Novel Approach for the Synthesis of Chlorophosphazene Cycles with a Defined Size via Controlled Cyclization of Linear Oligodichlorophosphazenes [Cl(PCl2=N)n–PCl3]+[PCl6]−
Abstract
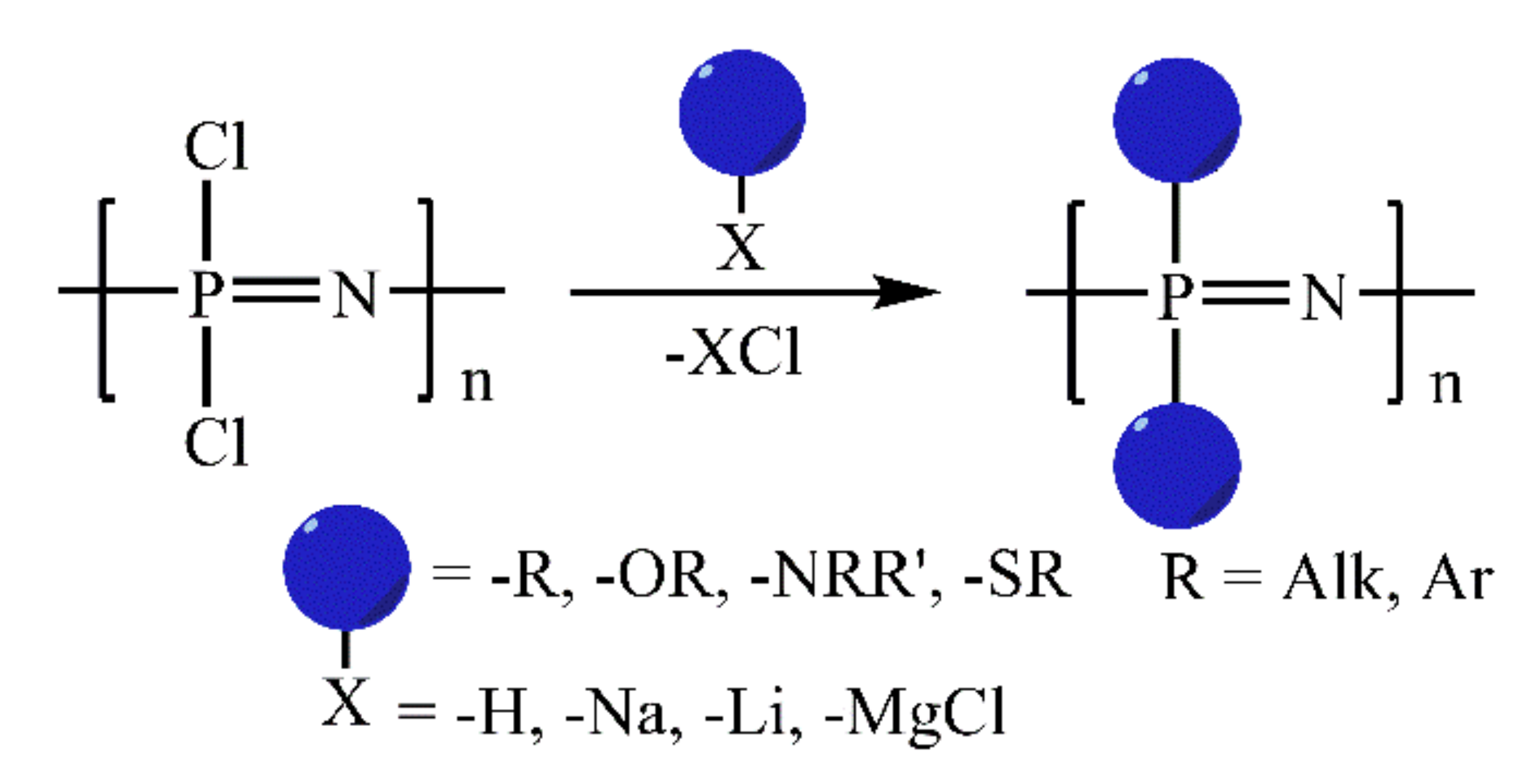
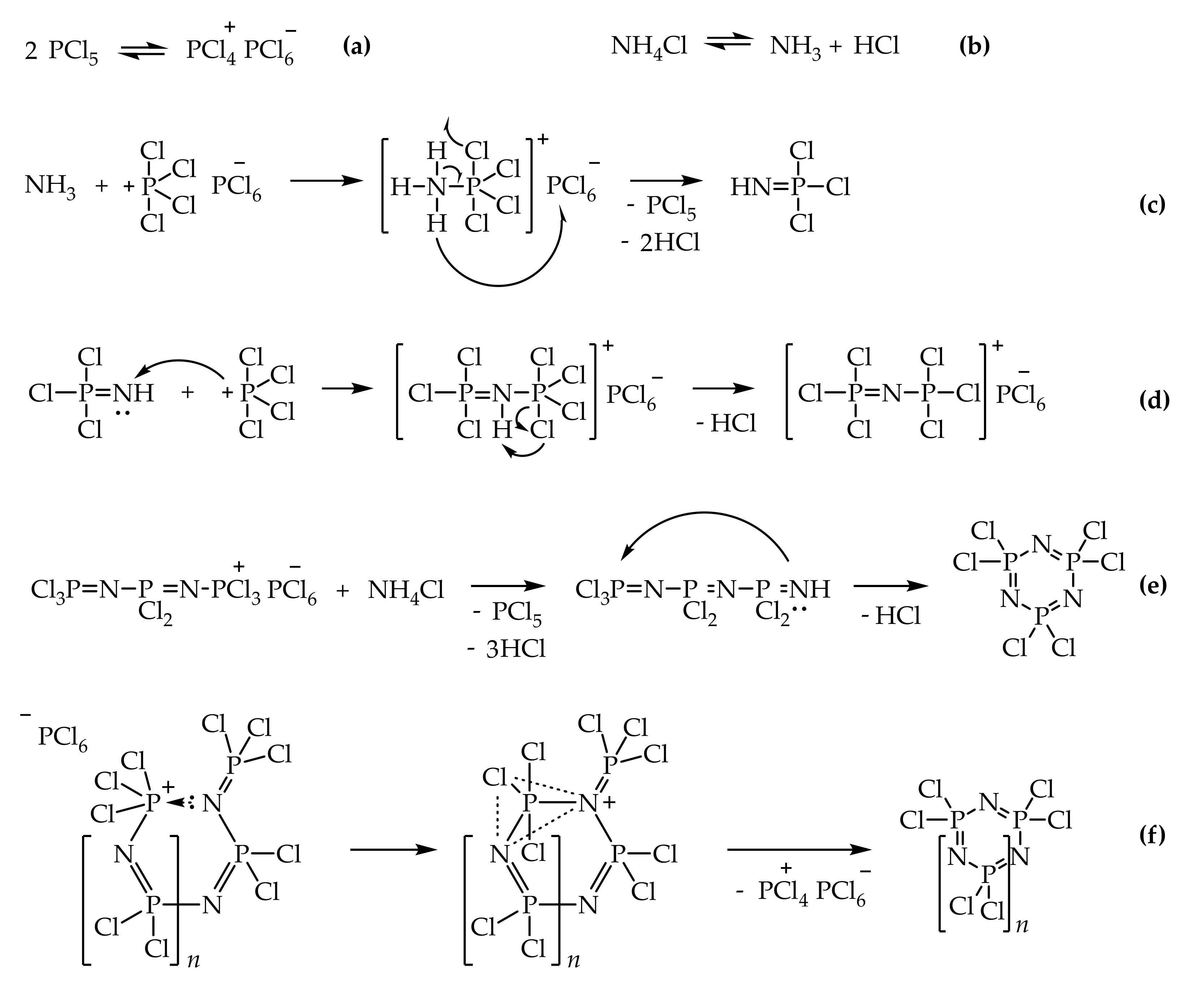
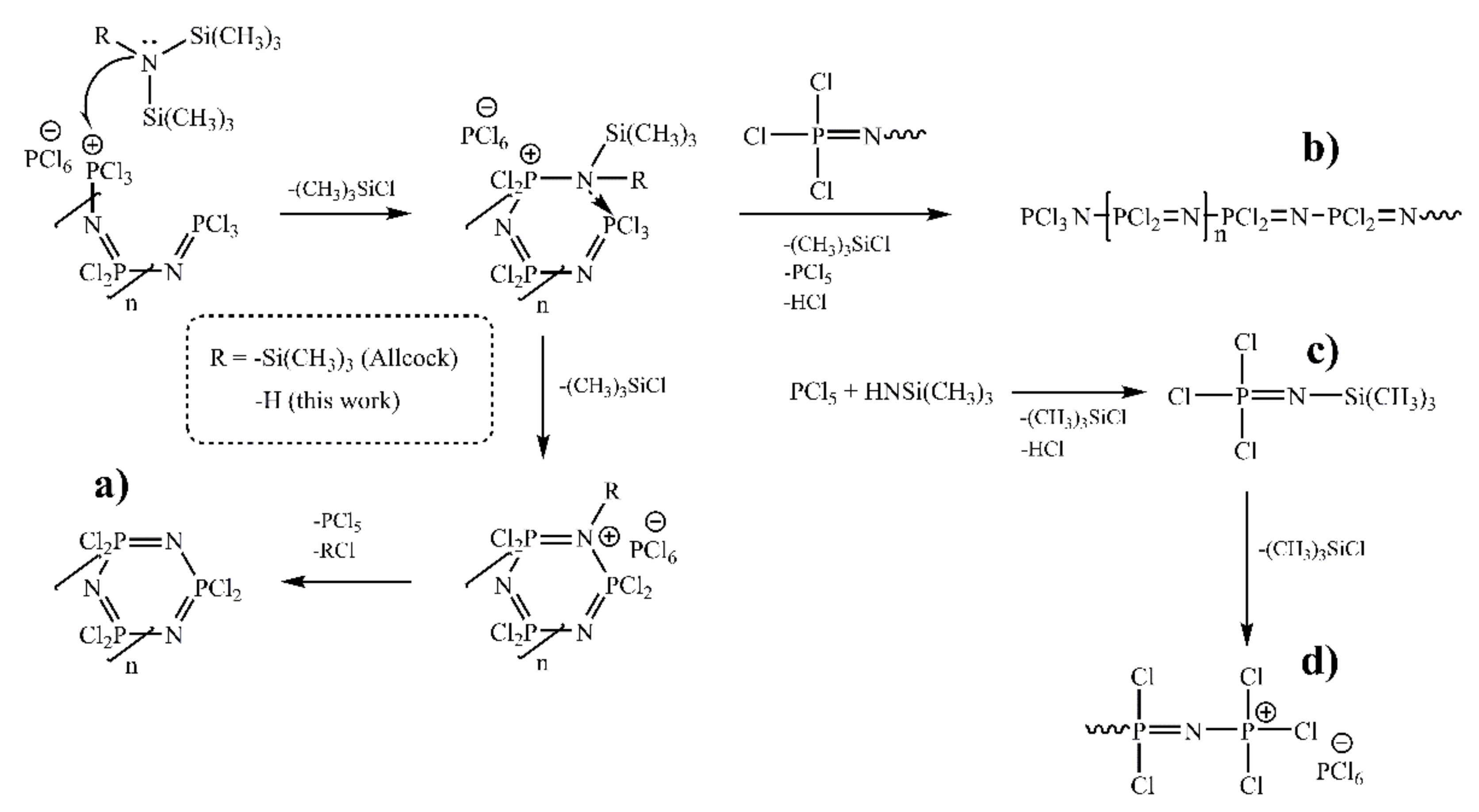
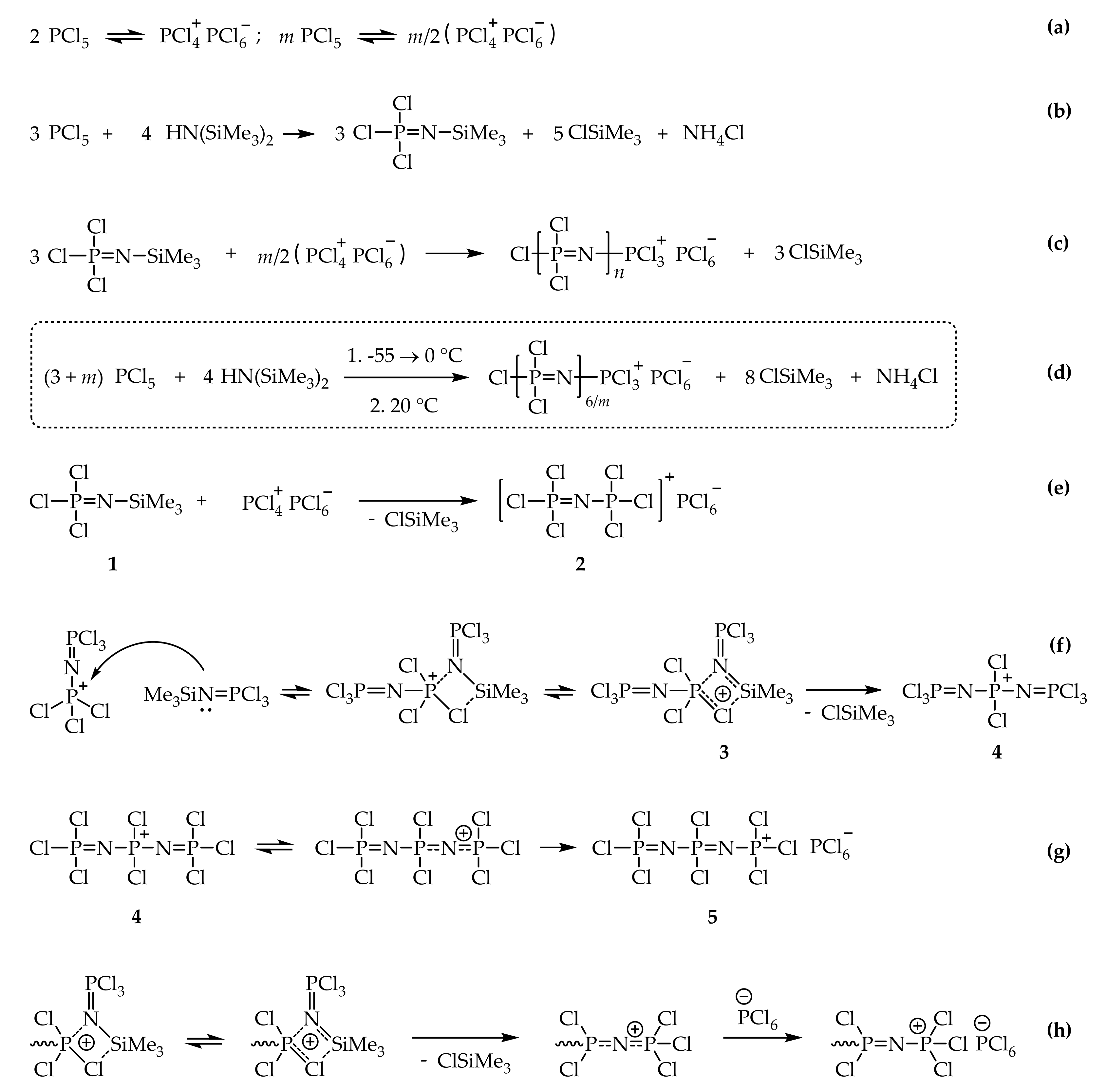
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Synthesis
2.2.1. General Procedure of Synthesis of Linear Oligodichlorophosphazenes
2.2.2. General Procedure for Cyclization Reaction
2.2.3. Synthesis of Phenoxycyclotriphosphazenes
2.3. Characterization
3. Linear Chains Preparation
4. Thermal Cyclization
5. Cyclization in the Presence of N-Containing Agents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vinogradova, S.V.; Tur, D.R.; Vasnev, V.A. Open-Chain Poly(Organophosphazenes). Synthesis and Properties. Russ. Chem. Rev. 1998, 67, 515–534. [Google Scholar] [CrossRef]
- Gleria, M.; Jaeger, R.D. Phosphazenes: A Worldwide Insight; Nova Publishers: New York, NY, USA, 2004; ISBN 978-1-59033-423-2. [Google Scholar]
- Li, Z.; Chen, C.; McCaffrey, M.; Yang, H.; Allcock, H.R. Polyphosphazene Elastomers with Alkoxy and Trifluoroethoxy Side Groups. ACS Appl. Polym. Mater. 2020, 2, 475–480. [Google Scholar] [CrossRef]
- Fei, S.-T.; Wood, R.M.; Lee, D.K.; Stone, D.A.; Chang, H.-L.; Allcock, H.R. Inorganic–Organic Hybrid Polymers with Pendent Sulfonated Cyclic Phosphazene Side Groups as Potential Proton Conductive Materials for Direct Methanol Fuel Cells. J. Membr. Sci. 2008, 320, 206–214. [Google Scholar] [CrossRef]
- Laine, R.M. Inorganic and Organometallic Polymers with Special Properties; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-94-011-2612-0. [Google Scholar]
- Golemme, G.; Drioli, E. Polyphosphazene Membrane Separations—Review. J. Inorg. Organomet. Polym. 1996, 6, 341–365. [Google Scholar] [CrossRef]
- Singler, R.E.; Willingham, R.A.; Lenz, R.W.; Furukawa, A.; Finkelmann, H. Liquid Crystalline Side-Chain Phosphazenes. Macromolecules 1987, 20, 1727–1728. [Google Scholar] [CrossRef]
- Gleria, M.; Bortolus, P.; Minto, F.; Flamigni, L. Aspects of Polyphosphazene Photochemistry. In Inorganic and Organometallic Polymers with Special Properties; Laine, R.M., Ed.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1992; pp. 375–393. ISBN 978-94-011-2612-0. [Google Scholar]
- Allcock, H.R.; Cameron, C.G.; Skloss, T.W.; Taylor-Meyers, S.; Haw, J.F. Molecular Motion of Phosphazene-Bound Nonlinear Optical Chromophores. Macromolecules 1996, 29, 233–238. [Google Scholar] [CrossRef]
- Kulichikhin, V.; Semakov, A.; Tur, D. New Flexible Piezoelectrics and Actuators Based on Polyorganophosphazenes. Sens. Actuators A Phys. 2016, 252, 48–53. [Google Scholar] [CrossRef]
- Bredov, N.S.; Gorlov, M.V.; Esin, A.S.; Bykovskaya, A.A.; Kireev, V.V.; Sinegribova, O.A.; Ryabochenko, M.D. Linear 2-Ethylhexyl Imidophosphoric Esters as Effective Rare-Earth Element Extractants. Appl. Sci. 2020, 10(4), 1229. [Google Scholar] [CrossRef]
- Medici, A.; Fantin, G.; Pedrini, P.; Gleria, M.; Minto, F. Functionalization of Phosphazenes. 1. Synthesis of Phosphazene Materials Containing Hydroxyl Groups. Macromolecules 1992, 25, 2569–2574. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, C.; Allcock, H.R. New Mixed-Substituent Fluorophosphazene High Polymers and Small Molecule Cyclophosphazene Models: Synthesis, Characterization, and Structure Property Correlations. Macromolecules 2015, 48, 1483–1492. [Google Scholar] [CrossRef]
- Wei, W.; Sun, X.; Ye, W.; Zhang, B.; Fei, X.; Li, X.; Liu, X. Thermal Latent Curing Agent for Epoxy Resins from Neutralization of 2-Methylimidazole with a Phosphazene-Containing Polyfunctional Carboxylic Acid. Polym. Adv. Technol. 2020, 31, 1553–1561. [Google Scholar] [CrossRef]
- Huang, W.-K.; Chen, K.-J.; Yeh, J.-T.; Chen, K.-N. Curing and Combustion Properties of a PU-Coating System with UV-Reactive Phosphazene. J. Appl. Polym. Sci. 2002, 85, 1980–1991. [Google Scholar] [CrossRef]
- Chistyakov, E.M.; Terekhov, I.V.; Shapagin, A.V.; Filatov, S.N.; Chuev, V.P. Curing of Epoxy Resin DER-331 by Hexakis(4-Acetamidophenoxy)Cyclotriphosphazene and Properties of the Prepared Composition. Polymers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Sirotin, I.S.; Sarychev, I.A.; Vorobyeva, V.V.; Kuzmich, A.A.; Bornosuz, N.V.; Onuchin, D.V.; Gorbunova, I.Y.; Kireev, V.V. Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines. Polymers 2020, 12, 1225. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Wu, G.; Zhang, X.; Zhao, C.; Lei, C. Synthesis of a Novel Nonflammable Eugenol-Based Phosphazene Epoxy Resin with Unique Burned Intumescent Char. Chem. Eng. J. 2020, 390, 124620. [Google Scholar] [CrossRef]
- Onuchin, D.V.; Sirotin, I.S.; Sarychev, I.A.; Bornosuz, N.V.; Kireev, V.V.; Gorbunova, I.Y.; Gorbatkina, Y.A. Physicochemical Properties of Epoxy Composites Modified with Epoxyphosphazene. Polym. Sci. Ser. B 2019, 61, 286–293. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H. A POSS-Phosphazene Based Porous Material for Adsorption of Metal Ions from Water. Chem. Asian J. 2019, 14, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Soldatov, M.; Wang, Q.; Liu, H. Phosphazene Functionalized Silsesquioxane-Based Porous Polymers for Absorbing I2, CO2 and Dyes. Polymer 2021, 218, 123491. [Google Scholar] [CrossRef]
- Ye, W.; Wei, W.; Fei, X.; Lu, R.; Liu, N.; Luo, J.; Zhu, Y.; Liu, X. Six-Arm Star-Shaped Polymer with Cyclophosphazene Core and Poly(ε-Caprolactone) Arms as Modifier of Epoxy Thermosets. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Qiao, Z.; Li, Q.; Li, X.; Wang, H. Facile Synthesis and Surface Activity of Poly(Ethylene Glycol) Star Polymers with a Phosphazene Core. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 17–25. [Google Scholar] [CrossRef]
- Chang, J.Y.; Ji, H.J.; Han, M.J.; Rhee, S.B.; Cheong, S.; Yoon, M. Preparation of Star-Branched Polymers with Cyclotriphosphazene Cores. Macromolecules 1994, 27, 1376–1380. [Google Scholar] [CrossRef]
- Inoue, K.; Miyamoto, H.; Itaya, T. Ionic Conductivity of Complexes of Novel Multiarmed Polymers with Phosphazene Core and LiClO4. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1839–1847. [Google Scholar] [CrossRef]
- Díaz, C.; Barbosa, M.; Godoy, Z. Monobranched and Hyperbranched Dendrimers Based on Cyclophosphazene Containing Nitrile and Phosphine Donors and Their Fe and Ru Organometallic Derivatives. Polyhedron 2004, 23, 1027–1035. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.-X.; Shi, X.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Cyclotriphosphazene Core-Based Dendrimers for Biomedical Applications: An Update on Recent Advances. J. Mater. Chem. B 2018, 6, 884–895. [Google Scholar] [CrossRef]
- Okutan, E.; Çoşut, B.; Kayıran, S.B.; Durmuş, M.; Kılıç, A.; Yeşilot, S. Synthesis of a Dendrimeric Phenoxy-Substituted Cyclotetraphosphazene and Its Non-Covalent Interactions with Multiwalled Carbon Nanotubes. Polyhedron 2014, 67, 344–350. [Google Scholar] [CrossRef]
- Allcock, H.R.; Allen, R.W.; O’Brien, J.P. Phosphorus-Nitrogen Compounds. 30. Synthesis of Platinum Derivatives of Polymeric and Cyclic Phosphazenes. J. Am. Chem. Soc. 1977, 99, 3984–3987. [Google Scholar] [CrossRef]
- Paddock, N.L.; Ranganathan, T.N.; Rettig, S.J.; Sharma, R.D.; Trotter, J. Complexes of Dodecamethylcyclohexaphosphazene with Palladium(II) Chloride and Platinum(II) Chloride. Crystal and Molecular Structure of Cis-Dichloro[H2-Dodecamethylcyclohexaphosphazene (N,N′)] Palladium(II) Sesquihydrate. Can. J. Chem. 1981, 59, 2429–2434. [Google Scholar] [CrossRef]
- Arıcı, T.A.; Örüm, S.M.; Demircioğlu, Y.S.; Özcan, A.; Özcan, A.S. Assessment of Adsorption Properties of Inorganic–Organic Hybrid Cyclomatrix Type Polyphosphazene Microspheres for the Removal of Pb(II) Ions from Aqueous Solutions. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 721–730. [Google Scholar] [CrossRef]
- Liebig, J.V. Nachtrag Der Redaction. Annalen Der Pharmacie 1834, 11, 139–150. [Google Scholar]
- Stokes, H.N. On Trimetaphosphimic Acid and Its Decomposition-Products. Amer. Chem. J. 1896, 18, 629–663. [Google Scholar]
- Schenck, R.; Römer, G. Über Die Phosphornitrilchloride Und Ihre Umsetzungen (I.). Ber. Dtsch. Chem. Ges. (A B Ser.) 1924, 57, 1343–1355. [Google Scholar] [CrossRef]
- Allcock, H. Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-323-14751-4. [Google Scholar]
- Becke-Goehring, M.; Lehr, W. Über Phosphor-Stickstoff-Verbindungen. XVI. Die Synthese der Phosphornitrid-dichloride. Z. Anorg. Allg. Chem. 1964, 327, 128–138. [Google Scholar] [CrossRef]
- Emsley, J.; Udy, P.B. Elucidation of the Reaction of Phosphorus Pentachloride and Ammonium Chloride by Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy. J. Chem. Soc. A 1970, 3025–3029. [Google Scholar] [CrossRef]
- Lund, L.G.; Paddock, N.L.; Proctor, J.E.; Searle, H.T. 514. Phosphonitrilic Derivatives. Part I. The Preparation of Cyclic and Linear Phosphonitrilic Chlorides. J. Chem. Soc. 1960, 2542–2547. [Google Scholar] [CrossRef]
- Bowers, D.J.; Wright, B.D.; Scionti, V.; Schultz, A.; Panzner, M.J.; Twum, E.B.; Li, L.-L.; Katzenmeyer, B.C.; Thome, B.S.; Rinaldi, P.L.; et al. Structure and Conformation of the Medium-Sized Chlorophosphazene Rings. Inorg. Chem. 2014, 53, 8874–8886. [Google Scholar] [CrossRef]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Nelson, J.M.; Reeves, S.D.; Honeyman, C.H.; Manners, I. “Living” Cationic Polymerization of Phosphoranimines as an Ambient Temperature Route to Polyphosphazenes with Controlled Molecular Weights. Macromolecules 1996, 29, 7740–7747. [Google Scholar] [CrossRef]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Olshavsky, M.A. A New Route to the Phosphazene Polymerization Precursors, Cl3PNSiMe3 and (NPCl2)3. Inorg. Chem. 1999, 38, 280–283. [Google Scholar] [CrossRef]
- Hammoutou, P.Y.; Heubel, J.; Jaeger, R.D. Differenciation Par L’anion De La Reaction De Deux Sels Ayant Un Cation Commun: Action De L’hexamethyldisilazane Sur Le Chlorure Et L’hexachlorophosphate D’hexachlorodiphosphazonium. Phosphorus Sulfur Silicon Relat. Elem. 1993, 79, 97–106. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Suraeva, O.V.; Solodukhin, A.N.; Kireev, V.V. Synthesis of Oligomeric Chlorophosphazenes in the Presence of ZnCl2. Polym. Sci. Ser. B 2013, 55, 63–68. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Ju, Z.; Zhong, S.; Zhao, Y. The Synthesis and 31P NMR Spectral Studies of Cyclophosphazenes. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1958–1963. [Google Scholar] [CrossRef]
- Yuan, F.; Zhu, Y.; Zhao, J.; Zhang, B.; Jiang, D. A Modified Method for Preparation of a Pure Octachlorocyclotetraphosphazene. Phosphorus Sulfur Silicon Relat. Elem. 2001, 176, 77–81. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification; Wiley: Hoboken, NJ, USA, 1986; ISBN 978-0-471-08467-9. [Google Scholar]
- Gorlov, M.V.; Bredov, N.S.; Esin, A.S.; Kireev, V.V. A Direct Synthesis of Cl3PNSiMe3 from PCl5 and Hexamethyldisilazane. J. Organomet. Chem. 2016, 818, 82–84. [Google Scholar] [CrossRef]
- Wang, B. Development of a One-Pot in Situ Synthesis of Poly(Dichlorophosphazene) from PCl3. Macromolecules 2005, 38, 643–645. [Google Scholar] [CrossRef]
- Wang, B.; Rivard, E.; Manners, I. A New High-Yield Synthesis of Cl3PNSiMe3, a Monomeric Precursor for the Controlled Preparation of High Molecular Weight Polyphosphazenes. Inorg. Chem. 2002, 41, 1690–1691. [Google Scholar] [CrossRef] [PubMed]
- Suárez Suárez, S.; Presa Soto, D.; Carriedo, G.A.; Presa Soto, A.; Staubitz, A. Experimental and Theoretical Study of the Living Polymerization of N-Silylphosphoranimines. Synthesis of New Block Copolyphosphazenes. Organometallics 2012, 31, 2571–2581. [Google Scholar] [CrossRef]


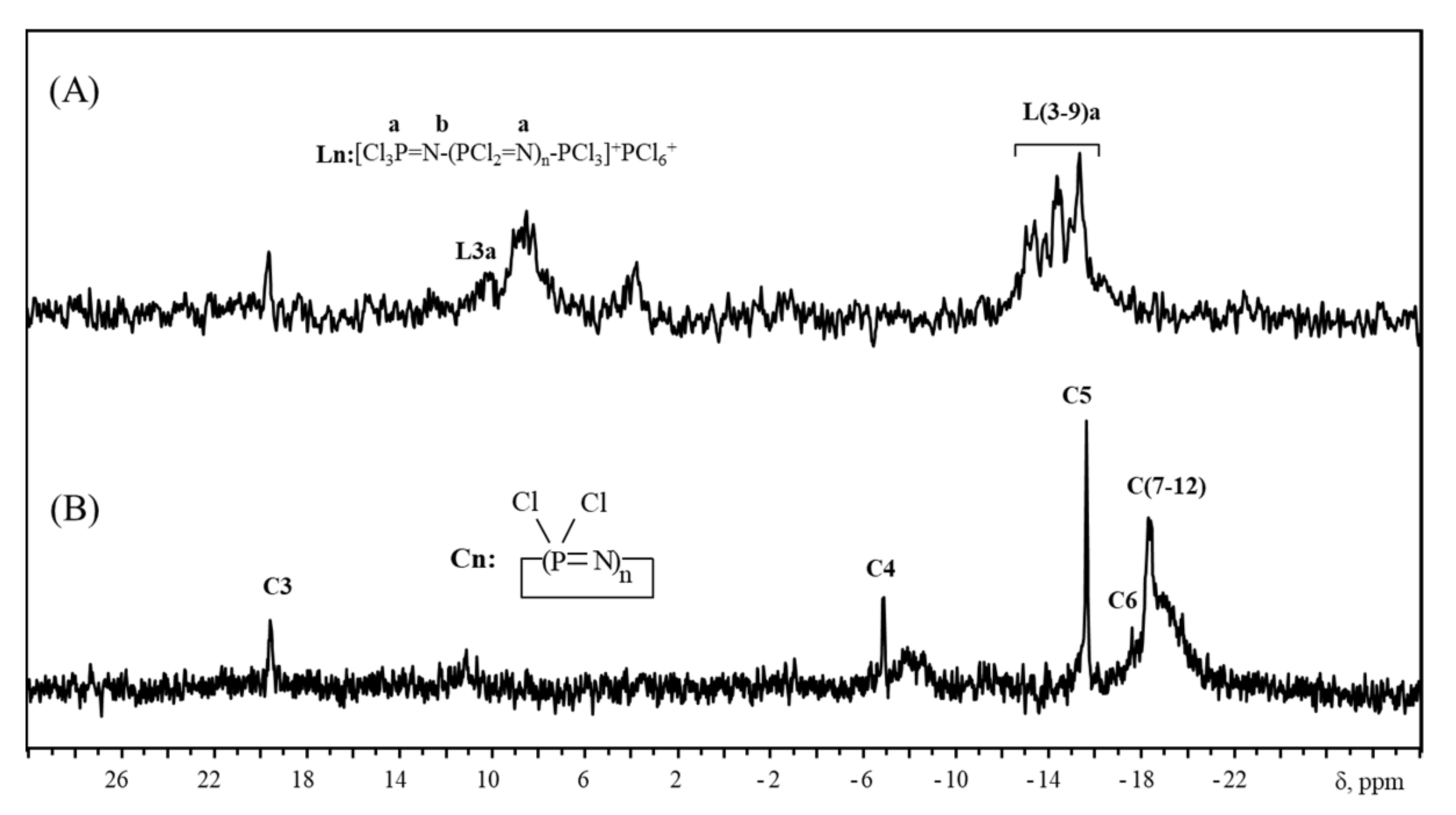
| Value of 31P NMR Chemical Shift of Chlorophosphazenes [Cl2P=N]n | Reference | |||
|---|---|---|---|---|
| 5 | 6 | 7 | ≥8 | |
| −17.0 | −16.0 | −18.0 | - | [35] (1972) |
| −15.1 | −15.3 | −17.0 | –17.7 | [39] (2014) |
| n | Weight (g) | mol | Yield of Product (%) |
|---|---|---|---|
| 2 | 2.58 | 0.016 | 75 |
| 6 | 3.88 | 0.024 | 82 |
| 7 | 4.03 | 0.025 | 84 |
| 9 | 4.23 | 0.027 | 88 |
| n | Weight (g) | mol | Yield of Product (%) |
|---|---|---|---|
| 2 | 1.49 | 0.00923 | 130 1 |
| 6 | 0.87 | 0.00539 | 80 |
| 7 | 0.79 | 0.00488 | 78 |
| 9 | 0.66 | 0.00410 | 71 |
| Cycle Size k of the Cyclic Homologue in the Product | m/z of Phenoxylated Derivatives [N=P(OPh)2]k | PCl5: HMDS Ratio Used to Obtain Linear Chlorophosphazene Oligomer/Calculated Average Number of P=N-Links in the Product after Cyclization | ||
|---|---|---|---|---|
| 2:3/k = 3 | 8:9/k = 5 | 1.04:1/k = 8 | ||
| Composition of the Cyclic Product, Determined by MALDI-TOF a) | ||||
| 3 | 694 | 95.00 | 38.40 | 0.7 |
| 4 | 925 | 3.00 | 40.30 | 0.8 |
| 5 | 1156 | - | 7.81 | 3.4 |
| 6 | 1387 | 0.50 | 2.32 | 47.9 |
| 7 | 1618 | 0.50 | 1.69 | 31.7 |
| 8 | 1849 | 1.00 | 5.70) | 7.4) |
| 9 | 2080 | - | 2.95 | 3.1 |
| 10 | 2311 | - | 0.84 | 2.7 |
| 11 | 2541 | - | - | 1.7 |
| 12 | 2773 | - | - | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorlov, M.; Bredov, N.; Esin, A.; Sirotin, I.; Soldatov, M.; Oberemok, V.; Kireev, V.V. Novel Approach for the Synthesis of Chlorophosphazene Cycles with a Defined Size via Controlled Cyclization of Linear Oligodichlorophosphazenes [Cl(PCl2=N)n–PCl3]+[PCl6]−. Int. J. Mol. Sci. 2021, 22, 5958. https://doi.org/10.3390/ijms22115958
Gorlov M, Bredov N, Esin A, Sirotin I, Soldatov M, Oberemok V, Kireev VV. Novel Approach for the Synthesis of Chlorophosphazene Cycles with a Defined Size via Controlled Cyclization of Linear Oligodichlorophosphazenes [Cl(PCl2=N)n–PCl3]+[PCl6]−. International Journal of Molecular Sciences. 2021; 22(11):5958. https://doi.org/10.3390/ijms22115958
Chicago/Turabian StyleGorlov, Mikhail, Nikolay Bredov, Andrey Esin, Igor Sirotin, Mikhail Soldatov, Volodymyr Oberemok, and Vyacheslav V. Kireev. 2021. "Novel Approach for the Synthesis of Chlorophosphazene Cycles with a Defined Size via Controlled Cyclization of Linear Oligodichlorophosphazenes [Cl(PCl2=N)n–PCl3]+[PCl6]−" International Journal of Molecular Sciences 22, no. 11: 5958. https://doi.org/10.3390/ijms22115958
APA StyleGorlov, M., Bredov, N., Esin, A., Sirotin, I., Soldatov, M., Oberemok, V., & Kireev, V. V. (2021). Novel Approach for the Synthesis of Chlorophosphazene Cycles with a Defined Size via Controlled Cyclization of Linear Oligodichlorophosphazenes [Cl(PCl2=N)n–PCl3]+[PCl6]−. International Journal of Molecular Sciences, 22(11), 5958. https://doi.org/10.3390/ijms22115958










