Human Polymerase δ-Interacting Protein 2 (PolDIP2) Inhibits the Formation of Human Tau Oligomers and Fibrils
Abstract
1. Introduction
2. Materials and Methods
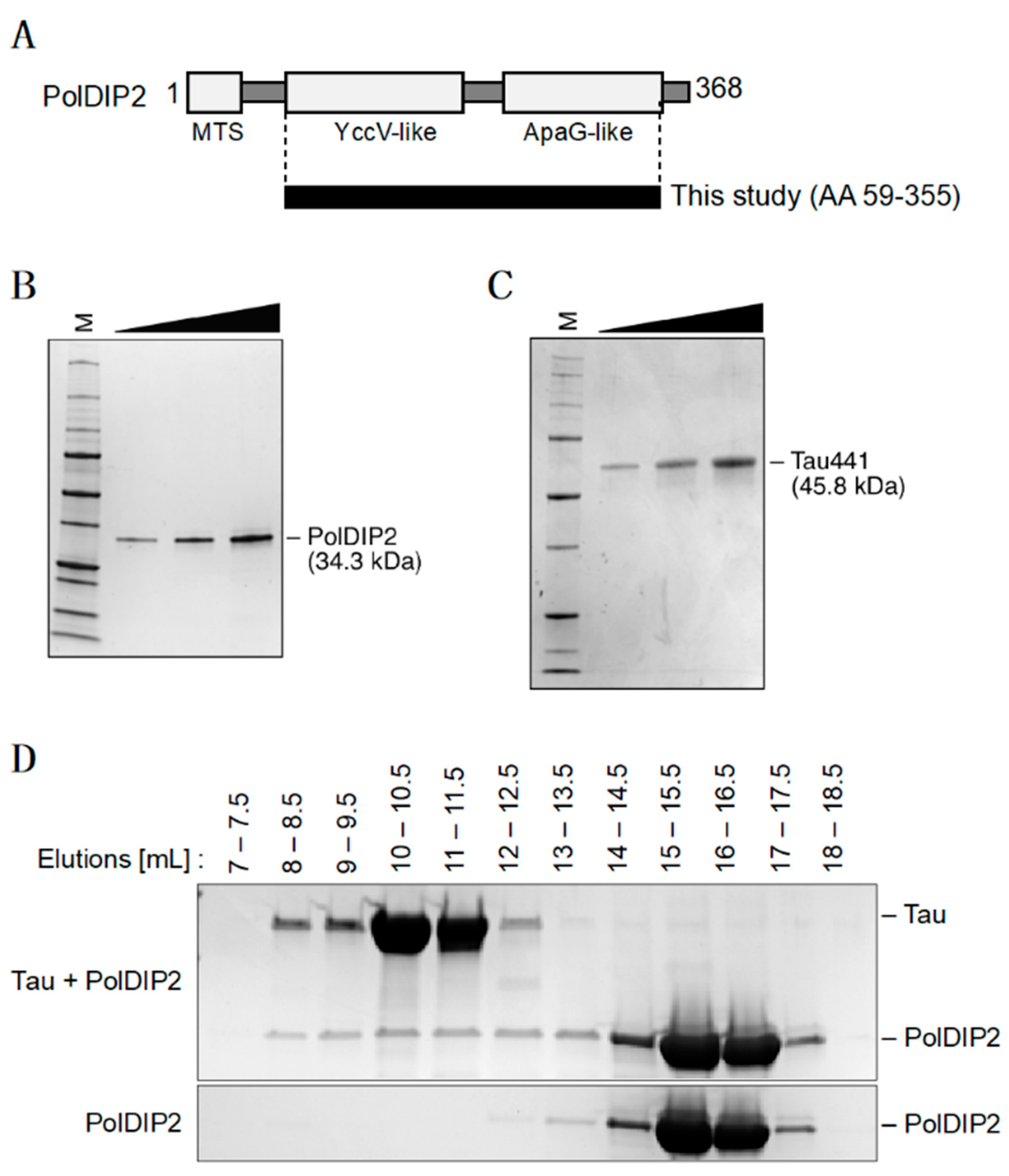
2.1. Preparation of Recombinant PolDIP2 and Tau
2.2. Reaction Buffers
2.3. Size Exclusion Chromatography
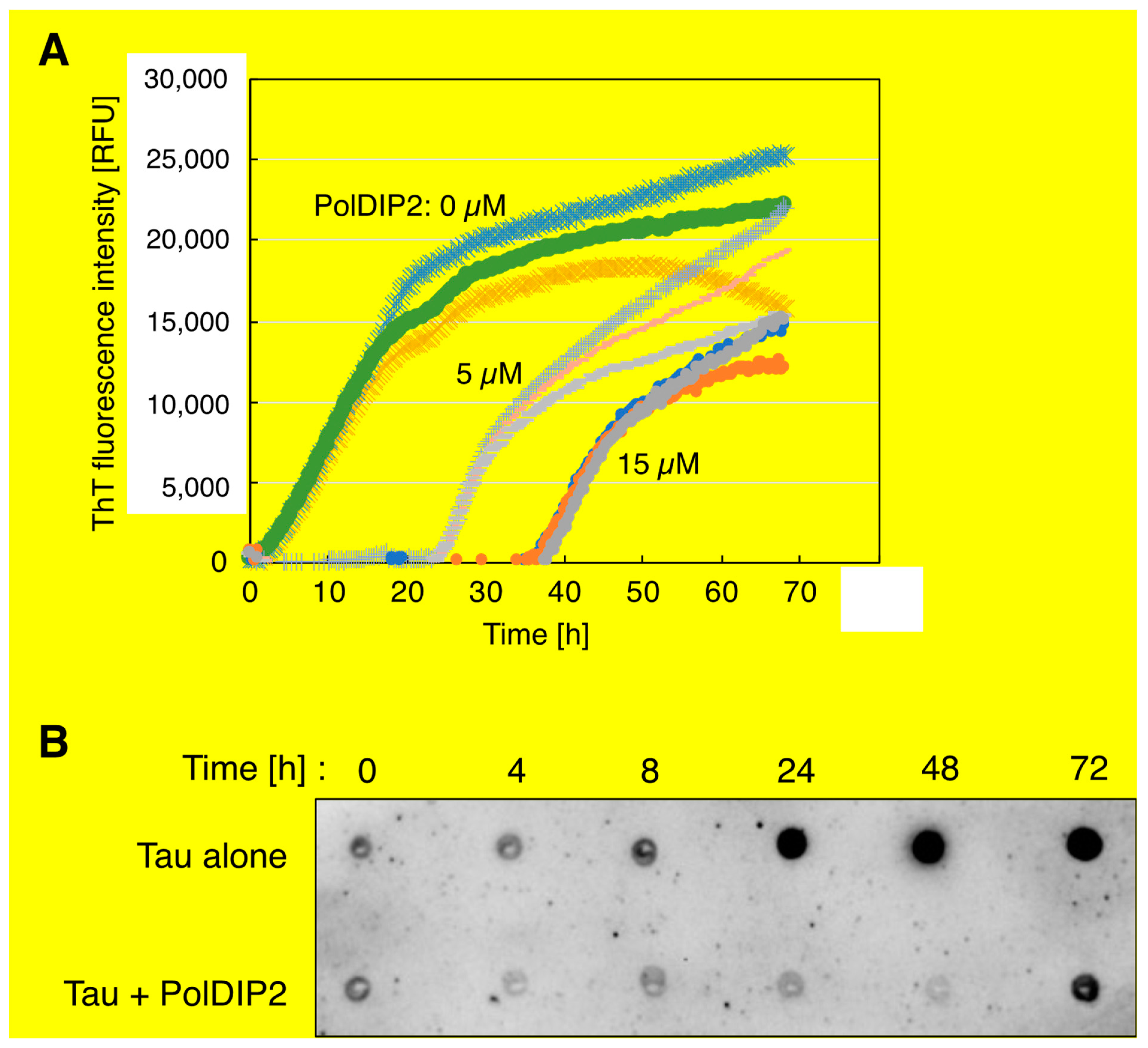
2.4. ThT-Based Tau Aggregation Assay
2.5. Filter Trap Assay
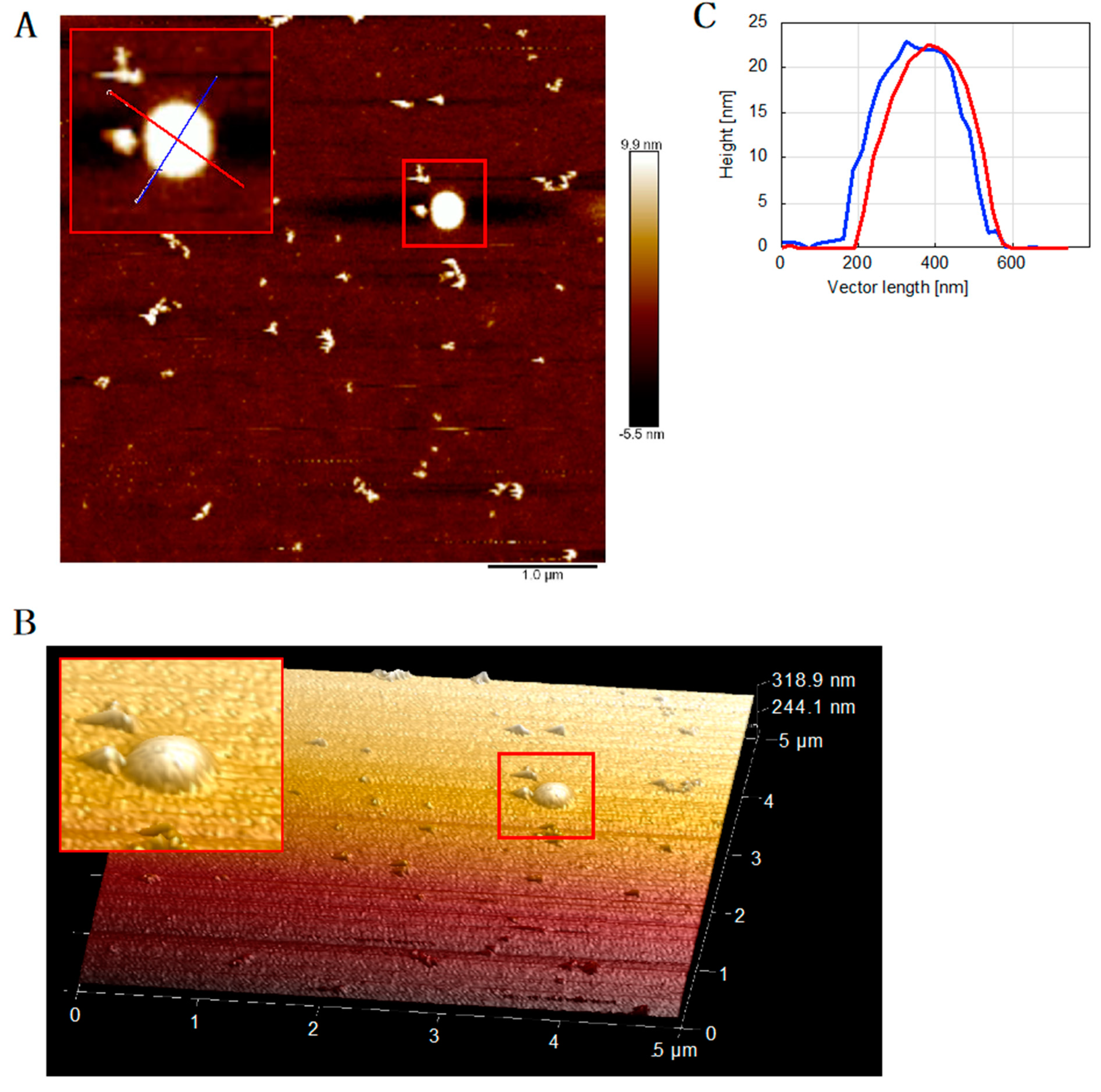
2.6. Atomic Force Microscopy (AFM)
2.7. Pull-Down Assay
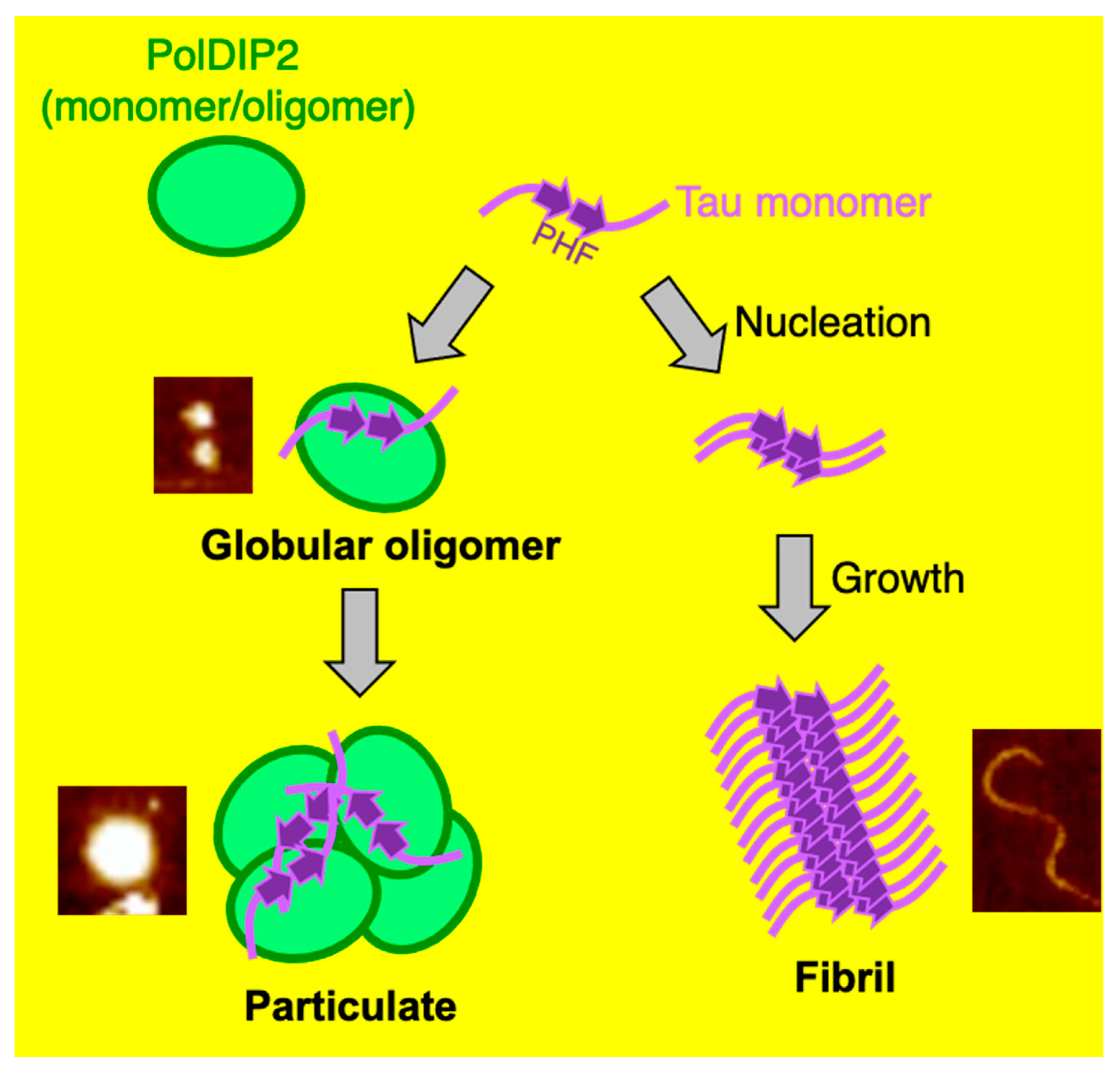
3. Results
3.1. PolDIP2 Interacts with Monomeric Tau
3.2. PolDIP2 Inhibits Tau Amyloid Formation
3.3. PolDIP2 Inhibits Tau Fibrillar Growth
4. Discussion
Limitations of This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irwin, D.J.; Lee, V.M.Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Bittar, A.; Bhatt, N.; Kayed, R. Advances and considerations in AD tau-targeted immunotherapy. Neurobiol. Dis. 2020, 134, 104707. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Uddin, M.S.; Mathew, B.; Ashraf, G.M. Toxic tau: Structural origins of tau aggregation in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 1417–1420. [Google Scholar] [CrossRef]
- Nizynski, B.; Dzwolak, W.; Nieznanski, K. Amyloidogenesis of Tau protein. Protein Sci. 2017, 26, 2126–2150. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Lee, V.M.Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Ferrari, L.; Stucchi, R.; Konstantoulea, K.; van de Kamp, G.; Kos, R.; Geerts, W.J.C.; van Bezouwen, L.S.; Förster, F.G.; Altelaar, M.; Hoogenraad, C.C.; et al. Arginine π-stacking drives binding to fibrils of the Alzheimer protein Tau. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rodriguez-Belmonte, E.M.; Mazloum, N.; Xie, B.; Lee, M.Y.W.T. Identification of a novel protein, PDIP38, that interacts with the p50 subunit of DNA polymerase δ and proliferating cell nuclear antigen. J. Biol. Chem. 2003, 278, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Kasho, K.; Stojkovič, G.; Velázquez-Ruiz, C.; Martínez-Jiménez, M.I.; Doimo, M.; Laurent, T.; Berner, A.; Pérez-Rivera, A.E.; Jenninger, L.; Blanco, L.; et al. A unique arginine cluster in PolDIP2 enhances nucleotide binding and DNA synthesis by PrimPol. Nucleic Acids Res. 2021, 49, 2179–2191. [Google Scholar] [CrossRef]
- Kim, Y.D.; Park, H.; Nah, J.; Moon, S.; Lee, W.J.; Hong, S.H.; Kam, T.I.; Jung, Y.K. Essential role of POLDIP2 in Tau aggregation and neurotoxicity via autophagy/proteasome inhibition. Biochem. Biophys. Res. Commun. 2015, 462, 112–118. [Google Scholar] [CrossRef]
- Pansieri, J.; Ostojić, L.; Iashchishyn, I.A.; Magzoub, M.; Wallin, C.; Wärmländer, S.K.T.S.; Gräslund, A.; Nguyen Ngoc, M.; Smirnovas, V.; Svedružić, Ž.; et al. Pro-Inflammatory S100A9 Protein Aggregation Promoted by NCAM1 Peptide Constructs. ACS Chem. Biol. 2019, 14, 1410–1417. [Google Scholar] [CrossRef]
- Horvath, I.; Iashchishyn, I.A.; Moskalenko, R.A.; Wang, C.; Wärmländer, S.K.T.S.; Wallin, C.; Gräslund, A.; Kovacs, G.G.; Morozova-Roche, L.A. Co-aggregation of pro-inflammatory S100A9 with α-synuclein in Parkinson’s disease: Ex vivo and in vitro studies. J. Neuroinflamm. 2018, 15, 1–16. [Google Scholar] [CrossRef]
- Crespo, R.; Koudstaal, W.; Apetri, A. In vitro assay for studying the aggregation of tau protein and drug screening. J. Vis. Exp. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Bittar, A.; Bhatt, N.; Hasan, T.F.; Montalbano, M.; Puangmalai, N.; McAllen, S.; Ellsworth, A.; Carretero Murillo, M.; Taglialatela, G.; Lucke-Wold, B.; et al. Neurotoxic tau oligomers after single versus repetitive mild traumatic brain injury. Brain Commun. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Lo Cascio, F.; Puangmalai, N.; Ellsworth, A.; Bucchieri, F.; Pace, A.; Palumbo Piccionello, A.; Kayed, R. Toxic Tau Oligomers Modulated by Novel Curcumin Derivatives. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Iashchishyn, I.A.; Pansieri, J.; Nyström, S.; Klementieva, O.; Kara, J.; Horvath, I.; Moskalenko, R.; Rofougaran, R.; Gouras, G.; et al. S100A9-Driven Amyloid-Neuroinflammatory Cascade in Traumatic Brain Injury as a Precursor State for Alzheimer’s Disease. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Apetri, A.; Crespo, R.; Juraszek, J.; Pascual, G.; Janson, R.; Zhu, X.; Zhang, H.; Keogh, E.; Holland, T.; Wadia, J.; et al. A common antigenic motif recognized by naturally occurring human V H 5-51/V L 4-1 anti-tau antibodies with distinct functionalities. Acta Neuropathol. Commun. 2018, 6. [Google Scholar] [CrossRef]
- Pir, G.J.; Choudhary, B.; Kaniyappan, S.; Chandupatla, R.R.; Mandelkow, E.; Mandelkow, E.M.; Wang, Y. Suppressing Tau Aggregation and Toxicity by an Anti-Aggregant Tau Fragment. Mol. Neurobiol. 2019, 56, 3751–3767. [Google Scholar] [CrossRef]
- Seidler, P.M.; Boyer, D.R.; Rodriguez, J.A.; Sawaya, M.R.; Cascio, D.; Murray, K.; Gonen, T.; Eisenberg, D.S. Structure-based inhibitors of tau aggregation. Nat. Chem. 2018, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Liu, M.; Kuo, M.H. In vitro aggregation assays using hyperphosphorylated tau protein. J. Vis. Exp. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- LeVine, H. Thioflavine T interaction with amyloid β-sheet structures. Amyloid 1995, 2, 1–6. [Google Scholar] [CrossRef]
- Ramachandran, G.; Udgaonkar, J.B. Mechanistic studies unravel the complexity inherent in tau aggregation leading to Alzheimer’s disease and the tauopathies. Biochemistry 2013, 52, 4107–4126. [Google Scholar] [CrossRef] [PubMed]
- Linse, S. Mechanism of amyloid protein aggregation and the role of inhibitors. Pure Appl. Chem. 2019, 91, 211–229. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Sarmiento, J.; Troncoso, J.; Jackson, G.R.; Kayed, R. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J. 2012, 26, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Foderà, V.; Vetri, V.; Wind, T.S.; Noppe, W.; Cornett, C.; Donald, A.M.; Morozova-Roche, L.A.; Vestergaard, B. Observation of the early structural changes leading to the formation of protein superstructures. J. Phys. Chem. Lett. 2014, 5, 3254–3258. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, S.; Eftekharzadeh, B.; Tepper, K.; Zoltowska, K.M.; Bennett, R.E.; Dujardin, S.; Laskowski, P.R.; MacKenzie, D.; Kamath, T.; Commins, C.; et al. Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J. 2018, 37, 1–21. [Google Scholar] [CrossRef]
- Boyko, S.; Surewicz, K.; Surewicz, W.K. Regulatory mechanisms of tau protein fibrillation under the conditions of liquid-liquid phase separation. Proc. Natl. Acad. Sci. USA 2020, 117, 31882–31890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Falcon, B.; Murzin, A.G.; Fan, J.; Crowther, R.A.; Goedert, M.; Scheres, S.H.W. Heparin-induced tau filaments are polymorphic and differ from those in alzheimer’s and pick’s diseases. eLife 2019, 8, e43584. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wells, E.A.; Robinson, A.S. Critical Molecular and Cellular Contributors to Tau Pathology. Biomedicines 2021, 9, 190. [Google Scholar] [CrossRef] [PubMed]

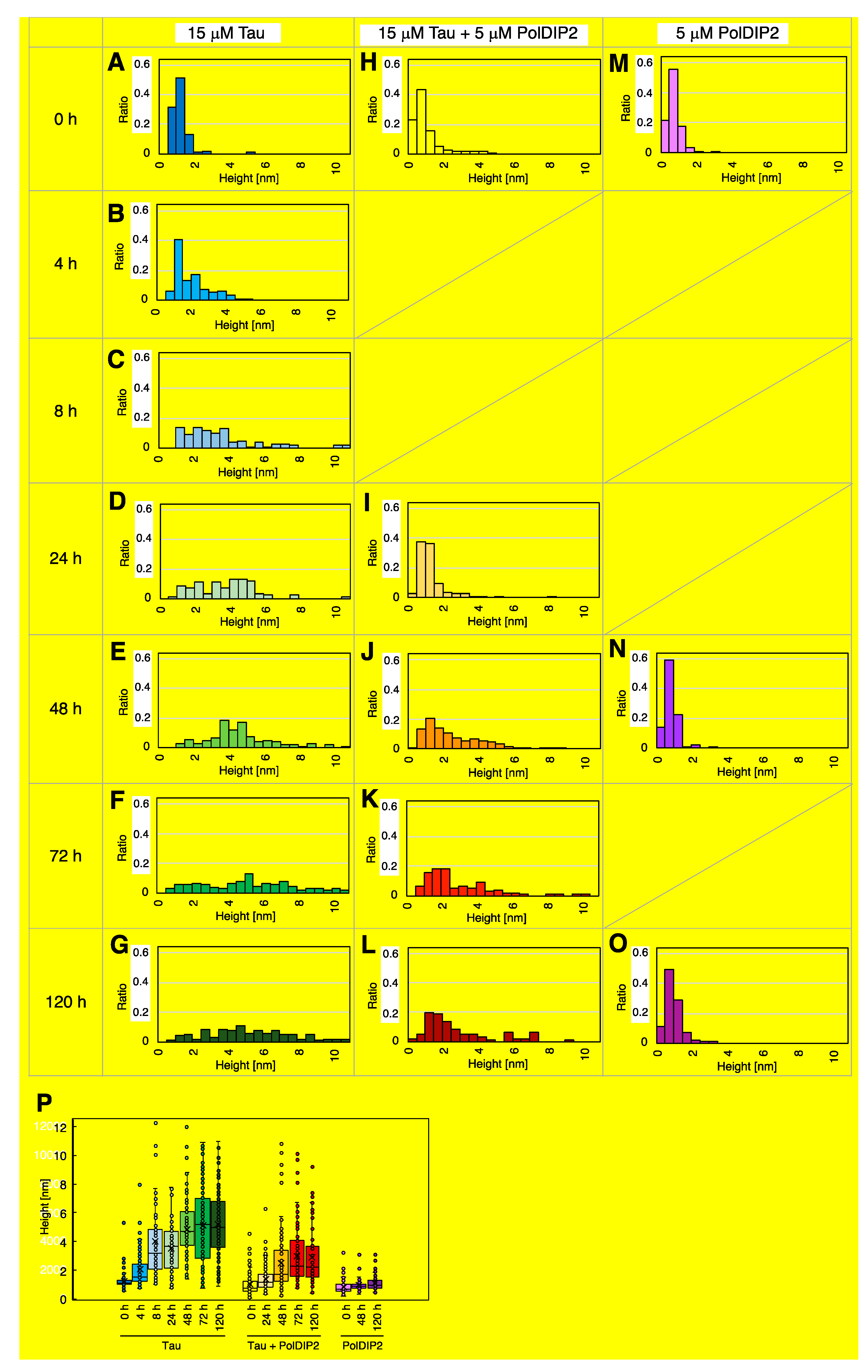
| Samples | Average Height (nm) | Median Height (nm) | Number |
|---|---|---|---|
| Tau, 0 h | 1.21 | 1.12 | 101 |
| Tau, 4 h | 2.03 | 1.53 | 100 |
| Tau, 8 h | 4.04 | 3.15 | 100 |
| Tau, 24 h | 3.51 | 3.67 | 82 |
| Tau, 48 h | 4.82 | 4.71 | 109 |
| Tau, 72 h | 5.11 | 5.20 | 110 |
| Tau, 120 h | 5.20 | 4.97 | 119 |
| Tau + PolDIP2, 0 h | 1.03 | 0.71 | 99 |
| Tau + PolDIP2, 24 h | 1.36 | 1.17 | 104 |
| Tau + PolDIP2, 48 h | 2.45 | 1.70 | 141 |
| Tau + PolDIP2, 72 h | 2.96 | 2.27 | 98 |
| Tau + PolDIP2, 120 h | 2.88 | 2.17 | 96 |
| PolDIP2, 0 h | 0.79 | 0.65 | 89 |
| PolDIP2, 48 h | 0.91 | 0.89 | 94 |
| PolDIP2, 120 h | 1.06 | 0.96 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasho, K.; Krasauskas, L.; Smirnovas, V.; Stojkovič, G.; Morozova-Roche, L.A.; Wanrooij, S. Human Polymerase δ-Interacting Protein 2 (PolDIP2) Inhibits the Formation of Human Tau Oligomers and Fibrils. Int. J. Mol. Sci. 2021, 22, 5768. https://doi.org/10.3390/ijms22115768
Kasho K, Krasauskas L, Smirnovas V, Stojkovič G, Morozova-Roche LA, Wanrooij S. Human Polymerase δ-Interacting Protein 2 (PolDIP2) Inhibits the Formation of Human Tau Oligomers and Fibrils. International Journal of Molecular Sciences. 2021; 22(11):5768. https://doi.org/10.3390/ijms22115768
Chicago/Turabian StyleKasho, Kazutoshi, Lukas Krasauskas, Vytautas Smirnovas, Gorazd Stojkovič, Ludmilla A. Morozova-Roche, and Sjoerd Wanrooij. 2021. "Human Polymerase δ-Interacting Protein 2 (PolDIP2) Inhibits the Formation of Human Tau Oligomers and Fibrils" International Journal of Molecular Sciences 22, no. 11: 5768. https://doi.org/10.3390/ijms22115768
APA StyleKasho, K., Krasauskas, L., Smirnovas, V., Stojkovič, G., Morozova-Roche, L. A., & Wanrooij, S. (2021). Human Polymerase δ-Interacting Protein 2 (PolDIP2) Inhibits the Formation of Human Tau Oligomers and Fibrils. International Journal of Molecular Sciences, 22(11), 5768. https://doi.org/10.3390/ijms22115768








