Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments
Abstract
1. Introduction
2. Results
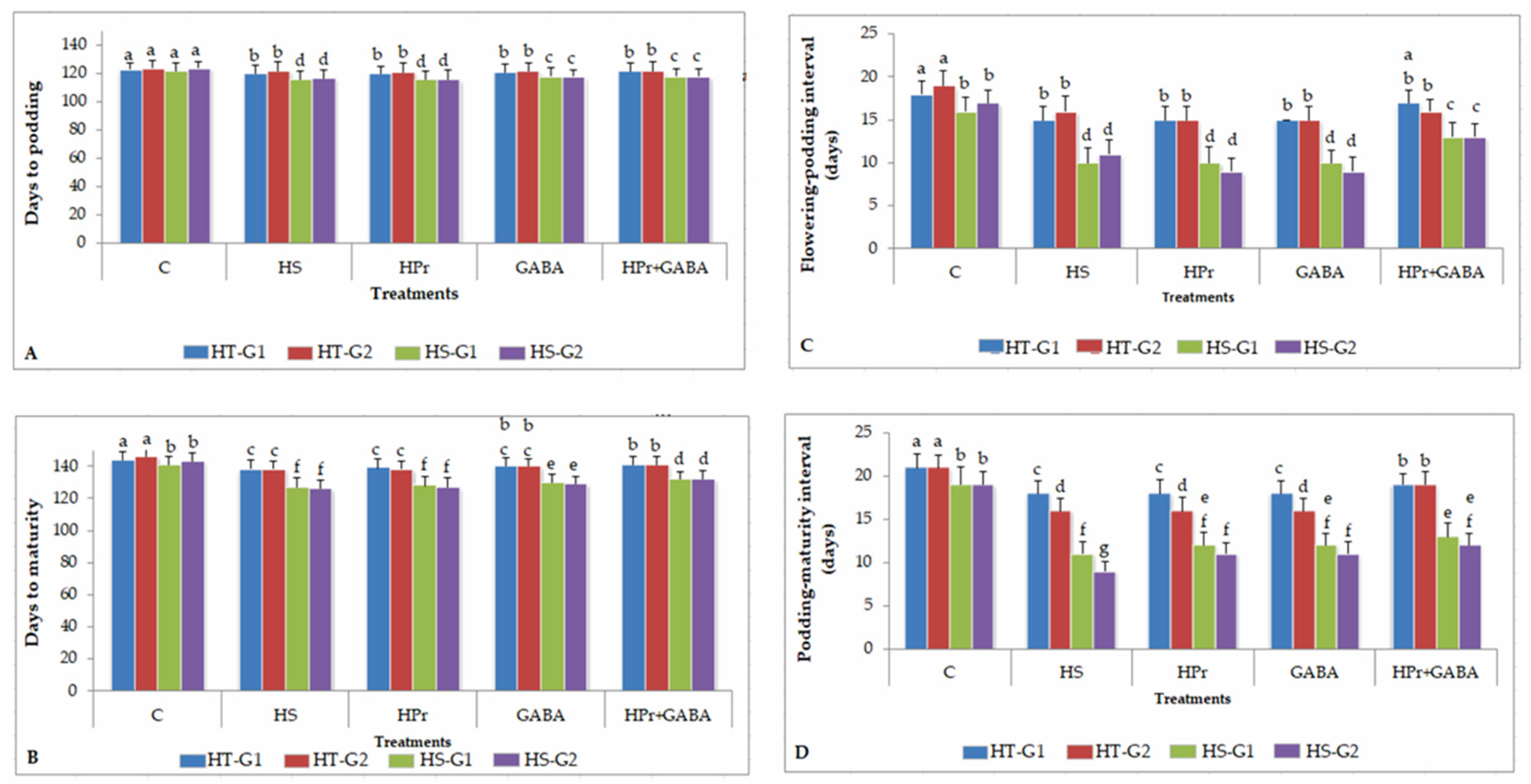
2.1. Phenology
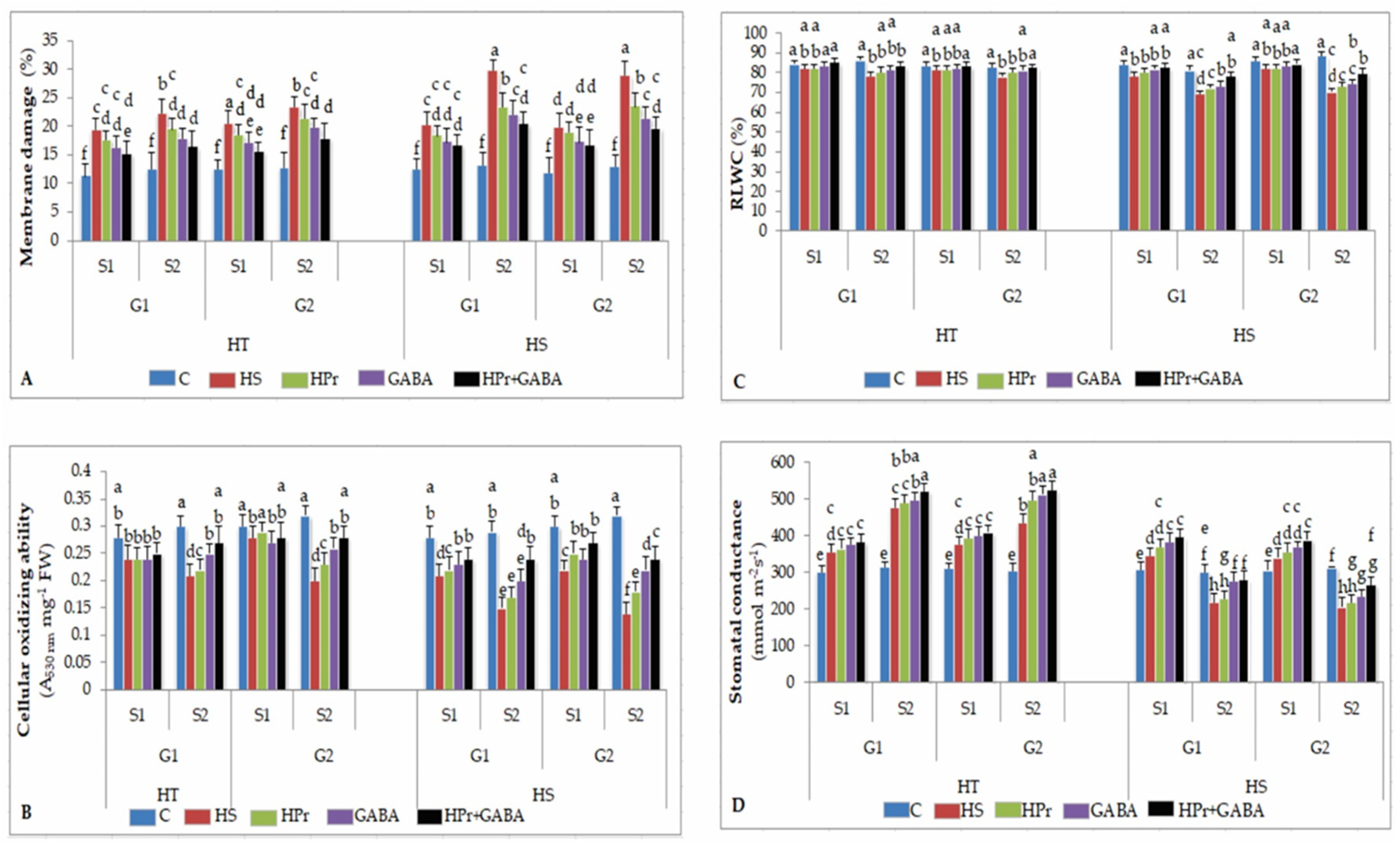
2.2. Stress Injury to Leaves
2.2.1. Membrane Damage
2.2.2. Cellular Oxidizing Ability
2.2.3. Leaf Water Status
2.2.4. Stomatal Conductance
2.3. Reproductive Function
2.3.1. Pollen Germination
2.3.2. Pollen Viability
2.3.3. Stigma Receptivity
2.3.4. Ovule Viability
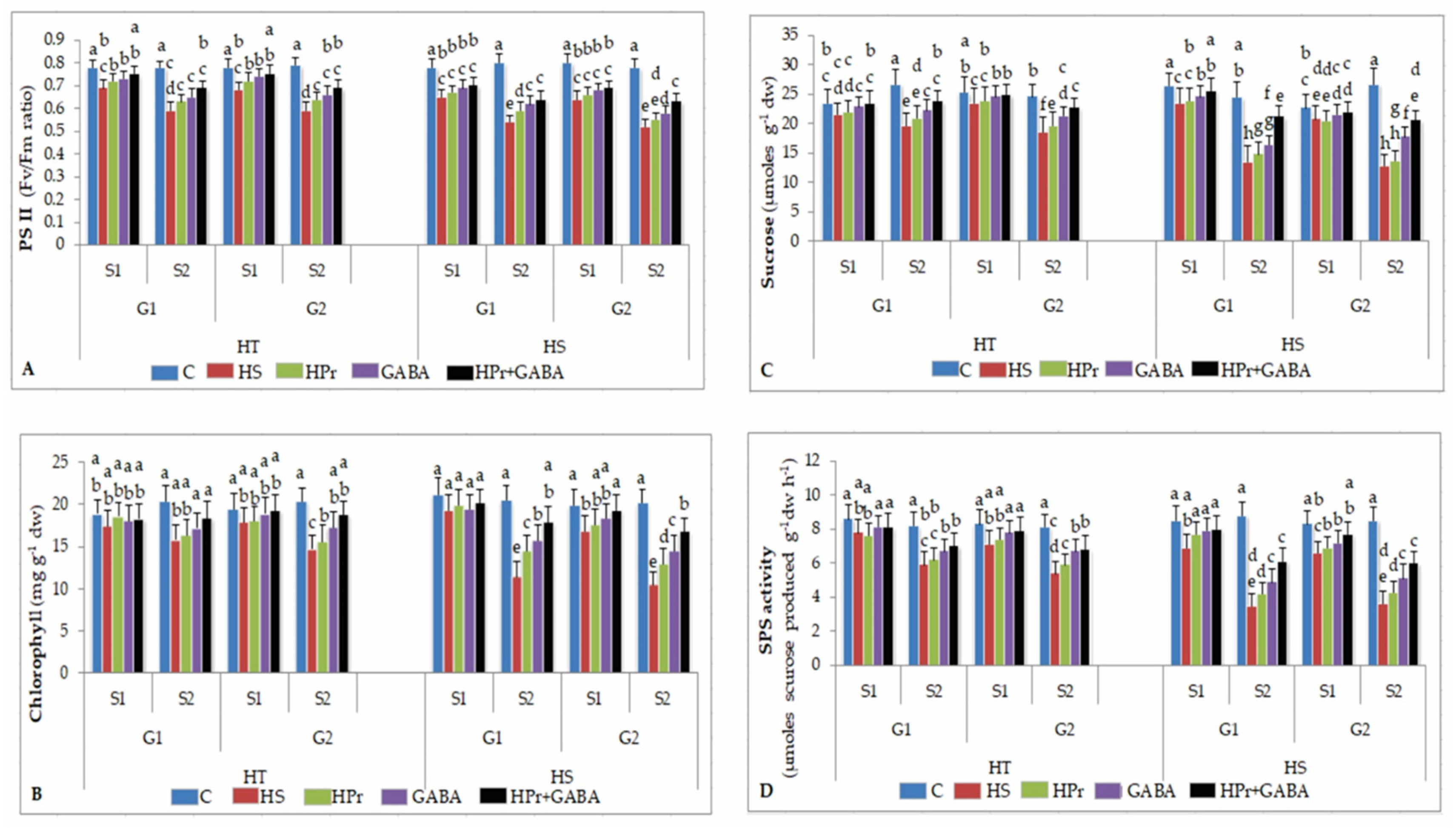
2.4. Photosynthetic Function
2.4.1. Photosynthetic Efficiency
2.4.2. Chlorophyll
2.4.3. Sucrose
2.4.4. Sucrose Phosphate Synthase
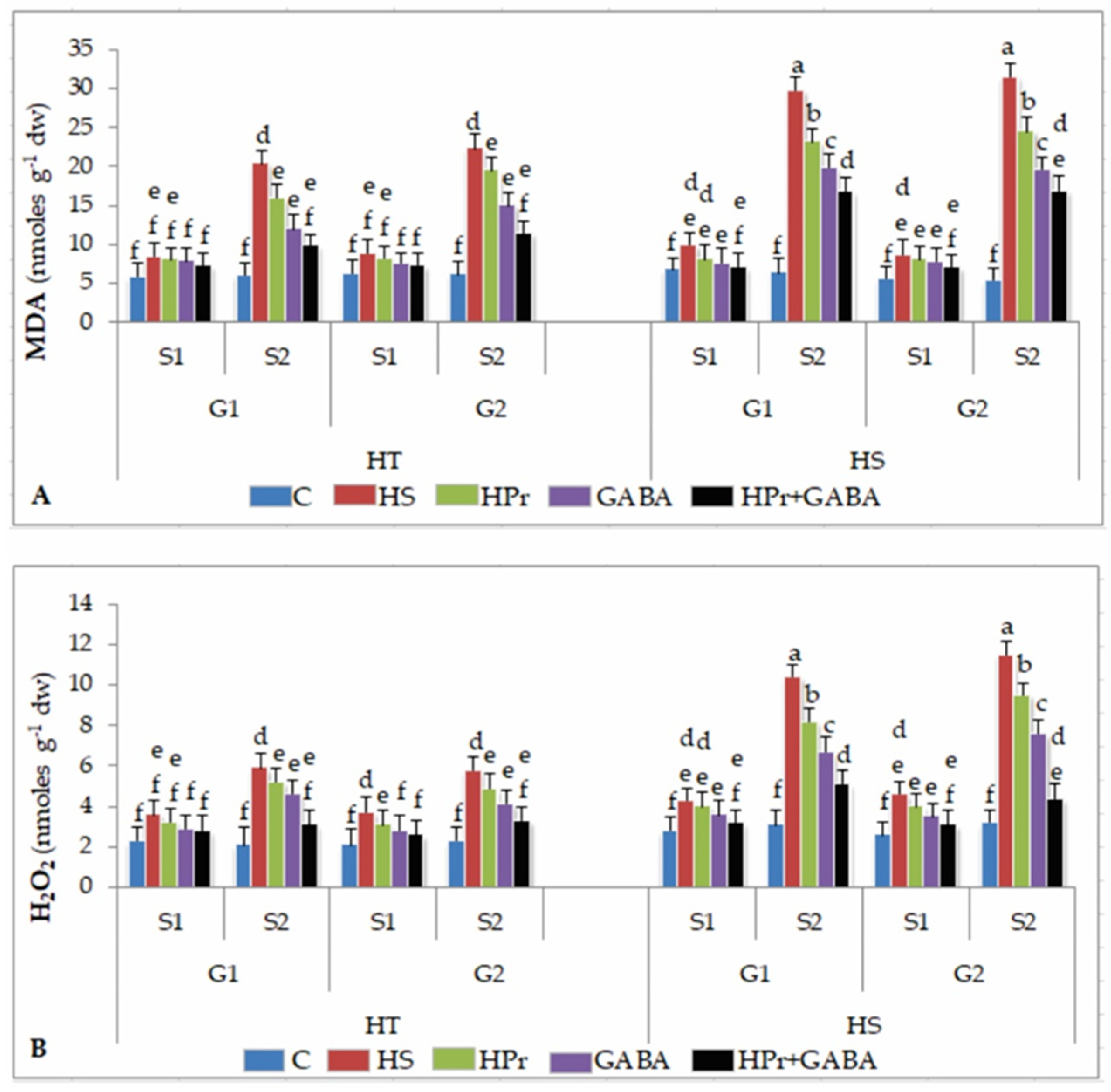
2.5. Oxidative Damage
2.5.1. Malondialdehyde
2.5.2. Hydrogen Peroxide
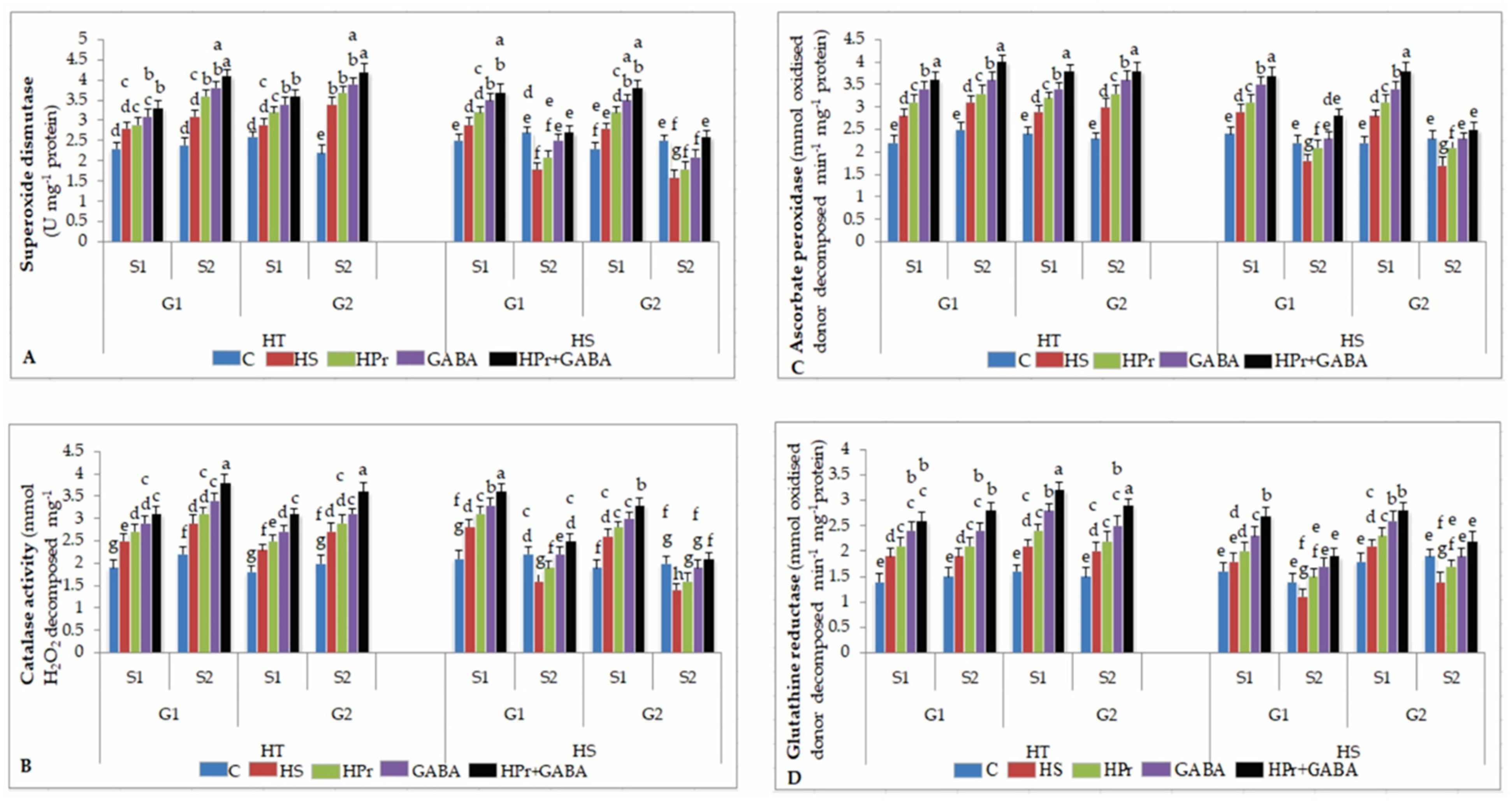
2.6. Antioxidants
2.6.1. Superoxide Dismutase (SOD)
2.6.2. Catalase (CAT)
2.6.3. Ascorbate Peroxidase (APX)
2.6.4. Glutathione Reductase (GR)
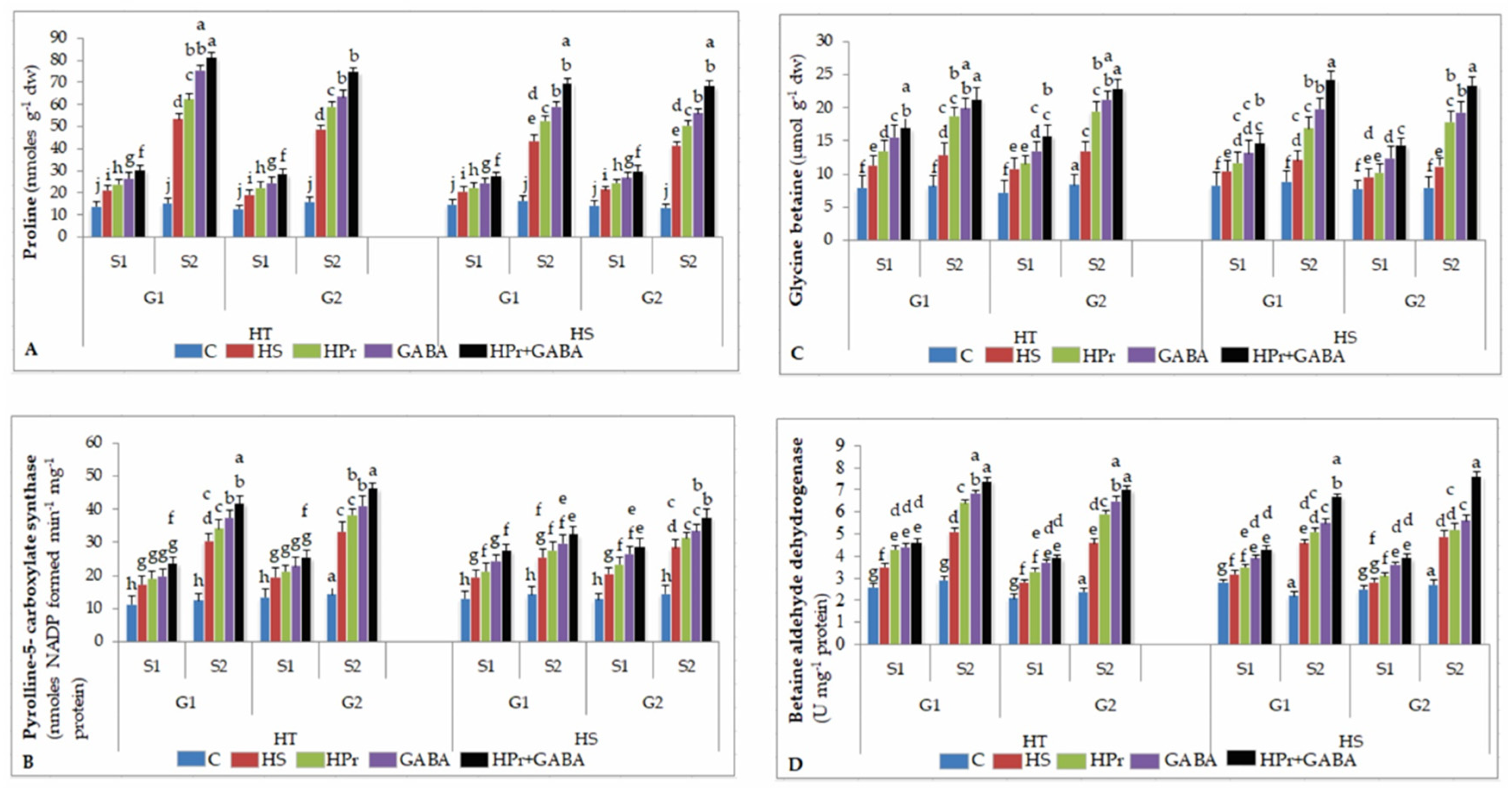
2.7. Osmolytes
2.7.1. Proline (Pro)
2.7.2. Pyrroline-5-Carboxylate Synthase (P5CS)
2.7.3. Glycine Betaine (GB)
2.7.4. Betaine Aldehyde Dehydrogenase (BADH)
2.7.5. Endogenous GABA
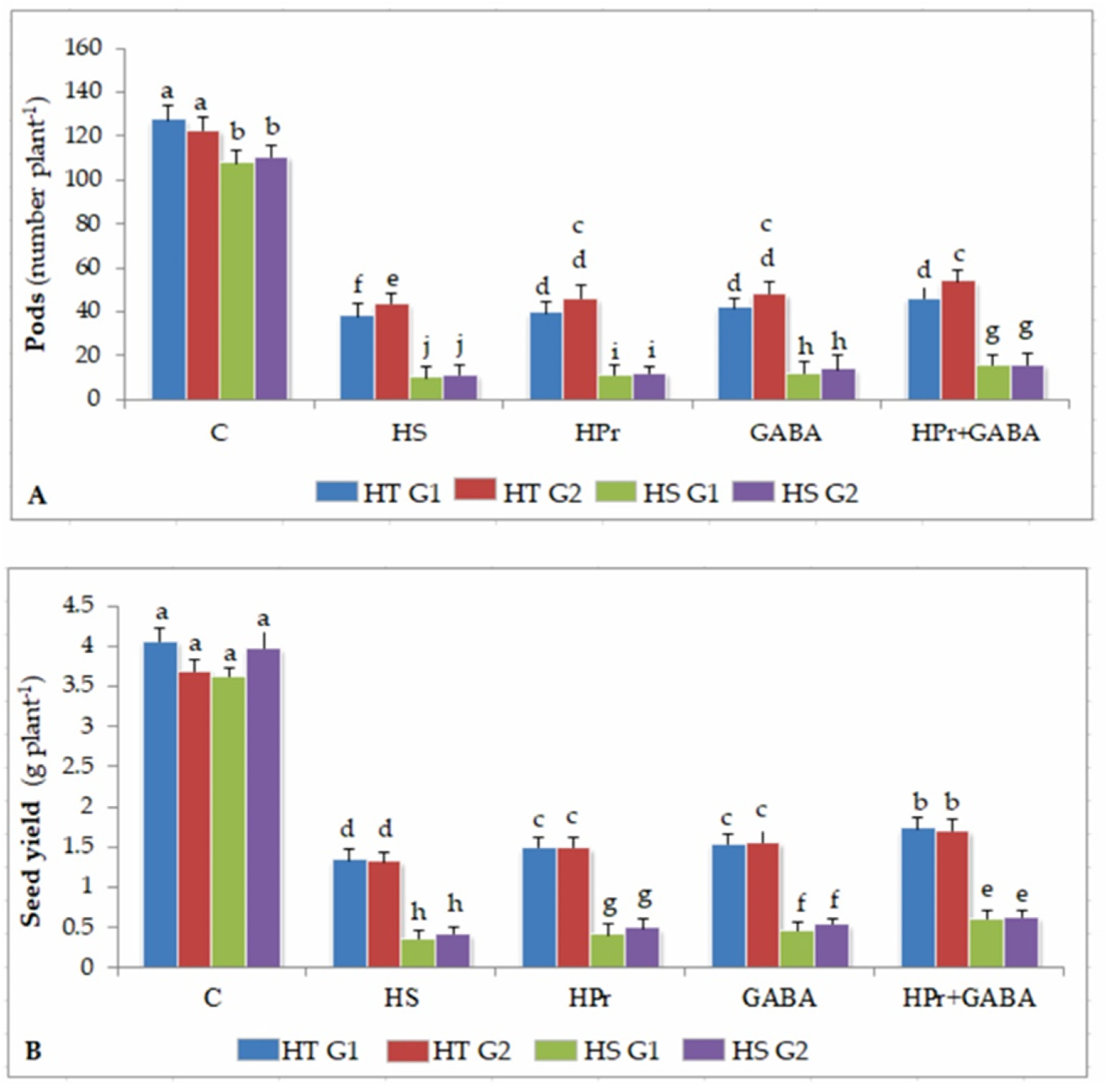
2.8. Yield Traits
2.8.1. Pod Number
2.8.2. Seed Yield
3. Discussion
3.1. Impacts of Heat Stress
3.2. Effect of Treatments
4. Materials and Methods
4.1. Plant Growth Conditions, Treatments, Phenology and Yield Traits
4.1.1. Plant Growth Conditions
4.1.2. Treatments
- Control;
- Heat-stress alone;
- Heat-primed seeds + Heat-stress;
- Heat-stress + GABA (1 mM) as a foliar treatment;
- Heat-primed seeds + Heat-stress + GABA (1 mM) as a foliar treatment.
4.1.3. Phenology and Yield Traits
4.2. Stress Injury
4.2.1. Membrane Damage (as Electrolyte Leakage)
4.2.2. Relative Leaf Water Content
4.2.3. Stomatal Conductance
4.2.4. Cellular Oxidizing Ability
4.3. Reproductive Function
4.3.1. Pollen Germination
4.3.2. Pollen Viability
4.3.3. Stigma Receptivity
4.3.4. Ovule Viability
4.4. Leaf Photosynthetic Function
4.4.1. Photochemical Efficiency
4.4.2. Chlorophyll
4.4.3. Sucrose
4.4.4. Sucrose Phosphate Synthase
4.5. Soluble Proteins
4.6. Osmolytes and Related Enzymes
4.6.1. Proline and Pyrroline-5-Carboxylate Synthase
4.6.2. Glycine Betaine and Betaine Aldehyde Dehydrogenase
4.6.3. Endogenous GABA
4.7. Oxidative Molecules and Antioxidants
4.7.1. Malondialdehyde
4.7.2. Hydrogen Peroxide
4.7.3. Superoxide Dismutase
4.7.4. Catalase
4.7.5. Ascorbate Peroxidase
4.7.6. Glutathione Reductase
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siebert, S.; Ewert, F. Future crop production threatened by extreme heat. Environ. Res. Lett. 2014, 9, 1–4. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.K. Field crops and the fear of heat stress-opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Kaushal, N.; Bhandari, K.; Siddique, K.H.; Nayyar, H. Food crops face rising temperatures: An overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food Agric. 2016, 2, 1–42. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Kaur, R.; Bains, T.S.; Bindumadhava, H.; Nayyar, H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015, 197, 527–541. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Kumar, S.; Nayyar, H. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017, 8, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Identification of high-temperature tolerant lentil (Lens culinaris Medik.) genotypes through leaf and pollen traits. Front. Plant Sci. 2017, 8, 744. [Google Scholar] [CrossRef]
- Roy, C.D.; Tarafdar, S.; Das, M.; Kundagrami, S. Screening lentil (Lens culinaris Medik.). Trends Biosci. 2012, 5, 143–146. [Google Scholar]
- Roy, S.; Islam, M.A.; Sarker, A.; Ismail, M.R.; Rafii, M.Y.; Mondal, M.M.A.; Malek, M.A. Morphological characterization of lentil accessions: Qualitative characters. Bangladesh J. Bot. 2012, 41, 187–190. [Google Scholar] [CrossRef]
- Delahunty, A.; Nuttall, J.; Nicolas, M.; Brand, J. Genotypic heat tolerance in lentil. In Proceedings of the 17th ASA Conference, Hobart, Australia, 20–24 September 2015. [Google Scholar]
- Mendanha, T.; Rosenqvist, E.; Hyldgaard, B.; Ottosen, C.O. Heat priming effects on anthesis heat stress in wheat cultivars (Triticum aestivum L.) with contrasting tolerance to heat stress. Plant Physiol. Biochem. 2018, 132, 213–221. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Han, X. Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.; Ling, Y.; Bahieldin, A.; Mahfouz, M.M. Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Nayyar, H. Plant growth-regulating molecules as thermoprotectants: Functional relevance and prospects for improving heat tolerance in food crops. J. Exp. Bot. 2020, 71, 569–594. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signaling in plants. Cell Mole. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Huang, B. Physiological effects of γ-aminobutyric acid application on improving heat and drought tolerance in creeping bentgrass. J. Am. Soc. Hortic. Sci. 2016, 141, 76–84. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Lin, F. Receptor modifiers indicate that 4-aminobutyric acid (GABA) is a potential modulator of ion transport in plants. Plant Growth Regul. 2000, 32, 65–76. [Google Scholar] [CrossRef]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Locy, R.D.; Wu, S.J.; Bisnette, J.; Barger, T.W.; McNabb, D.; Zik, M.; Cherry, J.H. The regulation of GABA accumulation by heat stress in Arabidopsis. In Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering; Springer: Dordrecht, The Netherlands, 2000; Volume 83, pp. 39–52. [Google Scholar]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.R. The plasma membrane as first responder to heat stress. Plant Cell 2009, 21, 2544. [Google Scholar] [CrossRef]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.; Siddique, K.H.; Nayyar, H. Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolas, E.; Torrecillas, A.; Sánchez-Blanco, M.J. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plant 2003, 47, 203. [Google Scholar] [CrossRef]
- Jaconis, S.Y.; Thompson, A.; Smith, S.L.; Trimarchi, C.; Cottee, N.S.; Bange, M.P.; Conaty, W.C. A standardised approach for determining heat tolerance in cotton using triphenyl tetrazolium chloride. Sci. Rep. 2021, 11, 5419. [Google Scholar] [CrossRef]
- Ibrahim, M.H.A.; Quick, J.S. Heritability of heat tolerance in winter and spring wheat. Crop. Sci. 2001, 41, 1401–1405. [Google Scholar] [CrossRef]
- Coria, N.A.; Sarquís, J.I.; Peñalosa, I.; Urzúa, M. Heat-induced damage in potato (Solanum tuberosum) tubers: Membrane stability, tissue viability, and accumulation of glycoalkaloids. J. Agric. Food Chem. 1998, 46, 4524–4528. [Google Scholar] [CrossRef]
- Tewari, A.K.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Vander Westhuizen, M.M.; Oosterhuis, D.M.; Berner, J.M.; Boogaers, N. Chlorophyll a fluorescence as an indicator of heat stress in cotton (Gossypium hirsutum L.). S. Afr. J. Plant Soil. 2020, 37, 116–119. [Google Scholar] [CrossRef]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Giri, J. Glycine betaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma functionality and fertility are reduced by heat and drought co-stress in wheat. Front. Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Gaur, P.; Turner, N.C.; Vadez, V.; Siddique, K.H.; Nayyar, H. Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop. Pasture Sci. 2017, 68, 823–841. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, D. High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. J. Plant Interact. 2011, 6, 43–52. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Mao, L.; Ying, T. Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem. 2016, 197, 333–339. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Tartari, A.; Cattivelli, L.; Forlani, G. Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 2006, 57, 3755–3766. [Google Scholar] [CrossRef]
- Hilker, M.; Schmulling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 743–761. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Strasser, R.J. Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): A chlorophyll a fluorescence study. Plant Biosyst. 2012, 146, 1037–1043. [Google Scholar] [CrossRef]
- Yang, A.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011, 129, 1619–1622. [Google Scholar] [CrossRef]
- Végh, B.; Marček, T.; Karsai, I.; Janda, T.; Darkó, É. Heat acclimation of photosynthesis in wheat genotypes of different origin. S. Afr. J. Plant Soil. 2018, 117, 184–192. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and non-aborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mattison, O.; Knox, R.B.; Heslop-Harrison, J.; Heslop-Harrison, Y. Protein pellicle of stigmatic papillae as a probable recognition site in incompatible reactions. Nature 1974, 247, 298–300. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplast. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Jones, M.G.; Outlaw, W.H.; Lowry, O.H. Enzymic assay of 10-7 to 10-14 moles of sucrose in plant tissues. Plant Physiol. 1997, 60, 379–383. [Google Scholar] [CrossRef]
- Déjardin, A.; Rochat, C.; Maugenest, S.; Boutin, J.P. Purification, characterization and physiological role of sucrose synthase in the pea seed coat (Pisum sativum L.). Planta 1997, 201, 128–137. [Google Scholar] [CrossRef]
- Huber, J.L.; Huber, S.C.; Nielsen, T.H. Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch. Biochem. Biophys. 1989, 270, 681–690. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Measurement of protein with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Filippou, P.; Bouchagier, P.; Skotti, E.; Fotopoulos, V. Proline and reactive oxygen/nitrogen species metabolism is involved in the tolerant response of the invasive plant species Ailanthus altissima to drought and salinity. Environ. Exp. Bot. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Daniell, H.; Muthukumar, B.; Lee, S.B. Marker free transgenic plants: Engineering the chloroplast genome without the use of antibiotic selection. Curr. Genet. 2001, 39, 109–116. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Sugiyama, M.; Watahiki, A.; Ohshima, I.; Iijima, Y.; Nishimura, S. Screening for γ-aminobutyric acid (GABA)-rich tomato varieties. J. Jpn. Soc. Hortic. Sci. 2008, 77, 242–250. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase activities of hydrocarbon-utilizing Candida yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Mavis, R.D.; Stellwagen, E. Purification and subunit structure of glutathione reductase from bakers’ yeast. Int. J. Biol. Chem. 1968, 243, 809–814. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, A.; Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, S.; Prasad, P.V.V.; Jha, U.; Kumar, J.; Siddique, K.H.M.; Nayyar, H. Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments. Int. J. Mol. Sci. 2021, 22, 5825. https://doi.org/10.3390/ijms22115825
Bhardwaj A, Sita K, Sehgal A, Bhandari K, Kumar S, Prasad PVV, Jha U, Kumar J, Siddique KHM, Nayyar H. Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments. International Journal of Molecular Sciences. 2021; 22(11):5825. https://doi.org/10.3390/ijms22115825
Chicago/Turabian StyleBhardwaj, Anjali, Kumari Sita, Akanksha Sehgal, Kalpna Bhandari, Shiv Kumar, P. V. Vara Prasad, Uday Jha, Jitendra Kumar, Kadambot H. M. Siddique, and Harsh Nayyar. 2021. "Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments" International Journal of Molecular Sciences 22, no. 11: 5825. https://doi.org/10.3390/ijms22115825
APA StyleBhardwaj, A., Sita, K., Sehgal, A., Bhandari, K., Kumar, S., Prasad, P. V. V., Jha, U., Kumar, J., Siddique, K. H. M., & Nayyar, H. (2021). Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments. International Journal of Molecular Sciences, 22(11), 5825. https://doi.org/10.3390/ijms22115825







