Unusual Quadrupedal Locomotion in Rat during Recovery from Lumbar Spinal Blockade of 5-HT7 Receptors
Abstract
1. Introduction
2. Results
2.1. Interlimb Coordination in Pairs of Limbs of Fore- and Hind Girdles
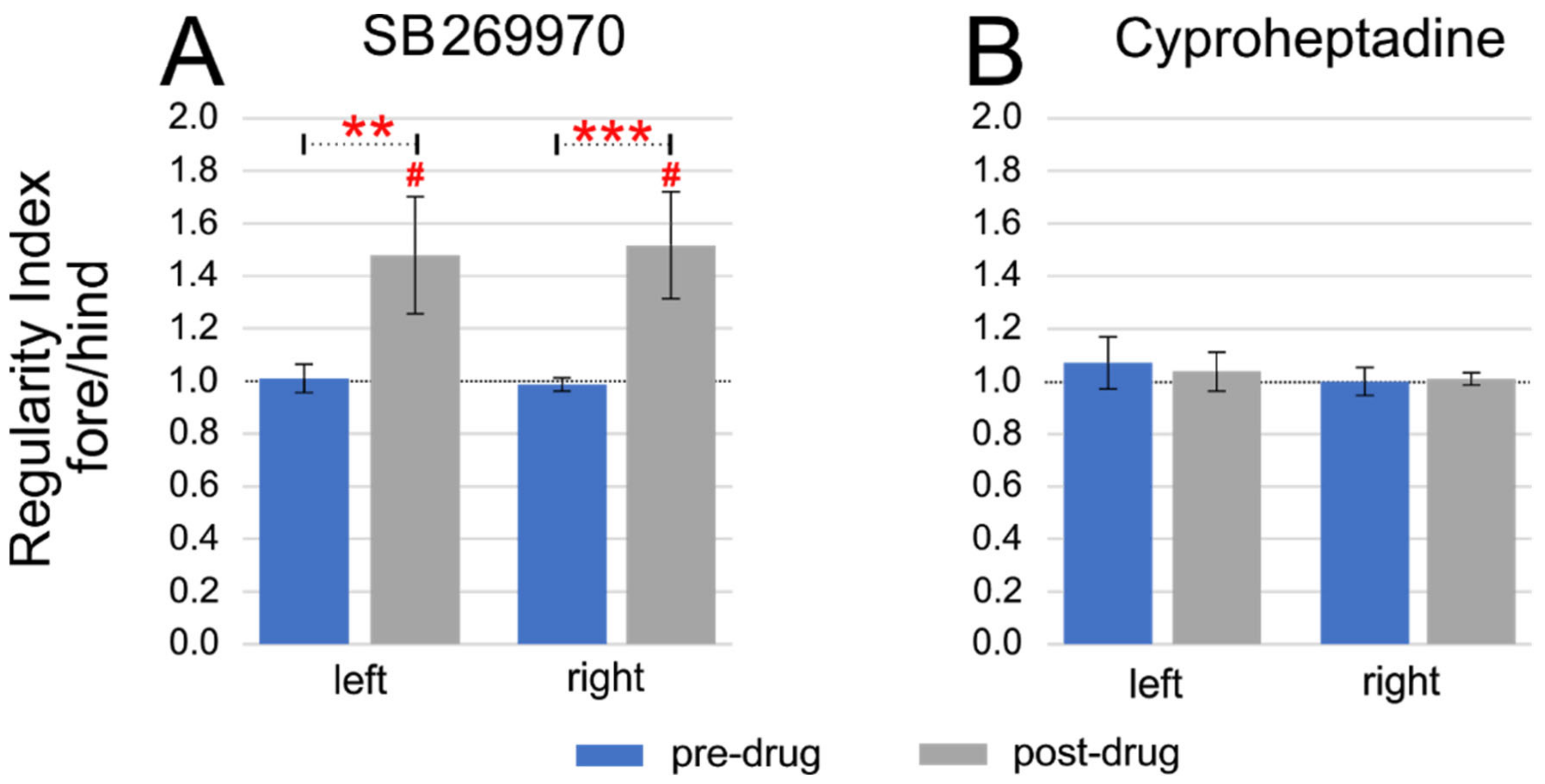
2.2. Fore-Hind Limb Coupling
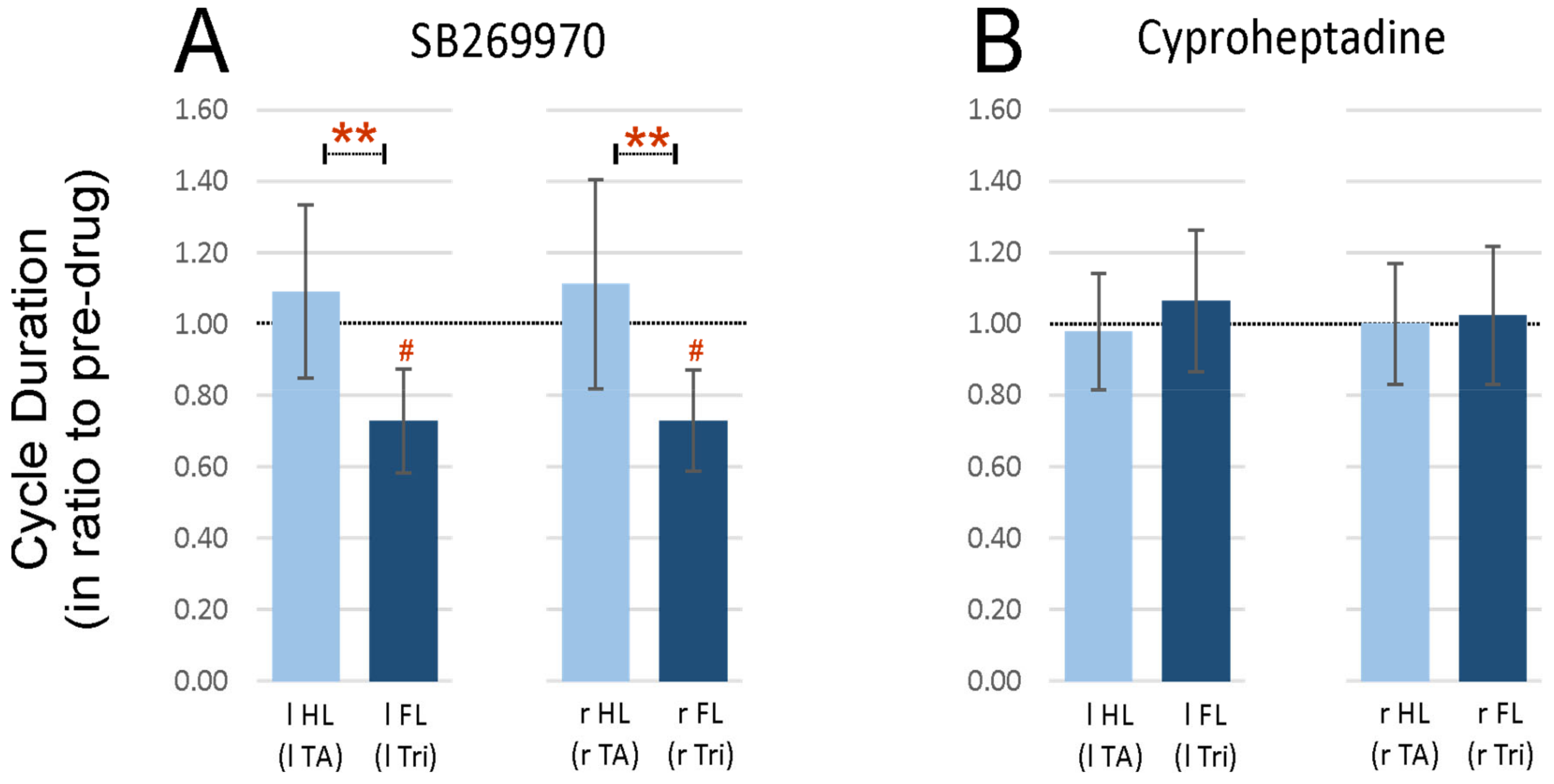
2.3. Step Cycle Duration Analysis
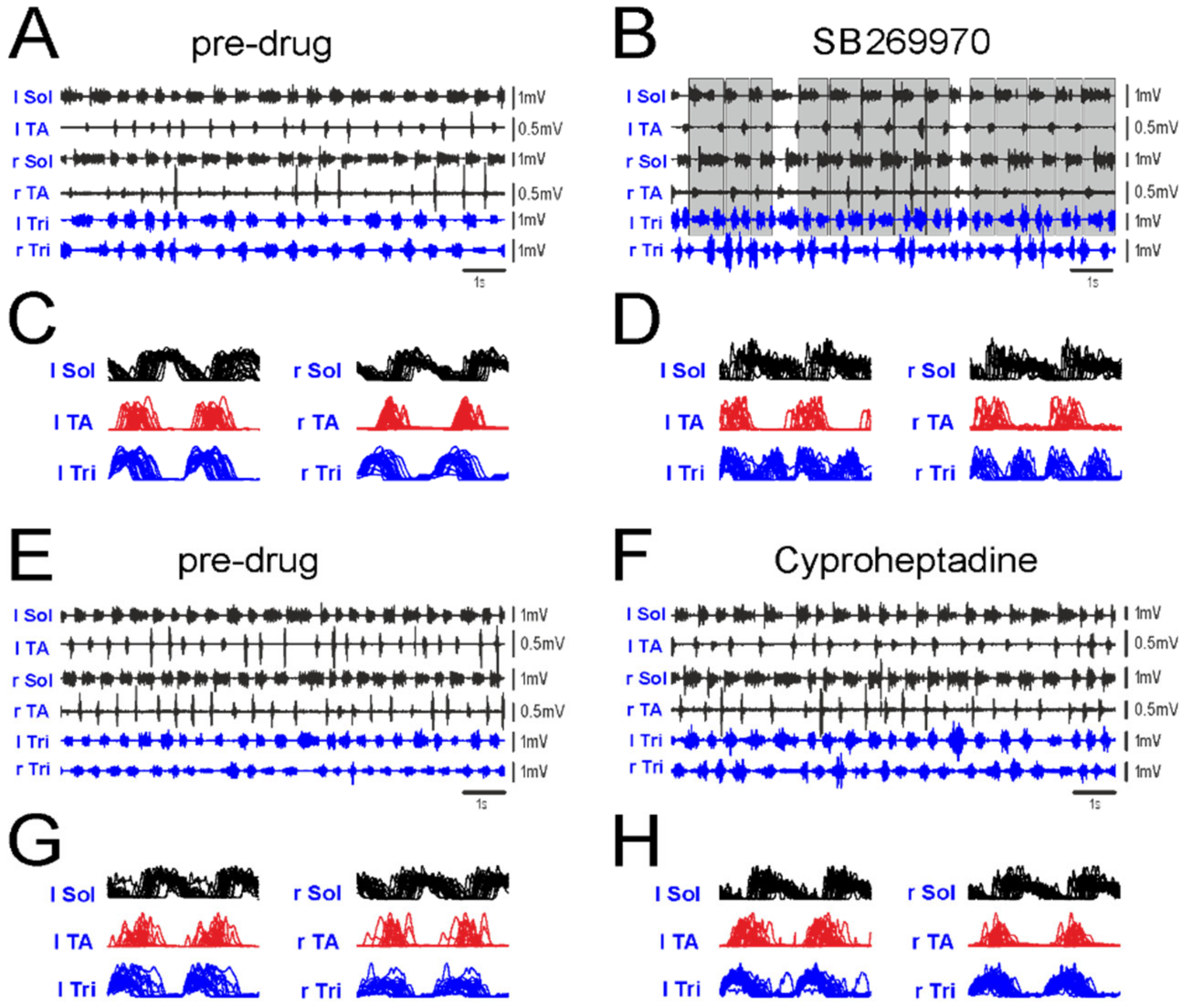
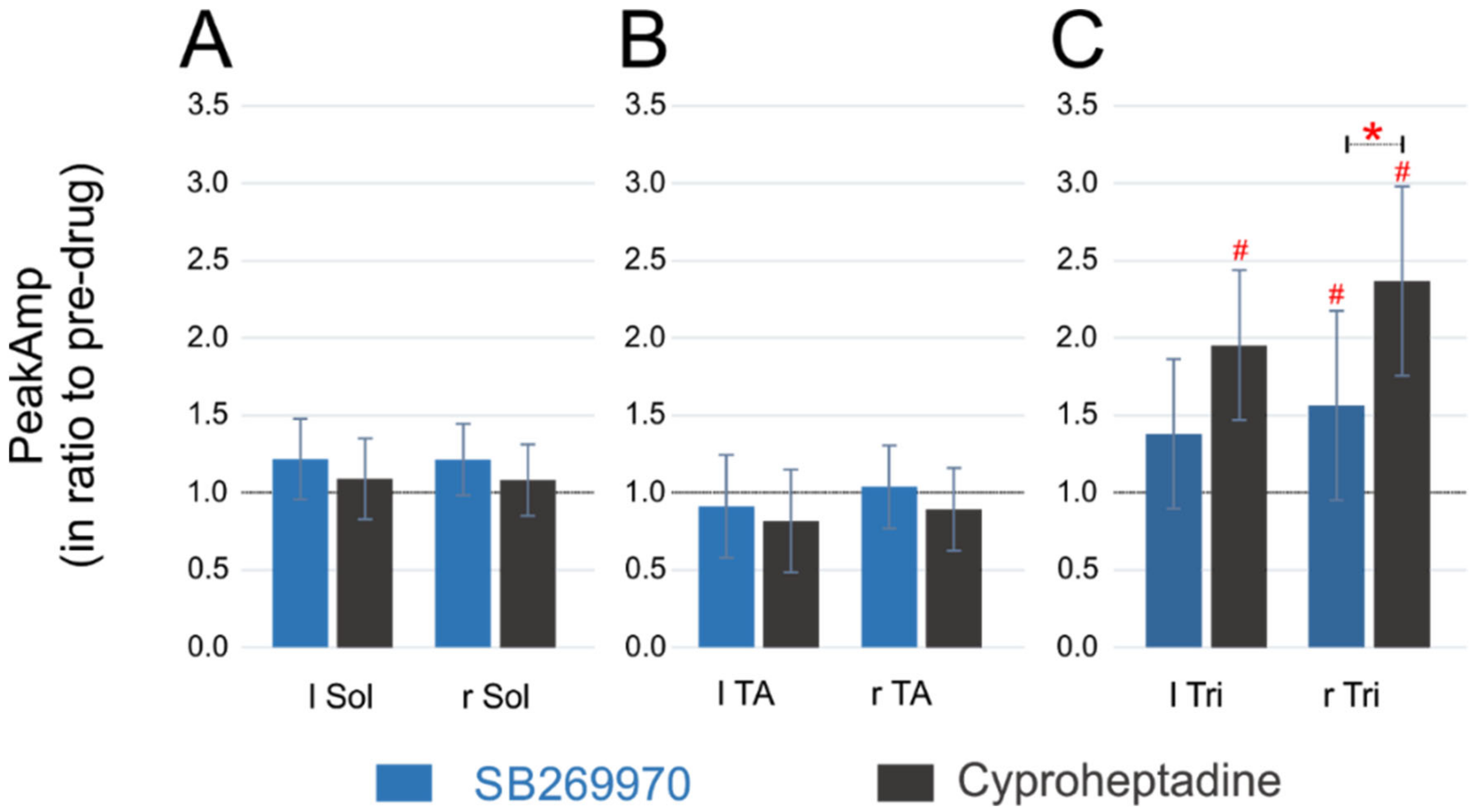
2.4. Amplitude of EMG Activity
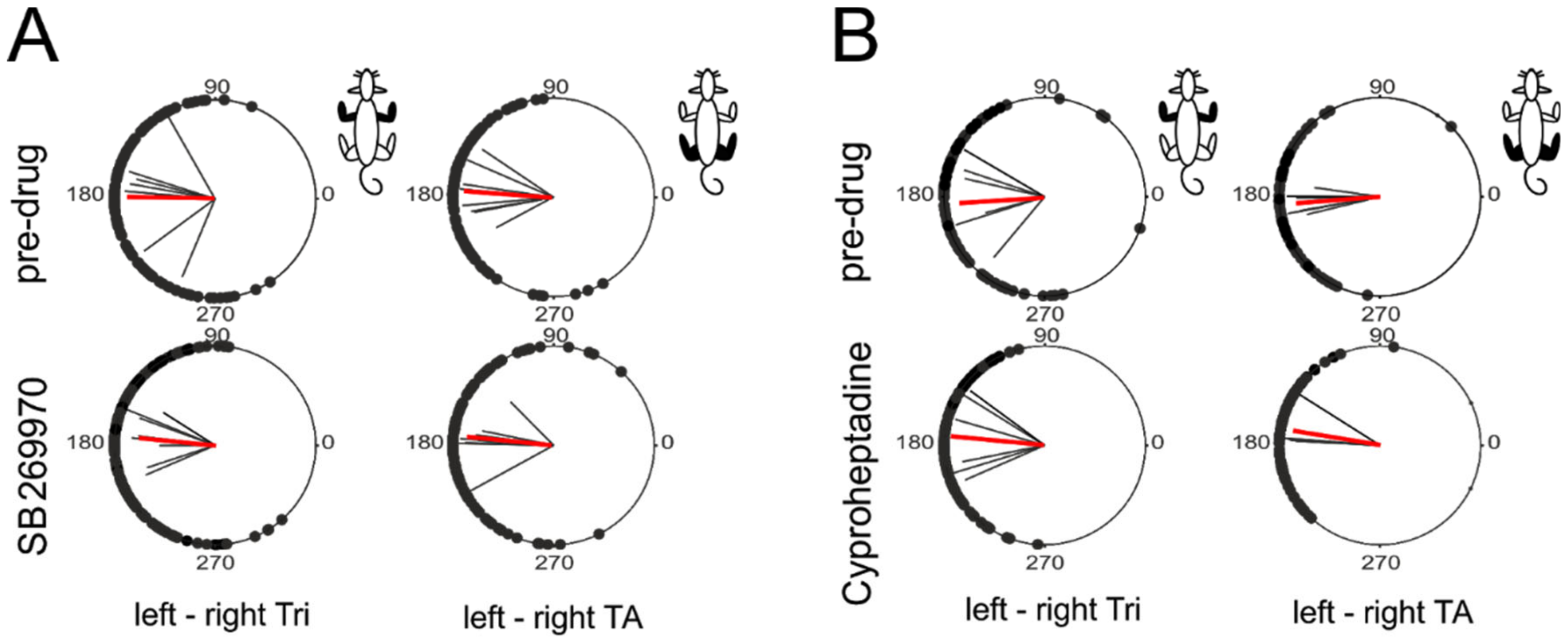
2.5. Intergirdle Ipsi- and Diagonal Coordination
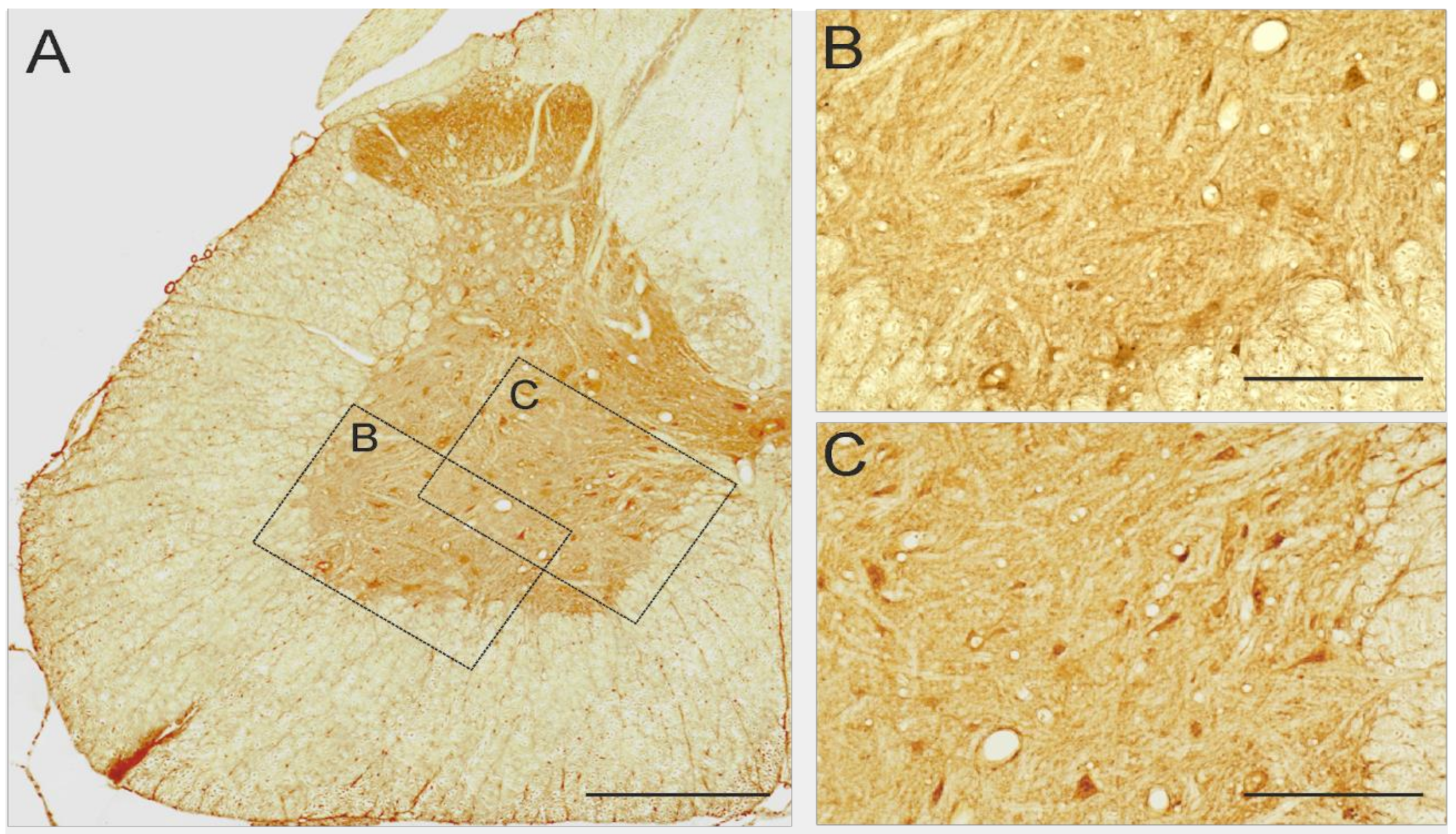
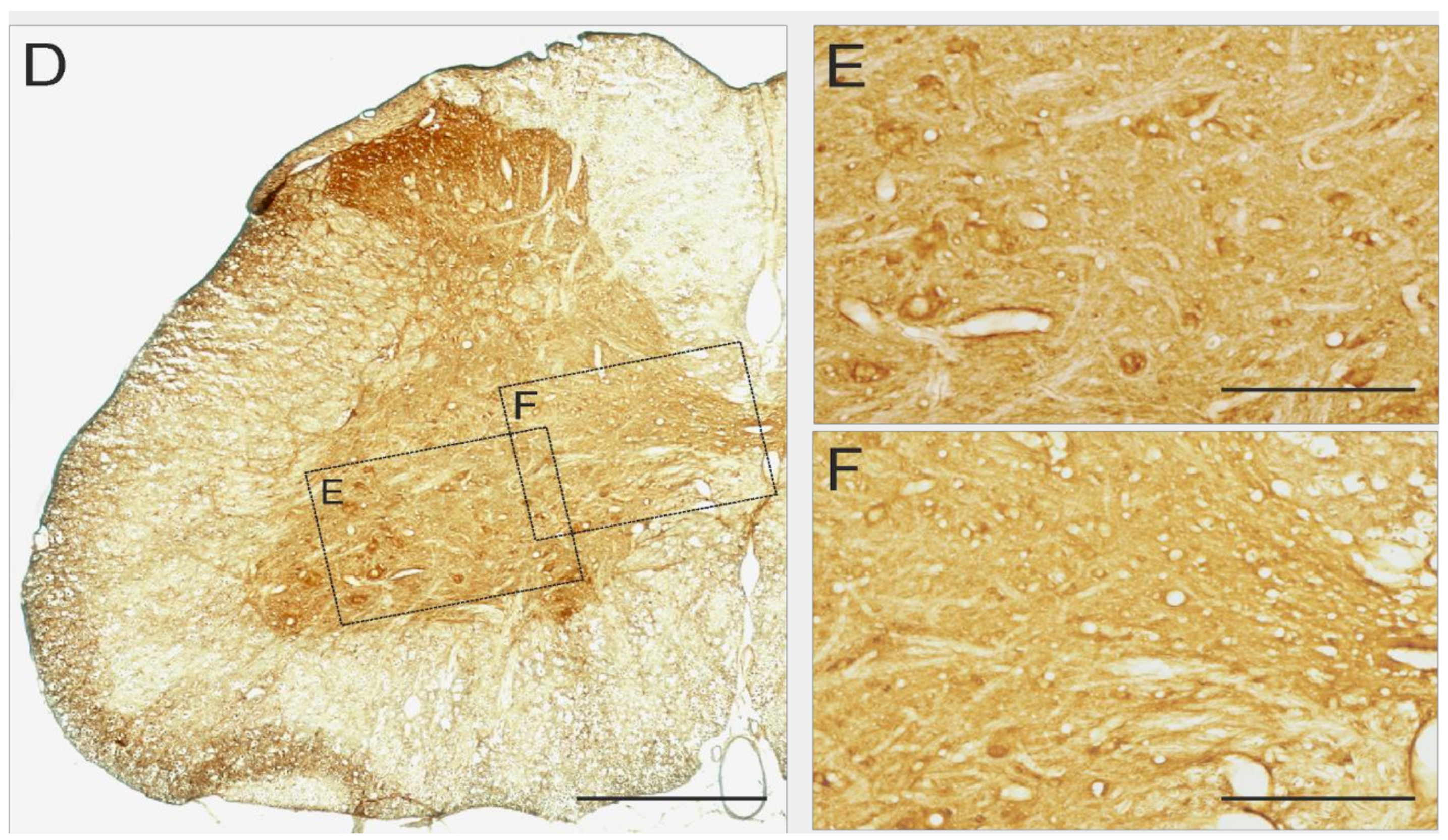
2.6. 5-HT7 and 5-HT2A Expression in Lumbar Segments of Adult Spinal Cord
3. Discussion
3.1. The Different Rhythms in Fore- and Hindlimbs in Locomotion of Intact Mammals
3.2. The Different Rhythms in Fore and Hindlimb in Locomotion of Mammals with Partial Spinal Cord Lesions
3.3. Candidates of Genetically Identified Propriospinal Connections for Control of Fore and Hindlimb Locomotor Pattern
3.4. Ascending Inter-Girdles (Lumbo-Cervical) Connections and 5-HT Innervation
3.5. 5-HT Sensory Feedback Control
4. Materials and Methods
4.1. Intrathecal Cannula Implantation
4.2. Implantation of EMG Electrodes
4.3. Testing the Effects of Intrathecal Drug Applications
4.4. Locomotor Performance and EMG Recordings
4.5. Locomotor EMG Pattern Analysis
4.6. Statistical Analysis
4.7. Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, T.G. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J. Physiol. 1914, 48, 18–46. [Google Scholar] [CrossRef]
- Grillner, S.; Zangger, P. How detailed is the central pattern generation for locomotion? Brain Res. Bull. 1975, 88, 367–371. [Google Scholar] [CrossRef]
- Grillner, S.; Zangger, P. The effect of dorsal root transection on the efferent motor pattern in the cat’s hindlimb during locomotion. Acta Physiol. Scand. 1984, 120, 393–405. [Google Scholar] [CrossRef]
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of locomotor automatism. Physiol. Rev. 1976, 56, 465–501. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, N.A.; Rybak, I.A. Organization of flexor-extensor interactions in the mammalian spinal cord: Insights from computational modelling. J. Physiol. 2016, 594, 6117–6131. [Google Scholar] [CrossRef]
- Rybak, I.A.; Dougherty, K.J.; Shevtsova, N.A. Organization of the Mammalian Locomotor CPG: Review of Computational Model and Circuit Architectures Based on Genetically Identified Spinal Interneurons(1,2,3). eNeuro 2015, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, N.A.; Talpalar, A.E.; Markin, S.N.; Harris-Warrick, R.M.; Kiehn, O.; Rybak, I.A. Organization of left-right coordination of neuronal activity in the mammalian spinal cord: Insights from computational modelling. J. Physiol. 2015, 593, 2403–2426. [Google Scholar] [CrossRef]
- Rossignol, S.; Dubuc, R.; Gossard, J.P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.J.; Lebret, J.M.; Kiehn, O. Organization of left-right coordination in the mammalian locomotor network. Brain Res. Brain Res. Rev. 2002, 40, 107–117. [Google Scholar] [CrossRef]
- Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006, 29, 279–306. [Google Scholar] [CrossRef]
- Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef]
- Kjaerulff, O.; Kiehn, O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: A lesion study. J. Neurosci. 1996, 16, 5777–5794. [Google Scholar] [CrossRef]
- Danner, S.M.; Wilshin, S.D.; Shevtsova, N.A.; Rybak, I.A. Central control of interlimb coordination and speed-dependent gait expression in quadrupeds. J. Physiol. 2016, 594, 6947–6967. [Google Scholar] [CrossRef]
- Viala, D.; Vidal, C. Evidence for distinct spinal locomotion generators supplying respectively fore- and hindlimbs in the rabbit. Brain Res. Bull. 1978, 155, 182–186. [Google Scholar] [CrossRef]
- English, A.W. Interlimb coordination during stepping in the cat: An electromyographic analysis. J. Neurophysiol. 1979, 42, 229–243. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Lennard, P.R. Interlimb coordination during stepping in the cat: In-phase stepping and gait transitions. Brain Res. Bull. 1982, 245, 353–364. [Google Scholar] [CrossRef]
- Thibaudier, Y.; Hurteau, M.F.; Dambreville, C.; Chraibi, A.; Goetz, L.; Frigon, A. Interlimb Coordination during Tied-Belt and Transverse Split-Belt Locomotion before and after an Incomplete Spinal Cord Injury. J. Neurotrauma 2017, 34, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Brockett, E.G.; Seenan, P.G.; Bannatyne, B.A.; Maxwell, D.J. Ascending and descending propriospinal pathways between lumbar and cervical segments in the rat: Evidence for a substantial ascending excitatory pathway. Neuroscience 2013, 240, 83–97. [Google Scholar] [CrossRef]
- Juvin, L.; Simmers, J.; Morin, D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J. Neurosci. 2005, 25, 6025–6035. [Google Scholar] [CrossRef] [PubMed]
- Dutton, R.C.; Carstens, M.I.; Antognini, J.F.; Carstens, E. Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain Res. Bull. 2006, 1119, 76–85. [Google Scholar] [CrossRef]
- Cote, M.P.; Detloff, M.R.; Wade, R.E., Jr.; Lemay, M.A.; Houle, J.D. Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front. Physiol. 2012, 3, 330. [Google Scholar] [CrossRef]
- Ruder, L.; Takeoka, A.; Arber, S. Long-Distance Descending Spinal Neurons Ensure Quadrupedal Locomotor Stability. Neuron 2016, 92, 1063–1078. [Google Scholar] [CrossRef]
- Flynn, J.R.; Conn, V.L.; Boyle, K.A.; Hughes, D.I.; Watanabe, M.; Velasquez, T.; Goulding, M.D.; Callister, R.J.; Graham, B.A. Anatomical and Molecular Properties of Long Descending Propriospinal Neurons in Mice. Front. Neuroanat. 2017, 11, 5. [Google Scholar] [CrossRef]
- Frigon, A. The neural control of interlimb coordination during mammalian locomotion. J. Neurophysiol. 2017, 117, 2224–2241. [Google Scholar] [CrossRef] [PubMed]
- Pocratsky, A.M.; Shepard, C.T.; Morehouse, J.R.; Burke, D.A.; Riegler, A.S.; Hardin, J.T.; Beare, J.E.; Hainline, C.; States, G.J.; Brown, B.L.; et al. Long ascending propriospinal neurons provide flexible, context-specific control of interlimb coordination. eLife 2020, 9, e53565. [Google Scholar] [CrossRef]
- Cazalets, J.R.; Sqalli-Houssaini, Y.; Clarac, F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J. Physiol. 1992, 455, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Cowley, K.C.; Schmidt, B.J. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci. Lett. 1994, 171, 147–150. [Google Scholar] [CrossRef]
- Schmidt, B.J.; Jordan, L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000, 53, 689–710. [Google Scholar] [CrossRef]
- Hochman, S.; Garraway, S.M.; Machacek, D.W.; Shay, B.L. 5-HT receptors and neuromodulatory control of spinal cord function. In Motor Neurobiology; Cope, T.C., Ed.; CRC Press: New York, NY, USA, 2001; pp. 48–87. [Google Scholar]
- Sławińska, U.; Miazga, K.; Jordan, L.M. 5-HT(2) and 5-HT(7) receptor agonists facilitate plantar stepping in chronic spinal rats through actions on different populations of spinal neurons. Front. Neural Circuits 2014, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Gackiere, F.; Vinay, L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Front. Neural Circuits 2014, 8, 102. [Google Scholar] [CrossRef]
- Cabaj, A.M.; Majczyński, H.; Couto, E.; Gardiner, P.F.; Stecina, K.; Sławińska, U.; Jordan, L.M. Serotonin controls initiation of locomotion and afferent modulation of coordination via 5-HT7 receptors in adult rats. J. Physiol. 2017, 595, 301–320. [Google Scholar] [CrossRef]
- Majczyński, H.; Cabaj, A.M.; Jordan, L.M.; Sławińska, U. Contribution of 5-HT2 Receptors to the Control of the Spinal Locomotor System in Intact Rats. Front. Neural Circuits 2020, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- De Leon, R.; Hodgson, J.A.; Roy, R.R.; Edgerton, V.R. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. Bull. 1994, 654, 241–250. [Google Scholar] [CrossRef]
- Thibaudier, Y.; Hurteau, M.F.; Telonio, A.; Frigon, A. Coordination between the fore- and hindlimbs is bidirectional, asymmetrically organized, and flexible during quadrupedal locomotion in the intact adult cat. Neuroscience 2013, 240, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Thibaudier, Y.; Frigon, A. Spatiotemporal control of interlimb coordination during transverse split-belt locomotion with 1:1 or 2:1 coupling patterns in intact adult cats. J. Neurophysiol. 2014, 112, 2006–2018. [Google Scholar] [CrossRef]
- Flynn, J.R.; Graham, B.A.; Galea, M.P.; Callister, R.J. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 2011, 60, 809–822. [Google Scholar] [CrossRef]
- Jankowska, E. Interneuronal relay in spinal pathways from proprioceptors. Prog. Neurobiol. 1992, 38, 335–378. [Google Scholar] [CrossRef]
- Giovanelli Barilari, M.; Kuypers, H.G. Propriospinal fibers interconnecting the spinal enlargements in the cat. Brain Res. Bull. 1969, 14, 321–330. [Google Scholar] [CrossRef]
- Reed, W.R.; Shum-Siu, A.; Onifer, S.M.; Magnuson, D.S. Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience 2006, 142, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.R.; Shum-Siu, A.; Whelan, A.; Onifer, S.M.; Magnuson, D.S. Anterograde labeling of ventrolateral funiculus pathways with spinal enlargement connections in the adult rat spinal cord. Brain Res. Bull. 2009, 1302, 76–84. [Google Scholar] [CrossRef]
- Górska, T.; Chojnicka-Gittins, B.; Majczyński, H.; Zmysłowski, W. Changes in forelimb-hindlimb coordination after partial spinal lesions of different extent in the rat. Behav. Brain Res. 2013, 239, 121–138. [Google Scholar] [CrossRef]
- Alluin, O.; Karimi-Abdolrezaee, S.; Delivet-Mongrain, H.; Leblond, H.; Fehlings, M.G.; Rossignol, S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J. Neurotrauma 2011, 28, 1963–1981. [Google Scholar] [CrossRef]
- Górska, T.; Bem, T.; Majczyński, H.; Zmysłowski, W. Different forms of impairment of the fore-hindlimb coordination after partial spinal lesions in cats. Acta Neurobiol. Exp. 1996, 56, 177–188. [Google Scholar]
- Leszczyńska, A.N.; Majczyński, H.; Wilczyński, G.M.; Sławińska, U.; Cabaj, A.M. Thoracic Hemisection in Rats Results in Initial Recovery Followed by a Late Decrement in Locomotor Movements, with Changes in Coordination Correlated with Serotonergic Innervation of the Ventral Horn. PLoS ONE 2015, 10, e0143602. [Google Scholar] [CrossRef]
- Cartmill, M.; Lemelin, P.; Schmitt, D. Support polygons and symmetrical gaits in mammals. Zool. J. Linn. Soc. 2002, 136, 401–420. [Google Scholar] [CrossRef]
- Arber, S. Motor circuits in action: Specification, connectivity, and function. Neuron 2012, 74, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Goulding, M. Circuits controlling vertebrate locomotion: Moving in a new direction. Nat. Rev. Neurosci. 2009, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Kiehn, O. Development and functional organization of spinal locomotor circuits. Curr. Opin. Neurobiol. 2011, 21, 100–109. [Google Scholar] [CrossRef]
- Bellardita, C.; Kiehn, O. Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr. Biol. 2015, 25, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Talpalar, A.E.; Bouvier, J.; Borgius, L.; Fortin, G.; Pierani, A.; Kiehn, O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 2013, 500, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Dyck, J.; Lanuza, G.M.; Gosgnach, S. Functional characterization of dI6 interneurons in the neonatal mouse spinal cord. J. Neurophysiol. 2012, 107, 3256–3266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lanuza, G.M.; Britz, O.; Wang, Z.; Siembab, V.C.; Zhang, Y.; Velasquez, T.; Alvarez, F.J.; Frank, E.; Goulding, M. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 2014, 82, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Britz, O.; Zhang, J.; Grossmann, K.S.; Dyck, J.; Kim, J.C.; Dymecki, S.; Gosgnach, S.; Goulding, M. A genetically defined asymmetry underlies the inhibitory control of flexor-extensor locomotor movements. eLife 2015, 4, e04718. [Google Scholar] [CrossRef]
- Crone, S.A.; Quinlan, K.A.; Zagoraiou, L.; Droho, S.; Restrepo, C.E.; Lundfald, L.; Endo, T.; Setlak, J.; Jessell, T.M.; Kiehn, O.; et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 2008, 60, 70–83. [Google Scholar] [CrossRef]
- Crone, S.A.; Zhong, G.; Harris-Warrick, R.; Sharma, K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J. Neurosci. 2009, 29, 7098–7109. [Google Scholar] [CrossRef]
- Lanuza, G.M.; Gosgnach, S.; Pierani, A.; Jessell, T.M.; Goulding, M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 2004, 42, 375–386. [Google Scholar] [CrossRef]
- Menelaou, E.; McLean, D.L. Speed control: Spinal interneurons with crossed purposes. Curr. Biol. 2013, 23, R716–R718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schnerwitzki, D.; Perry, S.; Ivanova, A.; Caixeta, F.V.; Cramer, P.; Gunther, S.; Weber, K.; Tafreshiha, A.; Becker, L.; Vargas Panesso, I.L.; et al. Neuron-specific inactivation of Wt1 alters locomotion in mice and changes interneuron composition in the spinal cord. Life Sci. Alliance 2018, 1, e201800106. [Google Scholar] [CrossRef]
- Haque, F.; Rancic, V.; Zhang, W.; Clugston, R.; Ballanyi, K.; Gosgnach, S. WT1-Expressing Interneurons Regulate Left-Right Alternation during Mammalian Locomotor Activity. J. Neurosci. 2018, 38, 5666–5676. [Google Scholar] [CrossRef]
- Haque, F.; Gosgnach, S. Mapping Connectivity Amongst Interneuronal Components of the Locomotor CPG. Front. Cell. Neurosci. 2019, 13, 443. [Google Scholar] [CrossRef]
- Griener, A.; Zhang, W.; Kao, H.; Haque, F.; Gosgnach, S. Anatomical and electrophysiological characterization of a population of dI6 interneurons in the neonatal mouse spinal cord. Neuroscience 2017, 362, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Larhammar, M.; Vieillard, J.; Nagaraja, C.; Hilscher, M.M.; Tafreshiha, A.; Rofo, F.; Caixeta, F.V.; Kullander, K. Characterization of Dmrt3-Derived Neurons Suggest a Role within Locomotor Circuits. J. Neurosci. 2019, 39, 1771–1782. [Google Scholar] [CrossRef]
- Andersson, L.S.; Larhammar, M.; Memic, F.; Wootz, H.; Schwochow, D.; Rubin, C.J.; Patra, K.; Arnason, T.; Wellbring, L.; Hjalm, G.; et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 2012, 488, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Vallstedt, A.; Kullander, K. Dorsally derived spinal interneurons in locomotor circuits. Ann. N. Y. Acad. Sci. 2013, 1279, 32–42. [Google Scholar] [CrossRef]
- Nakayama, K.; Nishimaru, H.; Kudo, N. Rhythmic motor activity in thin transverse slice preparations of the fetal rat spinal cord. J. Neurophysiol. 2004, 92, 648–652. [Google Scholar] [CrossRef][Green Version]
- Carlin, K.P.; Dai, Y.; Jordan, L.M. Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J. Neurophysiol. 2006, 95, 1278–1284. [Google Scholar] [CrossRef]
- Liu, J.; Akay, T.; Hedlund, P.B.; Pearson, K.G.; Jordan, L.M. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: In vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J. Neurophysiol. 2009, 102, 337–348. [Google Scholar] [CrossRef]
- Doly, S.; Fischer, J.; Brisorgueil, M.J.; Verge, D.; Conrath, M. Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: Immunocytochemical evidence. J. Comp. Neurol. 2005, 490, 256–269. [Google Scholar] [CrossRef]
- Noga, B.R.; Johnson, D.M.; Riesgo, M.I.; Pinzon, A. Locomotor-activated neurons of the cat. I. Serotonergic innervation and co-localization of 5-HT7, 5-HT2A, and 5-HT1A receptors in the thoraco-lumbar spinal cord. J. Neurophysiol. 2009, 102, 1560–1576. [Google Scholar] [CrossRef][Green Version]
- Leopoldo, M.; Lacivita, E.; Berardi, F.; Perrone, R.; Hedlund, P.B. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 2011, 129, 120–148. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Rossignol, S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. Bull. 1978, 146, 269–277. [Google Scholar] [CrossRef]
- Pearson, K.G.; Rossignol, S. Fictive motor patterns in chronic spinal cats. J. Neurophysiol. 1991, 66, 1874–1887. [Google Scholar] [CrossRef]
- Steeves, J.D.; Jordan, L.M. Localization of a descending pathway in the spinal cord which is necessary for controlled treadmill locomotion. Neurosci. Lett. 1980, 20, 283–288. [Google Scholar] [CrossRef]
- Shefchyk, S.J.; Jell, R.M.; Jordan, L.M. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp. Brain Res. 1984, 56, 257–262. [Google Scholar] [CrossRef]
- Drew, T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J. Neurophysiol. 1993, 70, 179–199. [Google Scholar] [CrossRef]
- Jankowska, E.; Nilsson, E.; Hammar, I. Processing information related to centrally initiated locomotor and voluntary movements by feline spinocerebellar neurones. J. Physiol. 2011, 589, 5709–5725. [Google Scholar] [CrossRef] [PubMed]
- Akay, T.; Tourtellotte, W.G.; Arber, S.; Jessell, T.M. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc. Natl. Acad. Sci. USA 2014, 111, 16877–16882. [Google Scholar] [CrossRef]
- Bui, T.V.; Brownstone, R.M. Sensory-evoked perturbations of locomotor activity by sparse sensory input: A computational study. J. Neurophysiol. 2015, 113, 2824–2839. [Google Scholar] [CrossRef] [PubMed]
- Zholudeva, L.V.; Abraira, V.E.; Satkunendrarajah, K.; McDevitt, T.C.; Goulding, M.D.; Magnuson, D.S.K.; Lane, M.A. Spinal Interneurons as Gatekeepers to Neuroplasticity after Injury or Disease. J. Neurosci. 2021, 41, 845–854. [Google Scholar] [CrossRef]
- Sławińska, U.; Majczyński, H.; Dai, Y.; Jordan, L.M. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J. Physiol. 2012, 590, 1721–1736. [Google Scholar] [CrossRef]
- Riddell, J.S.; Jankowska, E.; Eide, E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J. Physiol. 1993, 461, 723–741. [Google Scholar] [CrossRef]
- Quevedo, J.; Eguibar, J.R.; Jimenez, I.; Rudomin, P. Raphe magnus and reticulospinal actions on primary afferent depolarization of group I muscle afferents in the cat. J. Physiol. 1995, 482, 623–640. [Google Scholar] [CrossRef]
- Dougherty, K.J.; Bannatyne, B.A.; Jankowska, E.; Krutki, P.; Maxwell, D.J. Membrane receptors involved in modulation of responses of spinal dorsal horn interneurons evoked by feline group II muscle afferents. J. Neurosci. 2005, 25, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Meuser, T.; Pietruck, C.; Gabriel, A.; Xie, G.X.; Lim, K.J.; Pierce Palmer, P. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci. 2002, 71, 2279–2289. [Google Scholar] [CrossRef]
- Garraway, S.M.; Hochman, S. Pharmacological characterization of serotonin receptor subtypes modulating primary afferent input to deep dorsal horn neurons in the neonatal rat. Br. J. Pharmacol. 2001, 132, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Cowley, K.C.; Zaporozhets, E.; Maclean, J.N.; Schmidt, B.J. Is NMDA receptor activation essential for the production of locomotor-like activity in the neonatal rat spinal cord? J. Neurophysiol. 2005, 94, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Circular distribution. In Biostatistical Analysis, 5th ed.; McElroy, W.D., Swanson, C.P., Eds.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 605–668. [Google Scholar]
- Batschelet, E. Circular Statistics in Biology; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; p. 299. [Google Scholar]

| SB269970 | Cyproheptadine | ||||
|---|---|---|---|---|---|
| Forelimbs | Hindlimbs | Forelimbs | Hindlimbs | ||
| Left-Right Tri | Left-Right TA | Left-Right Tri | Left-Right TA | ||
| r-Value | pre-drug | 0.85 ± 0.11 | 0.85 ± 0.10 | 0.82 ± 0.12 | 0.82 ± 0.11 |
| post-drug | 0.75 ± 0.13 | 0.82 ± 0.12 | 0.91 ± 0.05 | 0.85 ± 0.15 | |
| p-value | 0.10 | 0.61 | 0.19 | 0.29 | |
| Phase (°) | pre-drug | 179.0 ± 38.3 | 177.8 ± 20.2 | 183.6 ± 30.0 | 184.5 ± 8.3 |
| post-drug | 174.3 ± 19.6 | 174.2 ± 20.3 | 174.5 ± 26.3 | 170.6 ± 11.6 | |
| p-value | 0.81 | 0.73 | 0.61 | 0.039 | |
| SB 269970 | |||||
|---|---|---|---|---|---|
| Ipsilateral | Diagonal | ||||
| Left Tri-Sol | Right Tri-Sol | Left Tri-Right TA | Right Tri-Left TA | ||
| R-Value 1st Tri Burst | pre-drug | 0.83 ± 0.11 | 0.84 ± 0.06 | 0.87 ± 0.16 | 0.89 ± 0.05 |
| post-drug | 0.71 ± 0.14 | 0.72 ± 0.11 | 0.70 ± 0.12 | 0.78 ± 0.15 | |
| p-value | 0.063 | 0.034 | 0.006 | 0.16 | |
| Phase (°) 1st Tri Burst | pre-drug | 187.3 ± 33.0 | 181.0 ± 19.4 | 68.9 ± 40.9 | 70.5 ± 26.0 |
| post-drug | 115.7 ± 37.5 | 109.3 ± 22.7 | 51.4 ± 21.5 | 49.9 ± 20.8 | |
| p-value | 0.00099 | 0.00005 | 0.37 | 0.12 | |
| r-Value 2nd Tri Burst | pre-drug | 0.83 ± 0.11 | 0.84 ± 0.06 | 0.87 ± 0.16 | 0.89 ± 0.05 |
| post-drug | 0.76 ± 0.10 | 0.68 ± 0.14 | 0.75 ± 0.19 | 0.76 ± 0.18 | |
| p-value | 0.13 | 0.008 | 0.085 | 0.088 | |
| Phase (°) 2nd Tri Burst | pre-drug | 187.3 ± 33.0 | 181.0 ± 19.4 | 68.9 ± 40.9 | 70.5 ± 26.0 |
| post-drug | 220.7 ± 38.8 | 224.9 ± 27.0 | 220.6 ± 33.0 | 208.9 ± 31.2 | |
| p-value | 0.069 | 0.003 | 0.00005 | 0.00005 | |
| Cyproheptadine | |||||
|---|---|---|---|---|---|
| Ipsilateral | Diagonal | ||||
| Left Tri-Sol | Right Tri-Sol | Left Tri-Right TA | Right Tri-Left TA | ||
| r-Value | pre-drug | 0.83 ± 0.16 | 0.84 ± 0.04 | 0.85 ± 0.13 | 0.83 ± 0.12 |
| post-drug | 0.87 ± 0.08 | 0.87 ± 0.1 | 0.94 ± 0.01 | 0.84 ± 0.12 | |
| p-value | 0.93 | 0.29 | 0.18 | 0.84 | |
| Phase (°) | pre-drug | 163.9 ± 15.2 | 159.5 ± 21.5 | 59.2 ± 17.5 | 54.9 ± 25.5 |
| post-drug | 161.7 ± 24.2 | 161.2 ± 34.6 | 58.7 ± 38.0 | 74.0 ± 35.7 | |
| p-value | 0.88 | 0.83 | 0.98 | 0.33 | |
| Agent | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT7 |
|---|---|---|---|---|
| SB269970 | Ki > 10,000 | Ki > 10,000 | Ki > 10,000 | Ki = 0.34–1258 |
| Cyproheptadine | Ki = 0.44 | Ki = 1.54 | Ki = 2.23 | Ki = 7.5 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławińska, U.; Majczyński, H.; Kwaśniewska, A.; Miazga, K.; Cabaj, A.M.; Bekisz, M.; Jordan, L.M.; Zawadzka, M. Unusual Quadrupedal Locomotion in Rat during Recovery from Lumbar Spinal Blockade of 5-HT7 Receptors. Int. J. Mol. Sci. 2021, 22, 6007. https://doi.org/10.3390/ijms22116007
Sławińska U, Majczyński H, Kwaśniewska A, Miazga K, Cabaj AM, Bekisz M, Jordan LM, Zawadzka M. Unusual Quadrupedal Locomotion in Rat during Recovery from Lumbar Spinal Blockade of 5-HT7 Receptors. International Journal of Molecular Sciences. 2021; 22(11):6007. https://doi.org/10.3390/ijms22116007
Chicago/Turabian StyleSławińska, Urszula, Henryk Majczyński, Anna Kwaśniewska, Krzysztof Miazga, Anna M. Cabaj, Marek Bekisz, Larry M. Jordan, and Małgorzata Zawadzka. 2021. "Unusual Quadrupedal Locomotion in Rat during Recovery from Lumbar Spinal Blockade of 5-HT7 Receptors" International Journal of Molecular Sciences 22, no. 11: 6007. https://doi.org/10.3390/ijms22116007
APA StyleSławińska, U., Majczyński, H., Kwaśniewska, A., Miazga, K., Cabaj, A. M., Bekisz, M., Jordan, L. M., & Zawadzka, M. (2021). Unusual Quadrupedal Locomotion in Rat during Recovery from Lumbar Spinal Blockade of 5-HT7 Receptors. International Journal of Molecular Sciences, 22(11), 6007. https://doi.org/10.3390/ijms22116007






