications for isotopic microsampling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 206, 355–365. [Google Scholar] [CrossRef]
- Rinaldi, C.E.; Cole, T.M. Environmental seasonality and incremental growth rates of beaver (Castor canadensis) incisors: Implications for palaeobiology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 206, 289–301. [Google Scholar] [CrossRef]
- Stanton, T.K.J.; Carlson, S.J. Microscale δ18O and δ13C Isotopic Analysis of an Ontogenetic Series of the Hadrosaurid Dinosaur Edmontosaurus: Implications for Physiology and Ecology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 206, ISBN 1530752035. [Google Scholar]
- Kohn, M.J.; Schoeninger, M.J.; Barker, W. Altered states: Effects of diagenesis on fossil tooth chemistry. Geochim. Cosmochim. Acta 1999, 63, 2737–2747. [Google Scholar] [CrossRef]
- Fong, H.; Sarikaya, M.; White, S.N.; Snead, M.L. Nano-mechanical properties profiles across dentin-enamel junction of human incisor teeth. Mater. Sci. Eng. C 1999, 7, 119–128. [Google Scholar] [CrossRef]
- Marshall, G.W.J.; Balooch, M.; Gallagher, R.R.; Gansky, S.A.; Marshall, S.J. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J. Biomed. Mater. Res. 2001, 54, 87–95. [Google Scholar] [CrossRef]
- Xu, C.; Yao, X.; Walker, M.P.; Wang, Y. Chemical/molecular structure of the dentin-enamel junction is dependent on the intratooth location. Calcif. Tissue Int. 2009, 84, 221–228. [Google Scholar] [CrossRef]
- Gallagher, R.R.; Balooch, M.; Balooch, G.; Wilson, R.S.; Marshall, S.J.; Marshall, G.W. Coupled nanomechanical and raman microspectroscopic investigation of human third molar DEJ. J. Dent. Biomech. 2010, 1. [Google Scholar] [CrossRef]
- Gallagher, R.R.; Demos, S.G.; Balooch, M.; Marshall, G.W.J.; Marshall, S.J. Optical spectroscopy and imaging of the dentin-enamel junction in human third molars. J. Biomed. Mater. Res. A 2003, 64, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Douglas, W.H.; Erlandsen, S.L. Scanning electron microscopy of type I collagen at the dentin-enamel junction of human teeth. J. Histochem. Cytochem. 1993, 41, 381–388. [Google Scholar] [CrossRef]
- Meredith, N.; Sherriff, M.; Setchell, D.J.; Swanson, S.A. Measurement of the microhardness and Young’s modulus of human enamel and dentine using an indentation technique. Arch. Oral Biol. 1996, 41, 539–545. [Google Scholar] [CrossRef]
- Kinney, J.H.; Balooch, M.; Marshall, S.J.; Marshall, G.W.J.; Weihs, T.P. Hardness and Young’s modulus of human peritubular and intertubular dentine. Arch. Oral Biol. 1996, 41, 9–13. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Z.R.; Zhang, J.; Li, H.; Yu, H.Y. On the friction and wear behaviour of human tooth enamel and dentin. Wear 2003, 255, 967–974. [Google Scholar] [CrossRef]
- Habelitz, S.; Marshall, S.; Marshall, G.; Balooch, M. Mechanical properties of human dental enamel on the nanometre scale. Arch. Oral Biol. 2001, 46, 173–183. [Google Scholar] [CrossRef]
- Habelitz, S.; Marshall, S.J.; Marshall, G.W.J.; Balooch, M. The functional width of the dentino-enamel junction determined by AFM-based nanoscratching. J. Struct. Biol. 2001, 135, 294–301. [Google Scholar] [CrossRef]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Balooch, G.; Marshall, G.W.; Marshall, S.J.; Warren, O.L.; Asif, S.A.S.; Balooch, M. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J. Biomech. 2004, 37, 1223–1232. [Google Scholar] [CrossRef]
- Marangos, O.; Misra, A.; Spencer, P.; Bohaty, B.; Katz, J.L. Physico-mechanical properties determination using microscale homotopic measurements: Application to sound and caries-affected primary tooth dentin. Acta Biomater. 2009, 5, 1338–1348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulze, K.A.; Balooch, M.; Balooch, G.; Marshall, G.W.; Marshall, S.J. Micro-Raman spectroscopic investigation of dental calcified tissues. J. Biomed. Mater. Res. A 2004, 69, 286–293. [Google Scholar] [CrossRef]
- Alebrahim, M.A.; Krafft, C.; Popp, J. Raman imaging to study structural and chemical features of the dentin enamel junction. IOP Conf. Ser. 2015, 92. [Google Scholar] [CrossRef]
- Nowak, J.; Florek, M.; Kwiatek, W.; Lekki, J.; Chevallier, P.; Zięba, E.; Mestres, N.; Dutkiewicz, E.M.; Kuczumow, A. Composite structure of wood cells in petrified wood. Mater. Sci. Eng. C 2005, 25, 119–130. [Google Scholar] [CrossRef]
- Nowak, M.D.; Florek, J.; Nowak, W.; Kwiatek, J.; Lekki, E.; Zięba, N.; Mestres, B.; Ben-Nissan, A.K. Micro-spectrometric investigations of inorganic components of the black corals. Key Eng. Mater. 2005, 284, 297–399. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Xu, J.; Stangel, I.; Butler, I.S.; Gilson, D.F.R. An FT-Raman spectroscopic investigation of dentin and collagen surfaces modified by 2-hydroxyethylmethacrylate. J. Dent. Res. 1997, 76, 596–601. [Google Scholar] [CrossRef]
- Takahashi, S.M.; Goto, J.H.; Zheng, K.K. Histology and elemental composition of the thick rodless enamel found in the enamel projection of human molar. Durham Anthropol. J. 2005, 12, 1–4. [Google Scholar]
- Annegarn, H.J.; Jodaikin, A.; Cleaton-Jones, P.E.; Sellschop, J.P.F.; Madiba, C.C.P.; Bibby, D. PIXE analysis of caries related trace elements in tooth enamel. Nucl. Instrum. Methods 1981, 181, 323–326. [Google Scholar] [CrossRef]
- Bodart, F.; Deconninck, G.; Martin, M.T. Large scale study of tooth enamel. IEEE Trans. Nucl. Sci. 1981, 28, 1401–1403. [Google Scholar] [CrossRef]
- Stock, S.R.; Vieira, A.E.M.; Delbem, A.C.B.; Cannon, M.L.; Xiao, X.; Carlo, F. De synchrotron microComputed tomography of the mature bovine dentinoenamel junction. J. Struct. Biol. 2008, 161, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Weatherell, J.A. Composition of dental enamel. Br. Med. Bull. 1975, 31, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Culjat, M.; Singh, R.S.; Yoon, D.C.; Brown, E.R. Imaging of human tooth enamel using ultrasound. IEEE Trans. Med. Imaging 2003, 22, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.H.; Gladden, J.R.; Marshall, G.W.; Marshall, S.J.; So, J.H.; Maynard, J.D. Resonant ultrasound spectroscopy measurements of the elastic constants of human dentin. J. Biomech. 2004, 37, 437–441. [Google Scholar] [CrossRef]
- Kolmas, J.; Kalinowski, E.; Wojtowicz, A.; Kolodziejski, W. Mid-infrared reflectance microspectroscopy of human molars: Chemical comparison of the dentin-enamel junction with the adjacent tissues. J. Mol. Struct. 2010, 966, 113–121. [Google Scholar] [CrossRef]
- Anderson, P.; Elliott, J.C.; Bose, U.; Jones, S.J. A comparison of the mineral content of enamel and dentine in human premolars and enamel pearls measured by X-ray microtomography. Arch. Oral Biol. 1996, 41, 281–290. [Google Scholar] [CrossRef]
- Kuczumow, A.; Nowak, J.; ChaŁas, R. Microchemical and structural regular variability of apatites in “overbuilt” enamel and dentin of human molar teeth. Radiat. Phys. Chem. 2011, 80, 1129–1134. [Google Scholar] [CrossRef]
- Kuczumow, A.; Chałas, R.; Nowak, J.; Smułek, W.; Jarzębski, M. Novel approach to tooth chemistry. Quantification of human enamel apatite in context for new biomaterials and nanomaterials development. Int. J. Mol. Sci. 2021, 22, 279. [Google Scholar] [CrossRef]
- Al-Jawad, M.; Steuwer, A.; Kilcoyne, S.H.; Shore, R.C.; Cywinski, R.; Wood, D.J. 2D mapping of texture and lattice parameters of dental enamel. Biomaterials 2007, 28, 2908–2914. [Google Scholar] [CrossRef] [PubMed]
- Hanlie, H.; Liyun, T.; Tao, J. The crystal characteristics of enamel and dentin by XRD method. J. Wuhan Univ. Technol. Sci. Ed. 2006, 21, 9. [Google Scholar] [CrossRef]
- Marshall, S.J.; Balooch, M.; Habelitz, S.; Balooch, G.; Gallagher, R.; Marshall, G.W. The dentin-enamel junction—A natural, multilevel interface. J. Eur. Ceram. Soc. 2003, 23, 2897–2904. [Google Scholar] [CrossRef]
- Lebed, S.; Stachura, Z.; Cholewa, M.; Legge, G.J.F.; Lekki, J.; Maranda, S.; Potempa, A.; Sarnecki, C.; Szklarz, Z.; Styczen, J.; et al. The new Cracow scanning nuclear microprobe. Nucl. Instrum. Methods Phys. Res. Sect. B 2001, 181, 95–98. [Google Scholar] [CrossRef]
- Genty, D.; Quinif, Y. Annually laminated sequences in the internal structure of some Belgian stalagmites—Importance for paleoclimatology. J. Sediment. Res. 1996, 66, 275–288. [Google Scholar]
- McGuire, J.D.; Gorski, J.P.; Dusevich, V.; Wang, Y.; Walker, M.P. Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy. J. Dent. Res. 2014, 93, 1028–1034. [Google Scholar] [CrossRef]
- McGuire, J.D.; Walker, M.P.; Mousa, A.; Wang, Y.; Gorski, J.P. Type VII collagen is enriched in the enamel organic matrix associated with the dentin-enamel junction of mature human teeth. Bone 2014, 63, 29–35. [Google Scholar] [CrossRef]
- Tsuda, H.; Arends, J. Orientational micro-Raman spectroscopy on hydroxyapatite single crystals and human enamel crystallites. J. Dent. Res. 1994, 73, 1703–1710. [Google Scholar] [CrossRef]
- Kirchner, M.T.; Edwards, H.G.M.; Lucy, D.; Pollard, A.M. Ancient and modern specimens of human teeth: A fourier transform Raman spectroscopic study. J. Raman Spectrosc. 1997, 28, 171–178. [Google Scholar] [CrossRef]
- Pouchou, L.J.; Pichoir, F. A new model of quantitative x-ray microanalysis 1. Application to the analysis of homogeneous samples. Reeherche Aérospatiale 1984, 3, 13–38. [Google Scholar]
- Lekki, J.; Lebed, S.; Paszkowski, M.L.; Kusiak, M.; Vogt, J.; Hajduk, R.; Polak, W.; Potempa, A.; Stachura, Z.; Styczeń, J. Age determination of monazites using the new experimental chamber of the Cracow proton microprobe. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 210, 472–477. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Drew, M.G.B. Comparison of calculations for interplanar distances in a crystal lattice. Crystallogr. Rev. 2017, 23, 252–301. [Google Scholar] [CrossRef]
- DeRocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical gradients in human enamel crystallites. Nature 2020, 583, 66–71. [Google Scholar] [CrossRef]
- Terpstra, R.A.; Driessens, F.C. Magnesium in tooth enamel and synthetic apatites. Calcif. Tissue Int. 1986, 39, 348–354. [Google Scholar] [CrossRef]
- Bertoni, E.; Bigi, A.; Cojazzi, G.; Gandolfi, M.; Panzavolta, S.; Roveri, N. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J. Inorg. Biochem. 1998, 72, 29–35. [Google Scholar] [CrossRef]
- Mayer, I.; Schlam, R.; Featherstone, J.D.B. Magnesium-containing carbonate apatites. J. Inorg. Biochem. 1997, 66, 1–6. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical-hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef]



| Density [g/cm3] | Hardness [GPa] | Elasticity Modulus | Friction | P [%] | Ca [%] | C [%] | |
|---|---|---|---|---|---|---|---|
| Enamel | 2.85 | 3.2 | 75 | 17.2 | 34.8 | 2 (19%) | |
| DEJ | 2.1 (73.7%) | 0.4 (12.5%) | 22 (29.3%) | 2.1 (36.2%) | 15.0 (87.2%) | 30.8 (88.5%) | |
| Dentin | 2.2 (77.2%) | 0.6 (18.75%) | 26 (34.7%) | 5.8 | 13.6 | 31.2 (89.7%) | 10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczumow, A.; Chałas, R.; Nowak, J.; Lekki, J.; Sarna-Boś, K.; Smułek, W.; Jarzębski, M. Novel Approach to Tooth Chemistry. Quantification of the Dental-Enamel Junction. Int. J. Mol. Sci. 2021, 22, 6003. https://doi.org/10.3390/ijms22116003
Kuczumow A, Chałas R, Nowak J, Lekki J, Sarna-Boś K, Smułek W, Jarzębski M. Novel Approach to Tooth Chemistry. Quantification of the Dental-Enamel Junction. International Journal of Molecular Sciences. 2021; 22(11):6003. https://doi.org/10.3390/ijms22116003
Chicago/Turabian StyleKuczumow, Andrzej, Renata Chałas, Jakub Nowak, Janusz Lekki, Katarzyna Sarna-Boś, Wojciech Smułek, and Maciej Jarzębski. 2021. "Novel Approach to Tooth Chemistry. Quantification of the Dental-Enamel Junction" International Journal of Molecular Sciences 22, no. 11: 6003. https://doi.org/10.3390/ijms22116003
APA StyleKuczumow, A., Chałas, R., Nowak, J., Lekki, J., Sarna-Boś, K., Smułek, W., & Jarzębski, M. (2021). Novel Approach to Tooth Chemistry. Quantification of the Dental-Enamel Junction. International Journal of Molecular Sciences, 22(11), 6003. https://doi.org/10.3390/ijms22116003








