New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure?
Abstract
1. Introduction
1.1. Environmental Pollution by Mercury
1.2. Seawater Mercury Pollution
1.3. Mytilus galloprovincialis as Sentinel Organism
1.4. Mercury and Mytilus galloprovincialis Reproduction
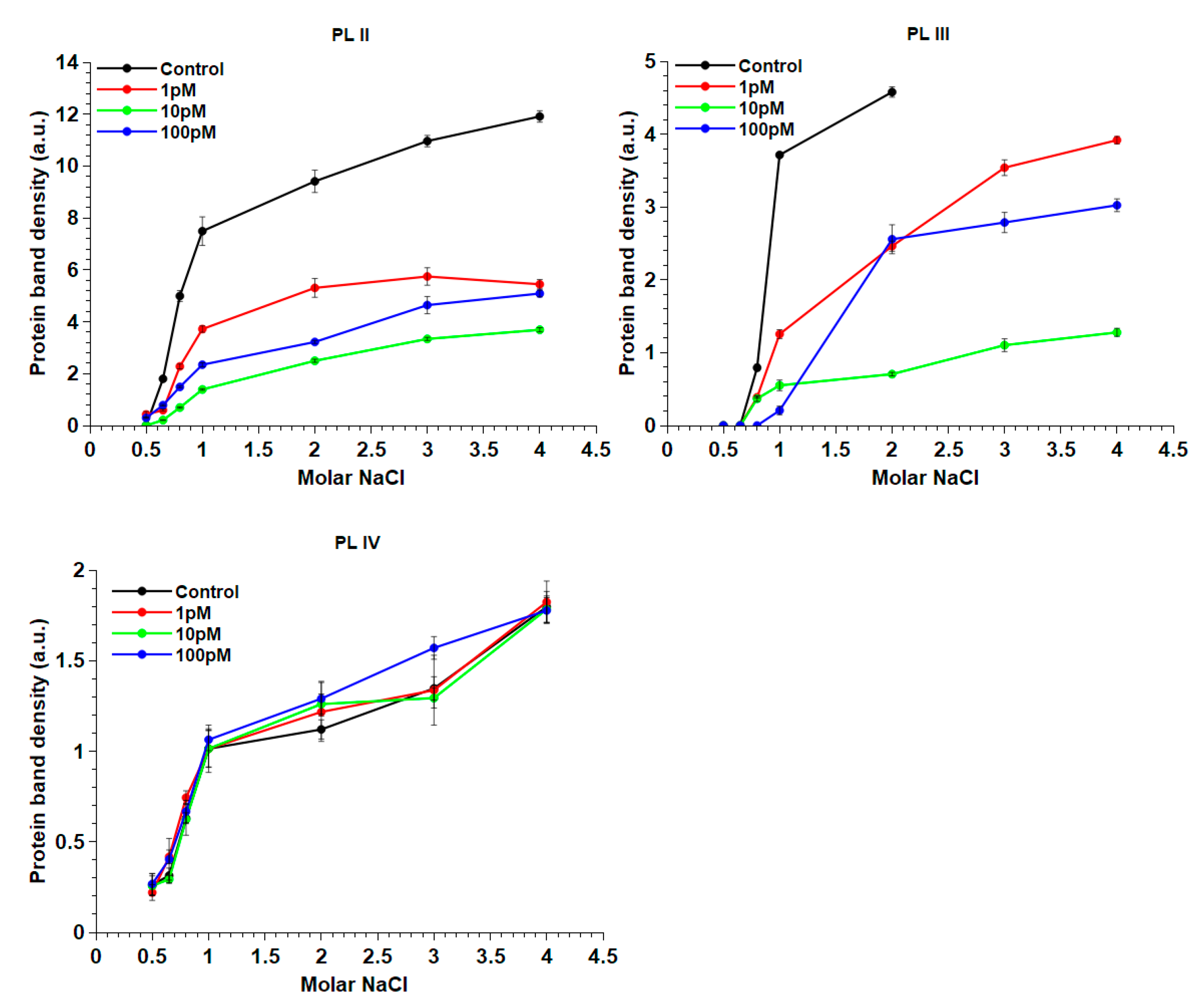
2. Results
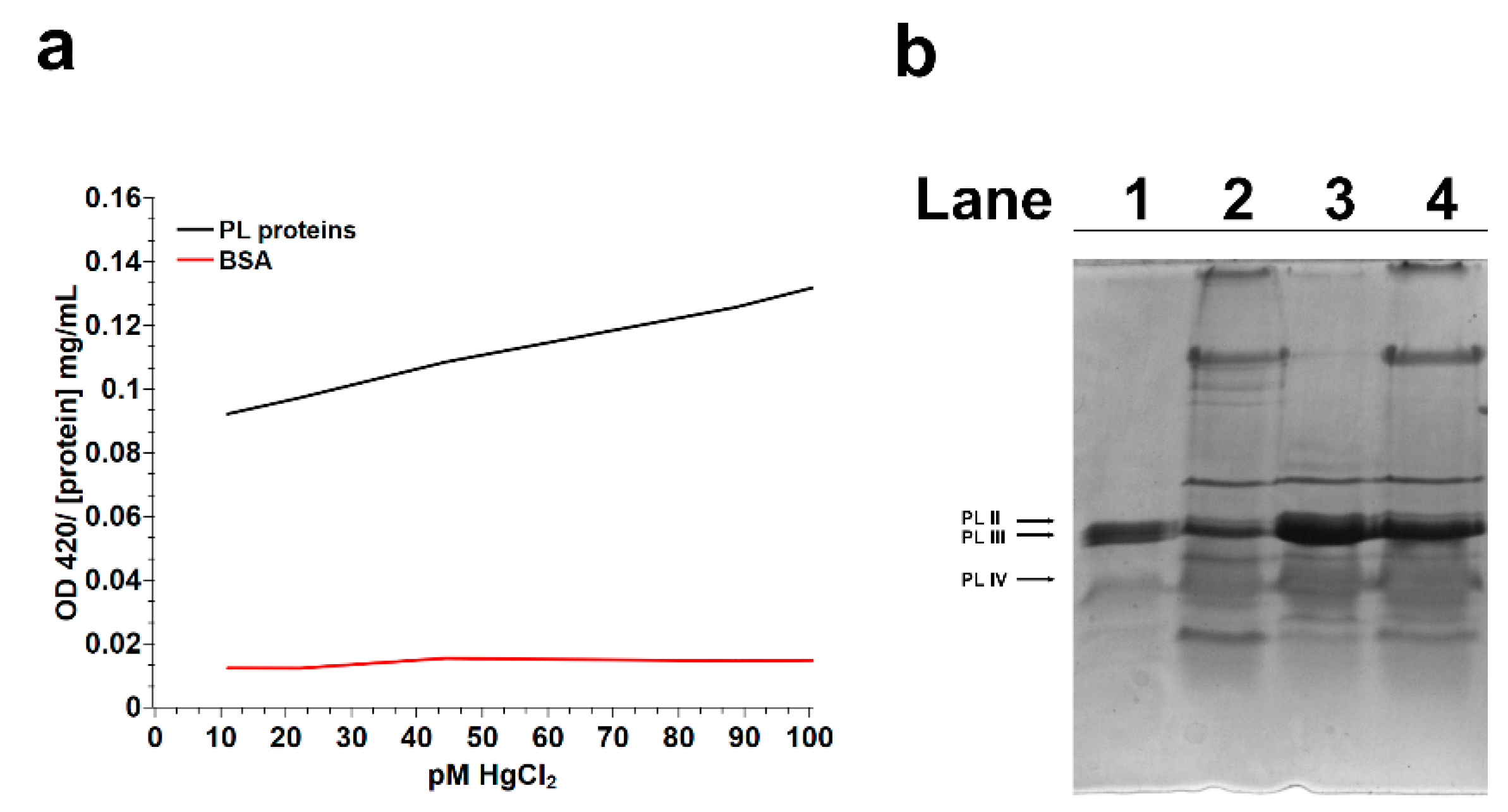
2.1. Turbidity Assays on PL Proteins
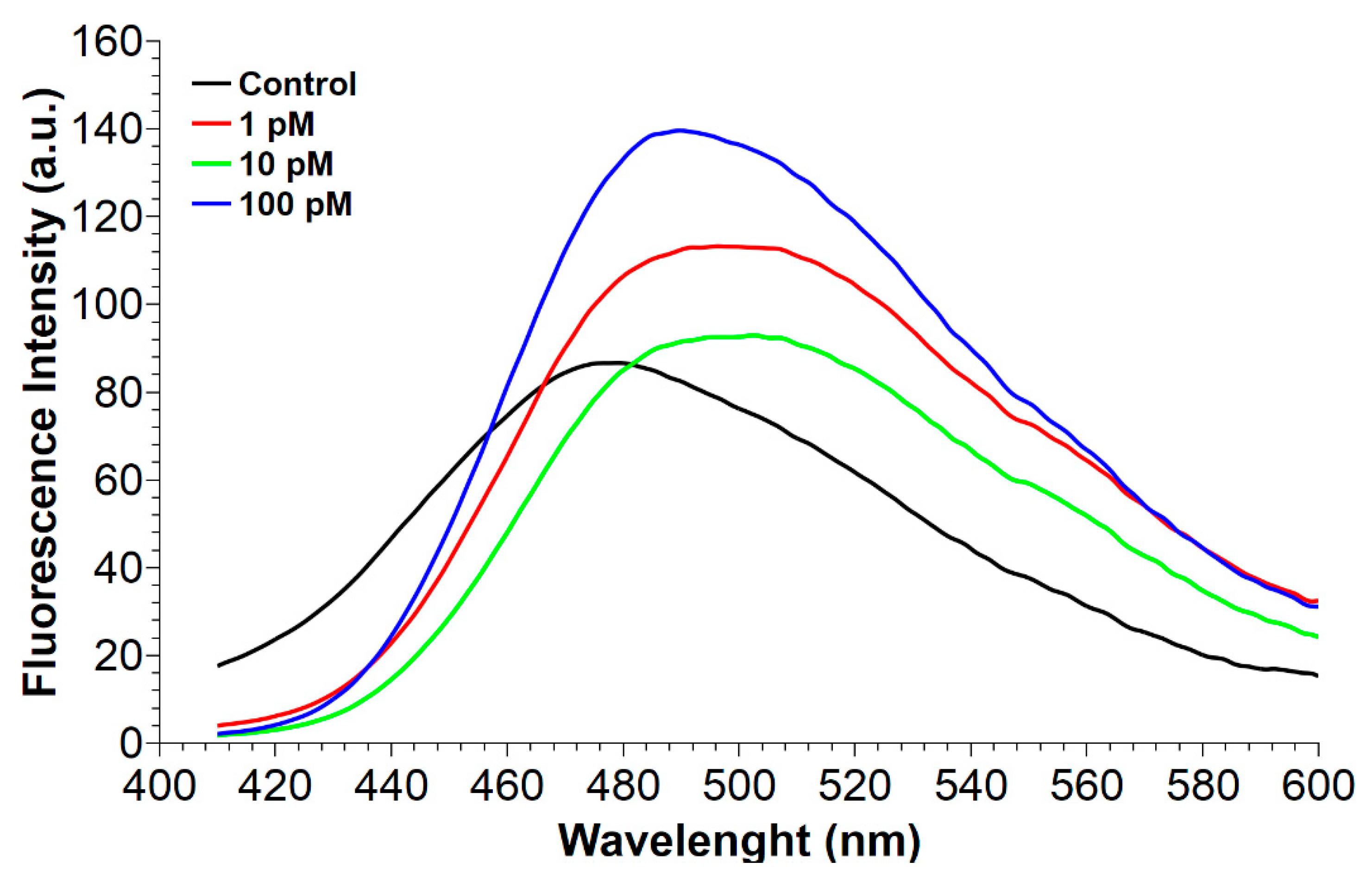
2.2. Fluorescence Spectroscopy
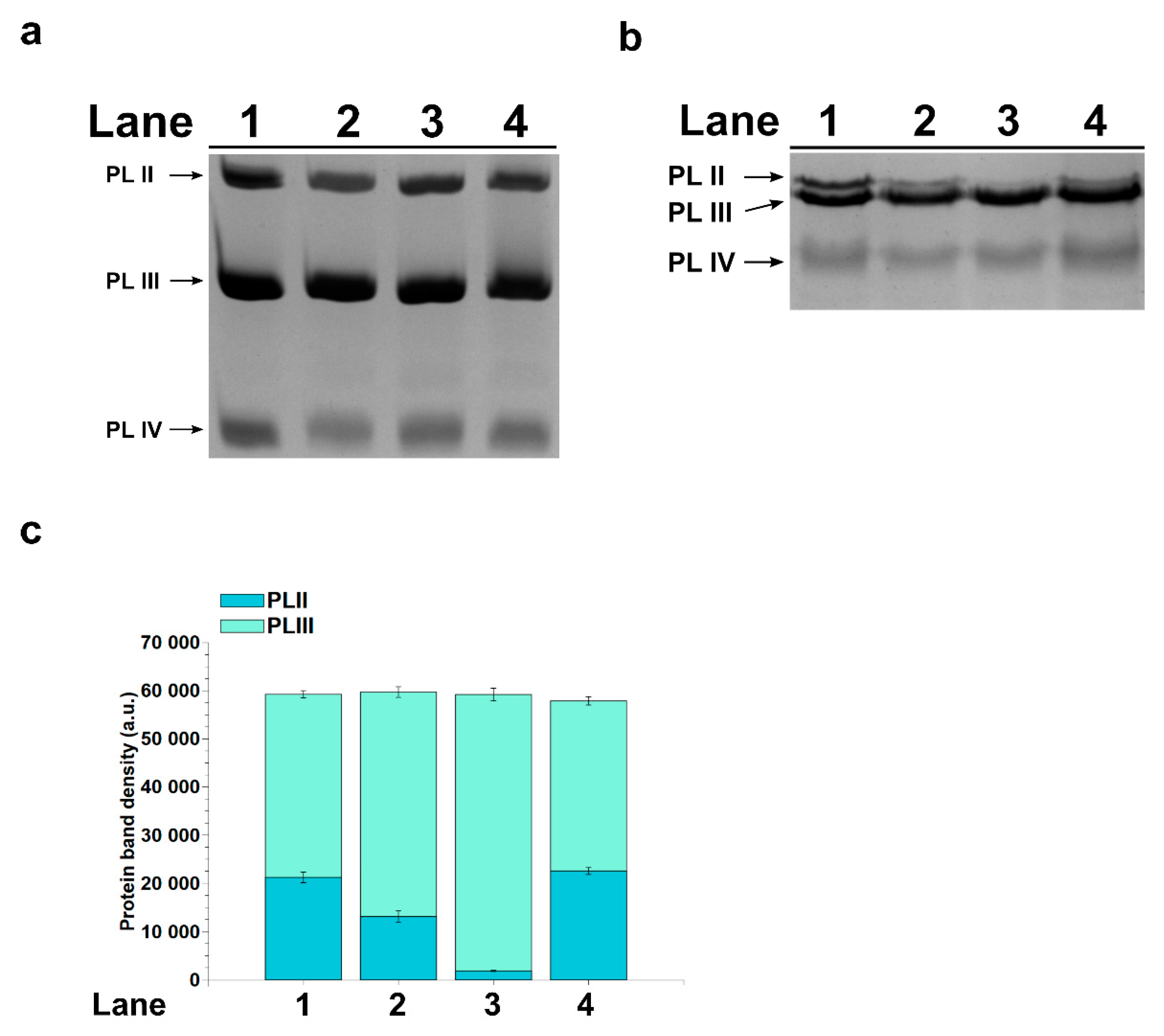
2.3. SDS-PAGE Analysis of PL Proteins
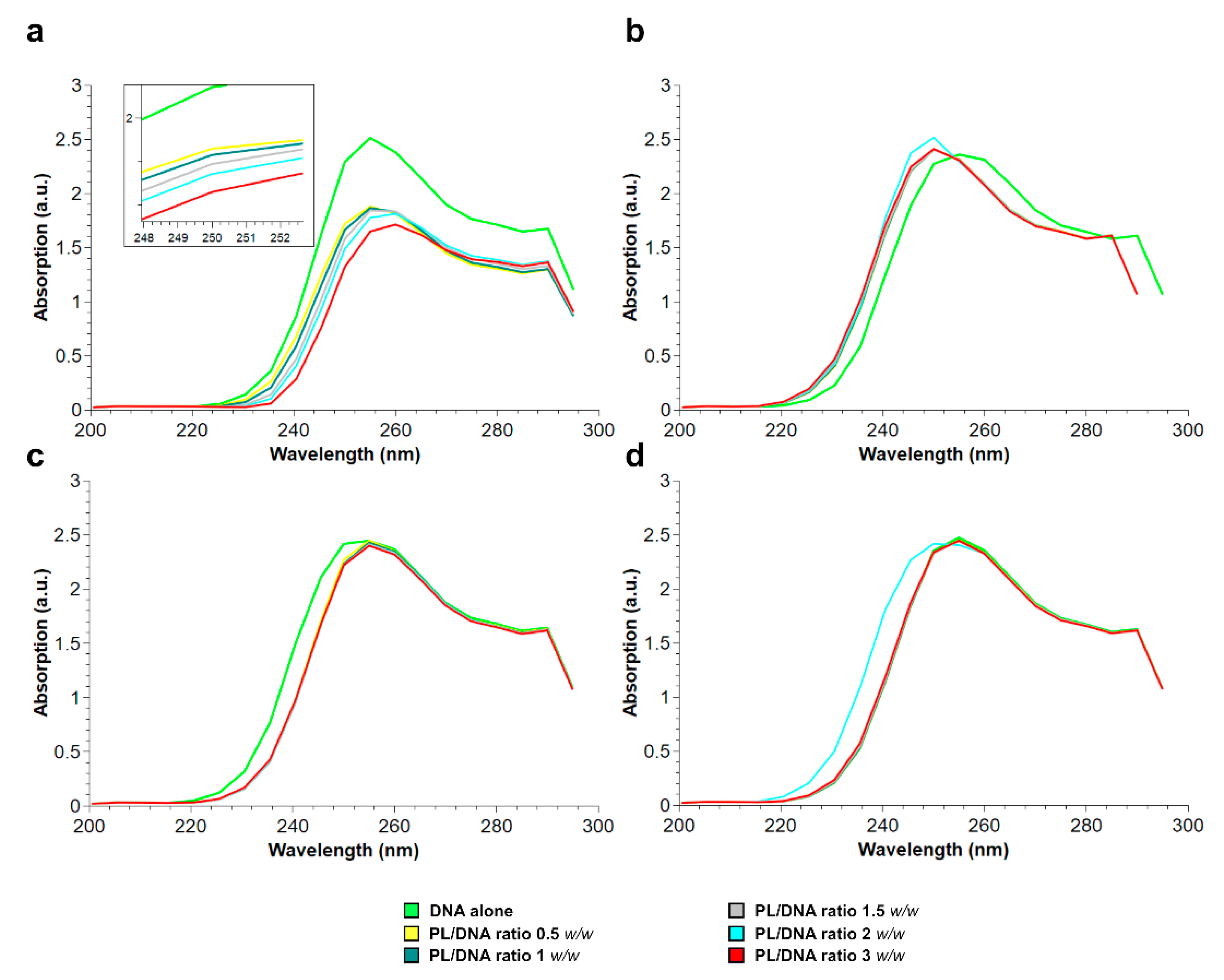
2.4. DNA Absorption Spectrum after PL Proteins Addition
2.5. Release of PL Proteins from Sperm Nuclei
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Mussels Sampling and Exposure to HgCl2
4.3. Spermatozoa Sampling and Processing
4.4. PL Proteins from M. galloprovincialis Spermatozoa Extraction and Analyses
4.5. Plasmid DNA Preparation and Analysis
4.6. Turbidity Analysis of M. galloprovincialis PL Proteins
4.7. Fluorescence Spectroscopy Analyses
4.8. Effect of PL Addiction from HgCl2-Exposed Mussels on the DNA Absorption Spectrum
4.9. Preparation of M. galloprovincialis Sperm Nuclei and Salt-Induced Release of Nuclear Proteins
4.10. Statistics Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASW | Artificial sea water |
| AU-PAGE | Acetic acid-urea polyacrylamide gel electrophoresis |
| SDS-PAGE | Sodium dodecyl sulphate–polyacrylamide gel electrophoresis |
| EDTA | Ethylenediaminetetraacetic acid |
| EMSA | Electrophoretic mobility shift assay |
| PCA | Perchloric acid |
| PL | Protamine-like |
| SNBP | Sperm nuclear basic protein |
| TBE | Tris/Borate/EDTA |
| OD | Optical density |
References
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.F.; Koyama, H. Cadmium, Copper, and Zinc Levels in Rice and Soil of Japan, Indonesia, and China by Soil Type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef]
- Depledge, M.H.; Aagaard, A.; Györkös, P. Assessment of Trace Metal Toxicity Using Molecular, Physiological and Behavioural Biomarkers. Mar. Pollut. Bull. 1995, 31, 19–27. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus Galloprovincialis (Lamarck, 1819) Spermatozoa: Hsp70 Expression and Protamine-like Protein Property Studies. Environ. Sci. Pollut. Res. Int. 2018, 25, 12957–12966. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; de Benedictis, D.; Brundo, M.V.; et al. Protamine-like Proteins’ Analysis as an Emerging Biotechnique for Cadmium Impact Assessment on Male Mollusk Mytilus Galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Tortora, F.; Lettieri, G.; Palumbo, G.; Manna, C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules 2020, 25, 3278. [Google Scholar] [CrossRef]
- Wiener, J.G. Mercury Exposed: Advances in Environmental Analysis and Ecotoxicology of a Highly Toxic Metal. Environ. Toxicol. Chem. 2013, 32, 2175–2178. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa Sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants (Basel) 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Pepi, M.; Filippelli, M. Methylmercury Resistance in Desulfovibrio Desulfuricans Strains in Relation to Methylmercury Degradation. Appl. Environ. Microbiol. 1993, 59, 2479–2485. [Google Scholar] [CrossRef]
- Cinnirella, S.; Bruno, D.E.; Pirrone, N.; Horvat, M.; Živković, I.; Evers, D.C.; Johnson, S.; Sunderland, E.M. Mercury Concentrations in Biota in the Mediterranean Sea, a Compilation of 40 Years of Surveys. Sci. Data 2019, 6, 205. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A Review of Toxicity and Mechanisms of Individual and Mixtures of Heavy Metals in the Environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Officioso, A.; Alzoubi, K.; Lang, F.; Manna, C. Hydroxytyrosol Inhibits Phosphatidylserine Exposure and Suicidal Death Induced by Mercury in Human Erythrocytes: Possible Involvement of the Glutathione Pathway. Food Chem. Toxicol. 2016, 89, 47–53. [Google Scholar] [CrossRef]
- Géret, F.; Jouan, A.; Turpin, V.; Bebianno, M.J.; Cosson, R.P. Influence of Metal Exposure on Metallothionein Synthesis and Lipid Peroxidation in Two Bivalve Mollusks: The Oyster (Crassostrea Gigas) and the Mussel (Mytilus Edulis). Aquat. Living Resour. 2002, 15, 61–66. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue Mussels (Mytilus Edulis Spp.) as Sentinel Organisms in Coastal Pollution Monitoring: A Review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Rittschof, D.; McClellan-Green, P. Molluscs as Multidisciplinary Models in Environment Toxicology. Mar. Pollut. Bull. 2005, 50, 369–373. [Google Scholar] [CrossRef]
- Piscopo, M. Seasonal Dependence of Cadmium Molecular Effects on Mytilus Galloprovincialis (Lamarck, 1819) Protamine-like Protein Properties. Mol. Reprod. Dev. 2019, 86, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Pedà, C.; Compa, M.; Tsangaris, C.; Alomar, C.; Claro, F.; Ioakeimidis, C.; Galgani, F.; Hema, T.; Deudero, S.; et al. Bioindicators for Monitoring Marine Litter Ingestion and Its Impacts on Mediterranean Biodiversity. Environ. Pollut. 2018, 237, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Ricciardiello, M.; Palumbo, G.; Troisi, J. Selectivity of Metal Bioaccumulation and Its Relationship with Glutathione S-Transferase Levels in Gonadal and Gill Tissues of Mytilus Galloprovincialis Exposed to Ni (II), Cu (II) and Cd (II). Rend. Fis. Acc. Lincei 2016, 27, 737–748. [Google Scholar] [CrossRef]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Panel on Health Risks and Toxicological Effects of Methylmercury Methylmercury Exposure and Health Effects in Humans: A Worldwide Concern. Ambio 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of Environmental Methylmercury on the Health of Wild Birds, Mammals, and Fish. Ambio 2007, 36, 12–18. [Google Scholar] [CrossRef]
- His, E.; Beiras, R.; Seaman, M.N.L. The Assessment of Marine Pollution—Bioassays with Bivalve Embryos and Larvae. In Advances in Marine Biology; Southward, A.J., Tyler, P.A., Young, C.M., Eds.; Academic Press: San Diego, CA, USA, 1999; Volume 37, pp. 1–178. [Google Scholar]
- Wessel, N.; Rousseau, S.; Caisey, X.; Quiniou, F.; Akcha, F. Investigating the Relationship between Embryotoxic and Genotoxic Effects of Benzo[a]Pyrene, 17α-Ethinylestradiol and Endosulfan on Crassostrea Gigas Embryos. Aquat. Toxicol. 2007, 85, 133–142. [Google Scholar] [CrossRef]
- Bellas, J. Comparative Toxicity of Alternative Antifouling Biocides on Embryos and Larvae of Marine Invertebrates. Sci. Total Environ. 2006, 367, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Connor, P.M. Acute Toxicity of Heavy Metals to Some Marine Larvae. Mar. Pollut. Bull. 1972, 3, 190–192. [Google Scholar] [CrossRef]
- Beiras, R.; His, E. Effects of Dissolved Mercury on Embryo-Genesis, Survival, Growth and Metamorphosis of Crassostrea Gigas Oyster Larvae. Mar. Ecol. Prog. Ser. 1994, 113, 95–103. [Google Scholar] [CrossRef]
- Beiras, R.; Albentosa, M. Inhibition of Embryo Development of the Commercial Bivalves Ruditapes Decussatus and Mytilus Galloprovincialis by Trace Metals; Implications for the Implementation of Seawater Quality Criteria. Aquaculture 2004, 230, 205–213. [Google Scholar] [CrossRef]
- Beiras, R.; His, E. Effects of Dissolved Mercury on Embryogenesis, Survival and Growth of Mytilus Galloprovincialis Mussel Larvae. Mar. Ecol. Prog. Ser. 1995, 126, 185–189. [Google Scholar] [CrossRef]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular Effects of Copper on the Reproductive System of Mytilus Galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus Galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Dellali, M.; Gnassia Barelli, M.; Romeo, M.; Aissa, P. The Use of Acetylcholinesterase Activity in Ruditapes Decussatus and Mytilus Galloprovincialis in the Biomonitoring of Bizerta Lagoon. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2001, 130, 227–235. [Google Scholar] [CrossRef]
- Smaoui-Damak, W.; Hamza-Chaffai, A.; Bebianno, M.J.; Amiard, J.C. Variation of Metallothioneins in Gills of the Clam Ruditapes Decussatus from the Gulf of Gabès (Tunisia). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 181–188. [Google Scholar] [CrossRef]
- Slavík, J. Anilinonaphthalene Sulfonate as a Probe of Membrane Composition and Function. Biochim. Biophys. Acta 1982, 694, 1–25. [Google Scholar] [CrossRef]
- Bothra, A.; Bhattacharyya, A.; Mukhopadhyay, C.; Bhattacharyya, K.; Roy, S. A Fluorescence Spectroscopic and Molecular Dynamics Study of Bis-ANS/Protein Interaction. J. Biomol. Struct. Dyn. 1998, 15, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, F.; Gührs, K.-H.; von Mikecz, A. Amyloid Domains in the Cell Nucleus Controlled by Nucleoskeletal Protein Lamin B1 Reveal a New Pathway of Mercury Neurotoxicity. PeerJ 2015, 3, e754. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, M.; Mizuguchi, K.; Ahmad, S. Conformational Changes in DNA-Binding Proteins: Relationships with Precomplex Features and Contributions to Specificity and Stability. Proteins Struct. Funct. Bioinform. 2014, 82, 841–857. [Google Scholar] [CrossRef]
- Stratton, A.; Ericksen, M.; Harris, T.V.; Symmonds, N.; Silverstein, T.P. Mercury(II) Binds to Both of Chymotrypsin’s Histidines, Causing Inhibition Followed by Irreversible Denaturation/Aggregation. Protein Sci. 2017, 26, 292–305. [Google Scholar] [CrossRef]
- Corbeil, M.-C.; Beauchamp, A.L. Crystal Structure and Vibrational Spectra of the Methylmercury Complex Withl-Alanine. J. Crystallogr. Spectrosc. Res. 1989, 19, 123–134. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef]
- Stebbing, A.R.D. A Theory for Growth Hormesis. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1998, 403, 249–258. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining Hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Why It Is Important to Toxicology and Toxicologists. Environ. Toxicol. Chem. 2008, 27, 1451–1474. [Google Scholar] [CrossRef]
- Chapman, P.M. The Implications of Hormesis to Ecotoxicology and Ecological Risk Assessment. Hum. Exp. Toxicol. 2001, 20, 499–505. [Google Scholar] [CrossRef] [PubMed]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the Properties of Sperm Protamine-like II Protein after Exposure of Mytilus Galloprovincialis (Lamarck 1819) to Sub-Toxic Doses of Cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.S.; Hocker, J.R.; Hanas, R.J.; Nwosu, E.C.; Hanas, J.S. Mercuric Ion Inhibition of Eukaryotic Transcription Factor Binding to DNA11Abbreviations: TFIIIA, Transcription Factor A for RNA Polymerase III; Cys2His2, Two Cysteine and Two Histidine Amino Acids; ICR, Internal Control Region; S, Sedimentation Constant; DNase I, Deoxyribonuclease I; DEAE, Diethylaminoethyl; RNase A, Ribonuclease A; and SV40, Simian Virus 40. Biochem. Pharmacol. 2001, 61, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-B.; Johs, A.; Parks, J.M.; Olliff, L.; Miller, S.M.; Summers, A.O.; Liang, L.; Smith, J.C. Structure and Conformational Dynamics of the Metalloregulator MerR upon Binding of Hg(II). J. Mol. Biol 2010, 398, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Lin, L.-Y.; Zou, X.-W.; Huang, C.-C.; Chan, N.-L. Structural Basis of the Mercury(II)-Mediated Conformational Switching of the Dual-Function Transcriptional Regulator MerR. Nucleic Acids Res. 2015, 43, 7612–7623. [Google Scholar] [CrossRef]
- Vassalli, Q.A.; Caccavale, F.; Avagnano, S.; Murolo, A.; Guerriero, G.; Fucci, L.; Ausió, J.; Piscopo, M. New Insights into Protamine-like Component Organization in Mytilus Galloprovincialis’ Sperm Chromatin. DNA Cell Biol. 2015, 34, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Loppi, S.; Piscopo, M.; Paoli, L.; Vannini, A.; Monaci, F.; Sorbo, S.; Lentini, M.; Esposito, S. The Biological Response Chain to Pollution: A Case Study from the “Italian Triangle of Death” Assessed with the Liverwort Lunularia Cruciata. Environ. Sci. Pollut. Res. Int. 2017, 24, 26185–26193. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.M.Y.; Yeung, Q.S.Y.; Briton-Jones, C.M.; Cheung, C.-K.; Lam, C.W.K.; Haines, C.J. Relationship between Semen Parameters and Mercury Concentrations in Blood and in Seminal Fluid from Subfertile Males in Hong Kong. Fertil. Steril. 2002, 78, 426–428. [Google Scholar] [CrossRef]
- Mocevic, E.; Specht, I.O.; Marott, J.L.; Giwercman, A.; Jönsson, B.A.G.; Toft, G.; Lundh, T.; Bonde, J.P. Environmental Mercury Exposure, Semen Quality and Reproductive Hormones in Greenlandic Inuit and European Men: A Cross-Sectional Study. Asian J. 2013, 15, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.S.; Escobar, A.G.; Torres, J.G.D.; Brum, D.S.; Santos, F.W.; Alonso, M.J.; Salaices, M.; Vassallo, D.V.; Peçanha, F.M.; Leivas, F.G.; et al. Chronic Exposure to Low Doses of Mercury Impairs Sperm Quality and Induces Oxidative Stress in Rats. J. Toxicol. Environ. Health A 2014, 77, 143–154. [Google Scholar] [CrossRef]
- Kushawaha, B.; Yadav, R.S.; Swain, D.K.; Rai, P.K.; Garg, S.K. Mercury-Induced Inhibition of Tyrosine Phosphorylation of Sperm Proteins and Altered Functional Dynamics of Buck Spermatozoa: An In Vitro Study. Biol. Trace Elem. Res. 2020, 198, 478–492. [Google Scholar] [CrossRef]
- Veprintsev, D.B.; Permyakov, E.A.; Kalinichenko, L.P.; Berliner, L.J. Pb2+ and Hg2+ Binding to Alpha-Lactalbumin. Biochem. Mol. Biol. Int. 1996, 39, 1255–1265. [Google Scholar]
- Someya, Y.; Kimura-Someya, T.; Yamaguchi, A. Role of the Charge Interaction between Arg(70) and Asp(120) in the Tn10-Encoded Metal-Tetracycline/H(+) Antiporter of Escherichia Coli. J. Biol. Chem. 2000, 275, 210–214. [Google Scholar] [CrossRef]
- Lettieri, G.; Maione, M.; Ranauda, M.A.; Mele, E.; Piscopo, M. Molecular Effects on Spermatozoa of Mytilus Galloprovincialis Exposed to Hyposaline Conditions. Mol. Reprod. Dev. 2019, 86, 650–660. [Google Scholar] [CrossRef]
- Notariale, R.; Basile, A.; Montana, E.; Romano, N.C.; Cacciapuoti, M.G.; Aliberti, F.; Gesuele, R.; De Ruberto, F.; Sorbo, S.; Tenore, G.C.; et al. Protamine-like Proteins Have Bactericidal Activity. The First Evidence in Mytilus Galloprovincialis. Acta Biochim. Pol. 2018, 65, 585–594. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Scarano, C.; Gori, C.; Giarra, A.; Febbraio, F. Relevance of Arginine Residues in Cu(II)-Induced DNA Breakage and Proteinase K Resistance of H1 Histones. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Tenore, G.C.; Notariale, R.; Maresca, V.; Maisto, M.; de Ruberto, F.; Heydari, M.; Sorbo, S.; Basile, A. Antimicrobial and Antioxidant Activity of Proteins from Feijoa Sellowiana Berg. Fruit before and after in Vitro Gastrointestinal Digestion. Nat. Prod. Res. 2020, 34, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Fioretti, F.M.; Fucci, L.; Ausió, J.; Piscopo, M. High Efficiency Method to Obtain Supercoiled DNA with a Commercial Plasmid Purification Kit. Acta Biochim. Pol. 2012, 59, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Ruiz, S. Nucleosomal Organization of Chromatin in Sperm Nuclei of the Bivalve Mollusc Aulacomya Ater. Mol. Cell Biochem. 1991, 101, 93–99. [Google Scholar] [CrossRef]
- Fioretti, F.M.; Febbraio, F.; Carbone, A.; Branno, M.; Carratore, V.; Fucci, L.; Ausió, J.; Piscopo, M. A Sperm Nuclear Basic Protein from the Sperm of the Marine Worm Chaetopterus Variopedatus with Sequence Similarity to the Arginine-Rich C-Termini of Chordate Protamine-Likes. DNA Cell Biol. 2012, 31, 1392–1402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. https://doi.org/10.3390/ijms22115893
Lettieri G, Notariale R, Carusone N, Giarra A, Trifuoggi M, Manna C, Piscopo M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? International Journal of Molecular Sciences. 2021; 22(11):5893. https://doi.org/10.3390/ijms22115893
Chicago/Turabian StyleLettieri, Gennaro, Rosaria Notariale, Nadia Carusone, Antonella Giarra, Marco Trifuoggi, Caterina Manna, and Marina Piscopo. 2021. "New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure?" International Journal of Molecular Sciences 22, no. 11: 5893. https://doi.org/10.3390/ijms22115893
APA StyleLettieri, G., Notariale, R., Carusone, N., Giarra, A., Trifuoggi, M., Manna, C., & Piscopo, M. (2021). New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? International Journal of Molecular Sciences, 22(11), 5893. https://doi.org/10.3390/ijms22115893








