Interaction between Metformin, Folate and Vitamin B12 and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies
Abstract
1. Introduction
2. Metformin in Pregnancy
2.1. Transplacental Transport of Metformin
2.2. Impact of Metformin on Placental Nutrient Transport and Nutrient Bioavailabilty
3. One Carbon Metabolism
4. One Carbon Metabolism in Pregnancy
4.1. Vitamin B12
4.2. Folate
5. Is Metformin Impacting on Fetal and Placental Development by Perturbing the One Carbon Metabolism Cycle?
5.1. Vitamin B12
5.2. Folate
6. Is Metformin Influencing Fetal Programming by Disturbing One Carbon Metabolism?
7. Vitamin Supplementation
8. Future Considerations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation (IDF). World Diabetes Day 2017 to Focus on Women and Diabetes, Belgium. 2017. Available online: https://www.idf.org/news/2:world-dia (accessed on 10 December 2020).
- Melchior, H.; Kurch-Bek, D.; Mund, M. The prevalence of gestational diabetes. Dtsch. Arztebl. Int. 2017, 114, 412–418. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Zhu, Y.; Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diab. Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Aiken, C.E.; Ozanne, S.E. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLOS Med. 2019, 16, e1002848. [Google Scholar] [CrossRef] [PubMed]
- Stacey, T.; Tennant, P.; McCowan, L.; Mitchell, E.; Budd, J.; Li, M.; Thompson, J.; Martin, B.; Roberts, D.; Heazell, A. Gestational diabetes and the risk of late stillbirth: A case–control study from England, UK. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 973–982. [Google Scholar] [CrossRef]
- Feig, D.S.; Donovan, L.E.; Zinman, B.; Sanchez, J.J.; Asztalos, E.; Ryan, E.A.; Fantus, I.G.; Hutton, E.; Armson, A.B.; Lipscombe, L.L.; et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): A multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 834–844. [Google Scholar] [CrossRef]
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes. A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883. [Google Scholar] [CrossRef]
- Engeland, A.; Bjørge, T.; Daltveit, A.K.; Skurtveit, S.; Vangen, S.; Vollset, S.E.; Furu, K. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur. J. Epidemiol. 2011, 26, 157–163. [Google Scholar] [CrossRef]
- Saravanan, P. Gestational diabetes: Opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020, 8, 793–800. [Google Scholar] [CrossRef]
- Whicher, C.A.; O’Neill, S.; Holt, R.I.G. Diabetes in the UK: 2019. Diabet. Med. 2020, 37, 242–247. [Google Scholar] [CrossRef]
- Murphy, H.R.; Howgate, C.; O’Keefe, J.; Myers, J.; Morgan, M.; Coleman, M.A.; Jolly, M.; Valabhji, J.; Scott, E.M.; Knighton, P.; et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: A 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 153–164. [Google Scholar] [CrossRef]
- Kelley, K.W.; Carroll, D.G.; Meyer, A. A review of current treatment strategies for gestational diabetes mellitus. Drugs Context 2015, 4. [Google Scholar] [CrossRef]
- Bahendeka, S.; Kaushik, R.; Swai, A.B.; Otieno, F.; Bajaj, S.; Kalra, S.; Bavuma, C.M.; Karigire, C. EADSG guidelines: Insulin storage and optimisation of injection technique in diabetes management. Diabetes Therapy. 2019, 10, 341–366. [Google Scholar] [CrossRef]
- Wakeman, M.; Archer, D.T. Metformin and micronutrient status in type 2 diabetes: Does polypharmacy involving acid-suppressing medications affect vitamin B12 levels? Diabetes Metab. Syndr. Obes. 2020, 13, 2093–2108. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Loeken, M.R. Metformin use in pregnancy: Promises and uncertainties. Diabetologia 2017, 60, 1612–1619. [Google Scholar] [CrossRef]
- Bridges, H.R.; Jones, A.J.Y.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Garcia Canaveras, J.C.; Chen, Z.; Wang, L.; Liang, L.; Jang, C.; Mayr, J.A.; Zhang, Z.; Ghergurovich, J.M.; Zhan, L.; et al. Serine catabolism feeds NADH when respiration is impaired. Cell Metab. 2020, 31, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hebert, M.F.; Wagner, D.J.; Easterling, T.R.; Liang, C.J.; Rice, K.; Wang, J. Organic cation transporter 3 facilitates fetal Exposure to metformin during pregnancy. Mol. Pharmacol. 2018, 94, 1125–1131. [Google Scholar] [CrossRef]
- Cuyàs, E.; Fernández-Arroyo, S.; Buxó, M.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; et al. Metformin induces a fasting- and antifolate-mimicking modification of systemic host metabolism in breast cancer patients. Aging 2019, 11, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; Scifres, C.; Valent, A.M.; Friedman, J.E.; Buchanan, T.A.; Coustan, D.; Aagaard, K.; Thornburg, K.L.; Catalano, P.M.; Galan, H.L.; et al. A cautionary response to SMFM statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 219. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- An, H.; He, L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J. Endocrinol. 2016, 228. [Google Scholar] [CrossRef]
- Hyer, S.; Balani, J.; Shehata, H. Metformin in pregnancy: Mechanisms and clinical applications. Int. J. Mol. Sci. 2018, 19, 1954. [Google Scholar] [CrossRef] [PubMed]
- Balani, J.; Hyer, S.L.; Rodin, D.A.; Shehata, H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: A case-control study. Diabet. Med. 2009, 26, 798–802. [Google Scholar] [CrossRef]
- Lee, P.A.; Chernausek, S.D.; Hokken-Koelega, A.C.S.; Czernichow, P. International small for gestational age advisory board consensus development conference statement: Management of short children born small for gestational age, april 24–october 1, 2001. Pediatrics 2003, 111, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Obolonkin, V.; Battin, M.; Wouldes, T.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 2011, 34, 2279–2284. [Google Scholar] [CrossRef]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res. Care 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Hanem, L.G.E.; Salvesen, Ø.; Juliusson, P.B.; Carlsen, S.M.; Nossum, M.C.F.; Vaage, M.; Ødegård, R.; Vanky, E. Intrauterine metformin exposure and offspring cardiometabolic risk factors (PedMet study): A 5–10 year follow-up of the PregMet randomised controlled trial. Lancet Child. Adolesc. Health 2019, 3, 166–174. [Google Scholar] [CrossRef]
- Ijäs, H.; Vääräsmäki, M.; Saarela, T.; Keravuo, R.; Raudaskoski, T. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: Growth and development of the children at the age of 18 months. BJOG 2015, 122, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Salomäki, H.; Vähätalo, L.H.; Laurila, K.; Jäppinen, N.T.; Penttinen, A.-M.; Ailanen, L.; Ilyasizadeh, J.; Pesonen, U.; Koulu, M. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS ONE 2013, 8, e56594. [Google Scholar] [CrossRef]
- Jamal, A.; Milani, F.; Al-Yasin, A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012, 10, 265–270. [Google Scholar]
- Jiang, S.; Teague, A.M.; Tryggestad, J.B.; Jensen, M.E.; Chernausek, S.D. Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Nguyen, T.V.; Tuohey, L.; Cluver, C.; Tong, S.; Kaitu’u-Lino, T.J. Combining metformin and sulfasalazine additively reduces the secretion of antiangiogenic factors from the placenta: Implications for the treatment of preeclampsia. Placenta 2020, 95, 78–83. [Google Scholar] [CrossRef]
- Cluver, C.; Walker, S.P.; Mol, B.W.; Hall, D.; Hiscock, R.; Brownfoot, F.C.; Kaitu’u-Lino, T.J.; Tong, S. A double blind, randomised, placebo-controlled trial to evaluate the efficacy of metformin to treat preterm pre-eclampsia (PI2 Trial): Study protocol. BMJ Open 2019, 9, e025809. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Beard, S.; Cannon, P.; Hastie, R.; Nguyen, T.V.; Binder, N.K.; Tong, S.; Hannan, N.J. Combining metformin and esomeprazole is additive in reducing sFlt-1 secretion and decreasing endothelial dysfunction—Implications for treating preeclampsia. PLoS ONE 2018, 13, e0188845. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.J.; Senadheera, S.; Illanes, S.E.; Kaitu’u-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Szewczyk, G.; Pyzlak, M.; Stangret, A.; Bachanek, M.; Trojanowski, S.; Alkhalayla, H.; Wejman, J. Anti-inflammatory action of metformin with respect to CX3CL1/CX3CR1 signaling in human placental circulation in normal-glucose versus high-glucose environments. Inflammation 2018, 41, 2246–2264. [Google Scholar] [CrossRef] [PubMed]
- Correia-Branco, A.; Keating, E.; Martel, F. Involvement of mTOR, JNK and PI3K in the negative effect of ethanol and metformin on the human first-trimester extravillous trophoblast HTR-8/SVneo cell line. Eur. J. Pharmacol. 2018, 833, 16–24. [Google Scholar] [CrossRef]
- Arshad, R.; Kanpurwala, M.A.; Karim, N.; Hassan, J.A. Effects of diet and metformin on placental morphology in gestational diabetes mellitus. Pak. J. Med. Sci. 2016, 32, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Herrin, M.A.; Pitruzzello, M.C.; Mulla, M.J.; Werner, E.F.; Pettker, C.M.; Flannery, C.A.; Abrahams, V.M. Glucose and metformin modulate human first trimester trophoblast function: A model and potential therapy for diabetes-associated uteroplacental insufficiency. Am. J. Reprod. Immunol. 2014, 73, 362–371. [Google Scholar] [CrossRef]
- Wang, F.; Cao, G.; Yi, W.; Li, L.; Cao, X. Effect of metformin on a preeclampsia-like mouse model induced by high-fat diet. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Del Zotto, H.; Castrogiovanni, D.; Romero, J.; Giovambattista, A.; Spinedi, E. Oral metformin treatment prevents enhanced insulin demand and placental dysfunction in the pregnant rat fed a fructose-rich diet. ISRN Endocrinol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Eyal, S.; Easterling, T.R.; Carr, D.; Umans, J.G.; Miodovnik, M.; Hankins, G.D.V.; Clark, S.M.; Risler, L.; Wang, J.; Kelly, E.J.; et al. Pharmacokinetics of metformin during pregnancy. Drug Metab. Dispos. 2010, 38, 833–840. [Google Scholar] [CrossRef]
- Charles, B.; Norris, R.; Xiao, X.; Hague, W. Population pharmacokinetics of metformin in late pregnancy. Ther. Drug Monit. 2006, 28, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Zahlsen, K.; Spigset, O.; Carlsen, S.M. Placental passage of metformin in women with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1575–1578. [Google Scholar] [CrossRef]
- Liao, M.Z.; Flood Nichols, S.K.; Ahmed, M.; Clark, S.; Hankins, G.D.; Caritis, S.; Venkataramanan, R.; Haas, D.; Quinney, S.K.; Haneline, L.S.; et al. Effects of pregnancy on the pharmacokinetics of metformin. Drug Metab. Dispos. 2020, 48, 264–271. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Sundelin, E.I.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Hougaard Christensen, M.M.; Brøsen, K.; Frøkiær, J.; Jessen, N. In vivo Imaging of human 11C-metformin in peripheral organs: Dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 2016, 57, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Prasad, P.D. Role of transporters in placental transfer of drugs. Toxicol. Appl. Pharmacol. 2005, 207, 381–387. [Google Scholar] [CrossRef]
- Han, T.; Proctor, W.R.; Costales, C.L.; Cai, H.; Everett, R.S.; Thakker, D.R. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 2015, 352, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Meyer, Z.; Schwabedissen, H.; Draber, K.; Präger, D.; Möritz, K.U.; Linnemann, K.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab. Dispos. 2005, 33, 31–37. [Google Scholar] [CrossRef]
- Lee, N.; Hebert, M.F.; Prasad, B.; Easterling, T.R.; Kelly, E.J.; Unadkat, J.D.; Wang, J. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab. Dispos. 2013, 41, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.R.; Dotters-Katz, S.K.; Zhou, C.; Manuck, T.; Boggess, K.; Bae-Jump, V. Effect of a high-fat diet and metformin on placental mtor signaling in mice. AJP Rep. 2019, 9, e138–e143. [Google Scholar] [CrossRef]
- Rosario, F.J.; Powell, T.; Jansson, T. Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-α and the RFC. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Jansson, T.; Aye, I.; Goberdhan, D. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 2012, 33, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Eliasson, L.; Rosario, F.; Powell, T.L.; Gupta, M.B. (Eds.) Remote control of fetal metabolism by placental mTOR signaling. In Reproductive Sciences; Sage Publications Inc.: Thousand Oaks, CA, USA, 2012; Volume 19. [Google Scholar]
- Kim, J.; Ahn, C.W.; Fang, S.; Lee, H.S.; Park, J.S. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine 2019, 98, e17918. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term metformin use and vitamin b12 deficiency in the diabetes prevention program outcomes study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Hart, H.E.; Kuijs, R.; Kooijman-Buiting, A.M.J.; Rutten, G.E.H.M. Influence of duration and dose of metformin on cobalamin deficiency in type 2 diabetes patients using metformin. Acta Diabetol. 2015, 52, 47–53. [Google Scholar] [CrossRef]
- Pflipsen, M.C.; Oh, R.C.; Saguil, A.; Seehusen, D.A.; Seaquist, D.; Topolski, R. The prevalence of vitamin B12 deficiency in patients with type 2 diabetes: A cross-sectional study. J. Am. Board Fam. Med. 2009, 22, 528–534. [Google Scholar] [CrossRef]
- Reinstatler, L.; Qi, Y.P.; Williamson, R.S.; Garn, J.V.; Oakley, G.P., Jr. Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: The national health and nutrition examination survey, 1999–2006. Diabetes Care 2012, 35, 327–333. [Google Scholar] [CrossRef]
- Corominas-Faja, B.; Quirantes-Piné, R.; Oliveras-Ferraros, C.; Vazquez-Martin, A.; Cufí, S.; Martin-Castillo, B.; Micol, V.; Joven, J.; Segura-Carretero, A.; Menendez, J.A. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging 2012, 4, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2013, 68, 2–7. [Google Scholar] [CrossRef]
- Maynard, A.G.; Kanarek, N. NADH ties one-carbon metabolism to cellular respiration. Cell Metab. 2020, 31, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.C.; Mackie, F.L.; Lean, S.C.; Greenwood, S.L.; Heazell, A.E.P.; Forbes, K.; Jones, R.L. Placental dysfunction is associated with altered microRNA expression in pregnant women with low folate status. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.N.; Wheeler, S.J.; Sanders, T.A.; Thomas, J.E.; Hutchinson, C.J.; Clarke, K.; Berry, J.L.; Jones, R.L.; Seed, P.T.; Poston, L. A prospective study of micronutrient status in adolescent pregnancy. Am. J. Clin. Nutr. 2009, 89, 1114–1124. [Google Scholar] [CrossRef]
- Rogne, T.; Tielemans, M.J.; Chong, M.F.-F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.V.; Steegers, E.A.P.; Joshi, S.; Chong, Y.-S.; et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: A systematic review and meta-analysis of individual participant data. Am. J. Epidemiol. 2017, 185, 212–223. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Gagliano, C.; Salomone, S.; Giordano, M.; Bucolo, C.; Pappalardo, A.; Drago, F.; Caraci, F.; Avitabile, T.; Motta, M. Folate status in type 2 diabetic patients with and without retinopathy. Clin. Ophthalmol. 2015, 9, 1437–1442. Available online: http://europepmc.org/abstract/MED/26300625 (accessed on 15 January 2021). [CrossRef] [PubMed]
- Henderson, A.M.; Tai, D.C.; Aleliunas, R.E.; Aljaadi, A.M.; Glier, M.B.; Xu, E.E.; Miller, J.W.; Verchere, C.B.; Green, T.J.; Devlin, A.M. Maternal folic acid supplementation with vitamin B(12) deficiency during pregnancy and lactation affects the metabolic health of adult female offspring but is dependent on offspring diet. FASEB J. 2018, 32, 5039–5050. [Google Scholar] [CrossRef] [PubMed]
- Guéant, J.-L.; Namour, F.; Guéant-Rodriguez, R.-M.; Daval, J.-L. Folate and fetal programming: A play in epigenomics? Trends Endocrinol. Metab. 2013, 24, 279–289. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Di Simone, N.; Riccardi, P.; Maggiano, N.; Piacentani, A.; D’Asta, M.; Capelli, A.; Caruso, A. Effect of folic acid on homocysteine-induced trophoblast apoptosis. Mol. Hum. Reprod. 2004, 10, 665–669. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Yajnik, C.S. Role of maternal vitamin B12 on the metabolic health of the offspring: A contributor to the diabetes epidemic? Br. J. Diabetes Vasc. Dis. 2010, 10, 109–114. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017, 3, doi. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.-L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front. Mol. Biosci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 status among pregnant women in the UK and its association with obesity and gestational diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef]
- Kouroglou, E.; Anagnostis, P.; Daponte, A.; Bargiota, A. Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: A systematic review and meta-analysis. Endocrine 2019, 66, 149–156. [Google Scholar] [CrossRef]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, K.P., Jr. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef]
- Ho, M.; Halim, J.H.; Gow, M.L.; El-Haddad, N.; Marzulli, T.; Baur, L.A.; Cowell, C.T.; Garnett, S.P. Vitamin B12 in obese adolescents with clinical features of insulin resistance. Nutrients 2014, 6, 5611–5618. [Google Scholar] [CrossRef]
- Chandyo, R.K.; Ulak, M.; Kvestad, I.; Shrestha, M.; Ranjitkar, S.; Basnet, S.; Hysing, M.; Shrestha, L.; Strand, T.A. The effects of vitamin B12 supplementation in pregnancy and postpartum on growth and neurodevelopment in early childhood: Study protocol for a randomized placebo controlled trial. BMJ Open 2017, 7, e016434. [Google Scholar] [CrossRef]
- Sukumar, N.; Rafnsson, S.B.; Kandala, N.-B.; Bhopal, R.; Yajnik, C.S.; Saravanan, P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1232–1251. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Panchanadikar, A.V.; Naik, S.S.; Deshpande, J.A.; Coyaji, K.J.; Fall, C.; Refsum, H. Maternal total homocysteine concentration and neonatal size in India. Asia Pac. J. Clin. Nutr. 2005, 14, 179–181. [Google Scholar]
- World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Kim, Y.I.; Fawaz, K.; Knox, T.; Lee, Y.M.; Norton, R.; Libby, E.; Mason, J.B. Colonic mucosal concentrations of folate are accurately predicted by blood measurements of folate status among individuals ingesting physiologic quantities of folate. Cancer Epidemiol. Biomark. Prev. 2001, 10, 715–719. [Google Scholar]
- Ramos, M.I.; Allen, L.H.; Mungas, D.M.; Jagust, W.J.; Haan, M.N.; Green, R.; Miller, J.W. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2005, 82, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; El Marroun, H.; Cecil, C.; Jaddoe, V.W.V.; Hillegers, M.; Tiemeier, H.; White, T. Maternal folate levels during pregnancy and offspring brain development in late childhood. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Ginsburg, E.S.; Missmer, S.A.; Chavarro, J.E. Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet. Gynecol. 2014, 124, 23–31. [Google Scholar] [CrossRef]
- Van Uitert, E.M.; Steegers-Theunissen, R.P. Influence of maternal folate status on human fetal growth parameters. Mol. Nutr. Food Res. 2013, 57, 582–595. [Google Scholar] [CrossRef]
- Hodgetts, V.A.; Morris, R.K.; Francis, A.; Gardosi, J.; Ismail, K.M. Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: A population study, systematic review and meta-analysis. BJOG 2015, 122, 478–490. [Google Scholar] [CrossRef]
- Maloney, C.A.; Hay, S.M.; Rees, W.D. Folate deficiency during pregnancy impacts on methyl metabolism without affecting global DNA methylation in the rat fetus. Br. J. Nutr. 2007, 97, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Engeham, S.F.; Haase, A.; Langley-Evans, S.C. Supplementation of a maternal low-protein diet in rat pregnancy with folic acid ameliorates programming effects upon feeding behaviour in the absence of disturbances to the methionine-homocysteine cycle. Br. J. Nutr. 2010, 103, 996–1007. [Google Scholar] [CrossRef]
- Burdge, G. Homocysteine: A role in fetal programming? Br. J. Nutr. 2006, 96. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr. Bull. 2008, 29, S101–S111. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, N.; Watson, E.D. Lessons from the one-carbon metabolism: Passing it along to the next generation. Repr. BioMed. Online 2013, 27, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Mani, C.; Kochhar, P.; Ravikumar, G.; Dwarkanath, P.; Sheela, C.N.; George, S.; Thomas, A.; Crasta, J.; Thomas, T.; Kurpad, A.V.; et al. Placental expression of ENG, VEGF, and FLT: Gender-specific associations with maternal vitamin B(12) status. Eur. J. Clin. Nutr. 2020, 74, 176–182. [Google Scholar] [CrossRef]
- Moussa, C.; Ross, N.; Jolette, P.; Macfarlane, A.J. Altered folate metabolism modifies cell proliferation and progesterone secretion in human placental choriocarcinoma JEG-3 cells. Br. J. Nutr. 2015, 114, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Joshi, K.; Mishra, S.; Otiv, S.; Kumbar, V. Molecular and cellular effects of vitamin B12 forms on human trophoblast cells in presence of excessive folate. Biomed. Pharmacother 2016, 84, 526–534. [Google Scholar] [CrossRef]
- Yin, X.; Gao, R.; Geng, Y.; Chen, X.; Liu, X.; Mu, X.; Ding, Y.; Wang, Y.; He, J. Autophagy regulates abnormal placentation induced by folate deficiency in mice. Mol. Hum. Reprod. 2019, 25, 305–319. [Google Scholar] [CrossRef]
- Carletti, J.V.; Correia-Branco, A.; Silva, C.R.; Andrade, N.; Silva, L.O.P.; Martel, F. The effect of oxidative stress induced by tert-butylhydroperoxide under distinct folic acid conditions: An in vitro study using cultured human trophoblast-derived cells. Reprod. Toxicol. 2018, 77, 33–42. [Google Scholar] [CrossRef]
- Ahmed, T.; Fellus, I.; Gaudet, J.; Macfarlane, A.J.; Fontaine-Bisson, B.; Bainbridge, S.A. Effect of folic acid on human trophoblast health and function in vitro. Placenta 2016, 37, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Powell, T.L.; Jansson, T. mTOR folate sensing links folate availability to trophoblast cell function. J. Physiol. 2017, 595, 4189–4206. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Smith, S.C.; Steegers, E.A.; Guilbert, L.J.; Baker, P.N. Folate affects apoptosis in human trophoblastic cells. BJOG. 2000, 107, 1513–1515. [Google Scholar] [CrossRef]
- Mahajan, A.; Sapehia, D.; Thakur, S.; Mohanraj, P.S.; Bagga, R.; Kaur, J. Effect of imbalance in folate and vitamin B12 in maternal/parental diet on global methylation and regulatory miRNAs. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Shah, T.; Mishra, S.; More, A.; Otiv, S.; Apte, K.; Joshi, K. Combination of vitamin B12 active forms improved fetal growth in Wistar rats through up-regulation of placental miR-16 and miR-21 levels. Life Sci. 2017, 191, 97–103. [Google Scholar] [CrossRef]
- Rosario, F.J.; Nathanielsz, P.W.; Powell, T.L.; Jansson, T. Maternal folate deficiency causes inhibition of mTOR signaling, down-regulation of placental amino acid transporters and fetal growth restriction in mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Berchtold, P.; Bolli, P.; Arbenz, U.; Keiser, G. Disturbance of intestinal absorption following metformin therapy (observations on the mode of action of biguanides. Diabetologia 1969, 5, 405–412. [Google Scholar] [CrossRef]
- Kos, E.; Liszek, M.J.; Emanuele, M.A.; Durazo-Arvizu, R.; Camacho, P. Effect of metformin therapy on vitamin D and vitamin B12 levels in patients with type 2 diabetes mellitus. Endocr. Pract. 2012, 18, 179–184. [Google Scholar] [CrossRef]
- Niafar, M.; Hai, F.; Porhomayon, J.; Nader, N.D. The role of metformin on vitamin B12 deficiency: A meta-analysis review. Intern. Emerg. Med. 2015, 10, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Houda, C.M.; Lu, Z.X.; Coat, S.; Baghurst, P.A.; Owens, J.A.; Sikaris, K.; Rowan, J.A.; Hague, W.M. Vitamin B12 and homocysteine status during pregnancy in the metformin in gestational diabetes trial: Responses to maternal metformin compared with insulin treatment. Diabetes Obes. Metab. 2013, 15, 660–667. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Gholinezhad-Chari, M.; Ghadimi, R. The effect of metformin treatment on the serum levels of homocysteine, folic acid, and vitamin B12 in patients with polycystic ovary syndrome. J. Hum. Reprod. Sci. 2017, 10, 95–101. [Google Scholar] [PubMed]
- Snow, C.F. Laboratory diagnosis of vitamin B12 and folate deficiency: A guide for the primary care physician. Arch. Intern. Med. 1999, 159, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.F.; Clark, J.S.; Ireland, J.T.; Kesson, C.M.; Watson, W.S. Malabsorption of vitamin B12 and intrinsic factor secretion during biguanide therapy. Diabetologia 1983, 24, 16–18. [Google Scholar] [CrossRef]
- Ting, R.Z.-W.; Szeto, C.C.; Chan, M.H.-M.; Ma, K.K.; Chow, K.M. Risk factors of vitamin B12 deficiency in patients receiving metformin. Arch. Intern. Med. 2006, 166, 1975–1979. [Google Scholar] [CrossRef]
- Friedman, P.; Shia, M.; Wallace, J.K. A saturable high affinity binding site for transcobalamin II-vitamin B12 complexes in human placental membrane preparations. J. Clin. Investig. 1977, 59, 51–58. [Google Scholar] [CrossRef]
- Quadros, E.; Sai, P.; Rothenberg, S.P. Characterization of the human placental membrane receptor for transcobalamin II-cobalamin. Arch. Biochem. Biophys. 1994, 308, 192–199. [Google Scholar] [CrossRef]
- Seligman, P.A.; Allen, R.H. Characterization of the receptor for transcobalamin II isolated from human placenta. J. Biol. Chem. 1978, 253, 1766–1772. [Google Scholar] [CrossRef]
- Schneider, H.; Miller, R.K. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int. J. Dev. Biol. 2009, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.W.; Catus, R.G.; Miller, R.K. Macromolecule transfer in the human trophoblast: Transcobalamin II-vitamin B12 uptake. Placenta Suppl. 1981, 3, 145–159. [Google Scholar]
- Perez-D’Gregorio, R.E.; Miller, R.K. Transport and endogenous release of vitamin B12 in the dually perfused human placenta. J. Pediatr. 1998, 132, S35–S42. [Google Scholar] [CrossRef]
- Graber, S.E.; Scheffel, U.; Hodkinson, B.; McIntyre, P.A. Placental transport of vitamin B12 in the pregnant rat. J. Clin. Investig. 1971, 50, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Dallaglio, K.; Bruno, A.; Cantelmo, A.R.; Esposito, A.I.; Ruggiero, L.; Orecchioni, S.; Calleri, A.; Bertolini, F.; Pfeffer, U.; Noonan, D.M. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis 2014, 35, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Venna, V.R.; Li, J.; Hammond, M.D.; Mancini, N.S.; McCullough, L.D. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur. J. Neurosci. 2014, 39, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Adya, R.; Chen, J.; Farhatullah, S.; Heutling, D.; Mitchell, D.; Lehnert, H.; Randeva, H.S. Metformin decreases angiogenesis via NF-κB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc. Res. 2009, 83, 566–574. [Google Scholar] [CrossRef]
- Di Pietro, M.; Parborell, F.; Irusta, G.; Pascuali, N.; Bas, D.; Bianchi, M.S.; Tesone, M.; Abramovich, D. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015, 156, 1453–1463. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCarthy, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McTernan, P.G.; et al. Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin. Epigen. 2015, 7. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Vatish, M.; Alam, M.T.; Ott, S.; Kumar, S.; Saravanan, P. Low vitamin b12 in pregnancy is associated with adipose-derived circulating mirs targeting pparγ and insulin resistance. J. Clin. Endocrinol. Metab. 2017, 102, 4200–4209. [Google Scholar] [CrossRef]
- Kumar, K.A.; Lalitha, A.; Pavithra, D.; Padmavathi, I.J.N.; Ganeshan, M.; Rao, K.R.; Venu, L.; Balakrishna, N.; Shanker, N.H.; Reddy, S.U.; et al. Maternal dietary folate and/or vitamin B12 restrictions alter body composition (adiposity) and lipid metabolism in Wistar rat offspring. J. Nutr. Biochem. 2013, 24, 25–31. [Google Scholar] [CrossRef]
- Kamaruddin, M.; Jusuf, E.; Sanusi, H. Correlation of vitamin B12 level with insulin resistance in infant and placenta outcomes. Enfermería Clín. 2020, 30, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Muntingh, G.; Rheeder, P. Vitamin B12 deficiency in metformin-treated type-2 diabetes patients, prevalence and association with peripheral neuropathy. BMC Pharmacol. Toxicol. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Ko, S.H.; Ahn, Y.B.; Song, K.H.; Han, K.D.; Park, Y.M.; Ko, S.H.; Kim, H.S. Association of vitamin B12 deficiency and metformin use in patients with type 2 diabetes. J. Korean Med. Sci. 2014, 29, 965–972. [Google Scholar] [CrossRef]
- Tomkin, G.H.; Hadden, D.R.; Weaver, J.A.; Montgomery, D.A.D. Vitamin-B12 status of patients on long-term metformin therapy. Br. Med. J. 1971, 2, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Scherneck, S.; Schlinke, N.; Beck, E.; Grupe, K.; Weber-Schoendorfer, C.; Schaefer, C. Pregnancy outcome after first-trimester exposure to metformin: A prospective cohort study. Reprod. Toxicol. 2018, 81, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Hubel, C.A.; Powers, R.W.; von Versen-Hoeynck, F.; Gammill, H.; Rajakumar, A.; Roberts, J.M. Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta. 2008, 29, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jansson, N.; Palmberg, I.; Säljö, K.; Powell, T.; Jansson, T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007, 582, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-H.; Hsieh, T.-T.; Wu, C.-P.; Li, M.-J.; Yeh, Y.-L.; Chen, S.-F. Mammalian target of rapamycin signaling is a mechanistic link between increased endoplasmic reticulum stress and autophagy in the placentas of pregnancies complicated by growth restriction. Placenta 2017, 60, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yung H-w Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Orozco, J.M.; Saxton, R.A.; Condon, K.J.; Liu, G.Y.; Krawczyk, P.A.; Scaria, S.M.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 2017, 358, 813–818. [Google Scholar] [CrossRef]
- Sahin, M.; Tutuncu, N.B.; Ertugrul, D.; Tanaci, N.; Guvener, N.D. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2007, 21, 118–123. [Google Scholar] [CrossRef]
- Pongchaidecha, M.; Srikusalanukul, V.; Chattananon, A.; Tanjariyaporn, S. Effect of metformin on plasma homocysteine, vitamin B12 and folic acid: A cross-sectional study in patients with type 2 diabetes mellitus. J. Med. Assoc. Thai. 2004, 87, 780–787. [Google Scholar] [PubMed]
- Wulffelé, M.G.; Kooy, A.; Lehert, P.; Bets, D.; Ogterop, J.C.; Borger van der Burg, B.; Donker, A.J.M.; Stehouwer, C.D.A. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: A randomized, placebo-controlled trial. J. Intern. Med. 2003, 254, 455–463. [Google Scholar] [CrossRef]
- Carlsen, S.M.; Følling, I.; Grill, V.; Bjerve, K.S.; Schneede, J.; Refsum, H. Metformin increases total serum homocysteine levels in non-diabetic male patients with coronary heart disease. Scand. J. Clin. Lab. Invest. 1997, 57, 521–527. [Google Scholar] [CrossRef]
- Solanky, N.; Requena Jimenez, A.; D’Souza, S.W.; Sibley, C.P.; Glazier, J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta 2010, 31, 134–143. [Google Scholar] [CrossRef]
- Piedrahita, J.A.; Oetama, B.; Bennett, G.D.; van Waes, J.; Kamen, B.A.; Richardson, J.; Lacey, S.W.; Anderson, R.G.; Finnell, R.H. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat. Genet. 1999, 23, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Caviedes, L.; Iñiguez, G.; Hidalgo, P.; Castro, J.J.; Castano, E.; Llanos, M.; Hirsch, S.; Ronco, A.M. Relationship between folate transporters expression in human placentas at term and birth weights. Placenta 2016, 38, 24–28. [Google Scholar] [CrossRef]
- Padmanabhan, N.; Jia, D.; Geary-Joo, C.; Wu, X.; Ferguson-Smith Anne, C.; Fung, E.; Bieda Mark, C.; Snyder Floyd, F.; Gravel Roy, A.; Cross James, C.; et al. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 2013, 155, 81–93. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Grynspan, D.; Van Mieghem, T.; Connor, K.L. Isolated fetal neural tube defects associate with increased risk of placental pathology: Evidence from the collaborative perinatal project. MedRxiv 2021. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; He, C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015, 29, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Jaiswal, D. One-Carbon Metabolism, Spermatogenesis, and Male Infertility. Reprod. Sci. 2013, 20, 622–630. [Google Scholar] [CrossRef]
- Zheng, L.D.; Linarelli, L.E.; Liu, L.; Wall, S.S.; Greenawald, M.H.; Seidel, R.W.; Estabrooks, P.A.; Almeida, F.A.; Cheng, Z. Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clin. Epigen. 2015, 7. [Google Scholar] [CrossRef]
- Kraus, D.; Yang, Q.; Kong, D.; Banks, A.S.; Zhang, L.; Rodgers, J.T.; Pirinen, E.; Pulinilkunnil, T.C.; Gong, F.; Wang, Y.-c.; et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 2014, 508, 258–262. [Google Scholar] [CrossRef]
- Roberti, A.; Fernández, A.F.; Fraga, M.F. Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation. Mol. Metab. 2021, 45. [Google Scholar] [CrossRef]
- Alshawi, A.; Agius, L. Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism. J. Biol. Chem. 2019, 294, 2839–5691. [Google Scholar] [CrossRef]
- Ferland-McCollough, D.; Fernandez-Twinn, D.S.; Cannell, I.G.; David, H.; Warner, M.; Vaag, A.A.; Bork-Jensen, J.; Brøns, C.; Gant, T.W.; Willis, A.E.; et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2012, 19, 1003–1012. [Google Scholar] [CrossRef]
- Coleman, C.B.; Lightell, D.J., Jr.; Moss, S.C.; Bates, M.; Parrino, P.E.; Woods, T.C. Elevation of miR-221 and-222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Mol. Cell. Endocrinol. 2013, 374, 125–129. [Google Scholar] [CrossRef]
- Goodspeed, D.; Seferovic, M.D.; Holland, W.; Mcknight, R.A.; Summers, S.A.; Branch, D.W.; Lane, R.H.; Aagaard, K.M. Essential nutrient supplementation prevents heritable metabolic disease in multigenerational intrauterine growth-restricted rats. FASEB J. 2015, 29, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, K.; Yajnik, P.; Lubree, H.; Joglekar, C.; Bhat, D.; Katre, P.; Joshi, S.; Ladkat, R.; Fall, C.; Yajnik, C. The pune rural intervention in young adolescents (PRIYA) study: Design and methods of a randomised controlled trial. BMC Nutr. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Excellence NIfHaC. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period: NICE Guideline, 2015; updated 2020. Available online: https://www.nice.org.uk/guidance/ng3/chapter/Recommendations (accessed on 15 January 2021).
- Hursthouse, N.A.; Gray, A.R.; Miller, J.C.; Rose, M.C.; Houghton, L.A. Folate status of reproductive age women and neural tube defect risk: The effect of long-term folic acid supplementation at doses of 140 µg and 400 µg per day. Nutrients 2011, 3, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Karat, S.C.; Yajnik, C.S.; Fall, C.H.D. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia 2014, 57, 110–121. [Google Scholar] [CrossRef]
- Rao, S.; Yajnik, C.S.; Kanade, A.; Fall, C.H.D.; Margetts, B.M.; Jackson, A.A.; Shier, R.; Joshi, S.; Rege, S.; Lubree, H.; et al. Intake of micronutrient-rich foods in rural indian mothers is associated with the size of their babies at birth: Pune maternal nutrition study. J. Nutr. 2001, 131, 1217–1224. [Google Scholar] [CrossRef]
- Whitrow, M.J.; Moore, V.M.; Rumbold, A.R.; Davies, M.J. Effect of supplemental folic acid in pregnancy on childhood asthma: A prospective birth cohort study. Am. J. Epidemiol. 2009, 170, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Valera-Gran, D.; Navarrete-Muñoz, E.M.; Garcia de la Hera, M.; Fernández-Somoano, A.; Tardón, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; González-Safont, L.; Romaguera, D.; et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4-5 y of age: The prospective birth cohort infancia y medio ambiente (INMA) study. Am. J. Clin Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Pickell, L.; Brown, K.; Li, D.; Wang, X.L.; Deng, L.; Wu, Q.; Selhub, J.; Luo, L.; Jerome-Majewska, L.; Rozen, R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. A. Clin. Mol. Teratol. 2011, 91, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, R.; Pannia, E.; Kubant, R.; Wasek, B.; Bottiglieri, T.; Malysheva, O.V.; Caudill, M.A.; Anderson, G.H. Choline and folic acid in diets consumed during pregnancy interact to program food intake and metabolic regulation of male wistar rat offspring. J. Nutr. 2021, 151. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The pune maternal nutrition study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Boachie, J.; Adaikalakoteswari, A.; Gázquez, A.; Zammit, V.; Larque, E.; Saravanan, P. Vitamin B12 induces hepatic fatty infiltration through altered fatty acid metabolism. Cell. Physiol. Biochem. 2021, 55, 241–255. [Google Scholar]
- Fisher, J.J.; Vanderpeet, C.L.; Bartho, L.A.; McKeating, D.R.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V. Mitochondrial dysfunction in placental trophoblast cells experiencing gestational diabetes mellitus. J. Physiol. 2021, 599, 1291–1305. [Google Scholar] [CrossRef]
- Lawrence, S.A.; Titus, S.A.; Ferguson, J.; Heineman, A.L.; Taylor, S.M.; Moran, R.G. Mammalian mitochondrial and cytosolic folylpolyglutamate synthetase maintain the subcellular compartmentalization of folates. J. Biol Chem. 2014, 289, 29386–29396. [Google Scholar] [CrossRef]
- Behboudi-Gandevani, S.; Amiri, M.; Yarandi, R.B.; Tehrani, F.R. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11. [Google Scholar] [CrossRef]
- Jaffe, A.; Giveon, S.; Rubin, C.; Novikov, I.; Ziv, A.; Kalter-Leibovici, O. Gestational diabetes risk in a multi-ethnic population. Acta Diabetologica 2020, 57, 263–269. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Aiken, C.E.; Ozanne, S.E. Comparative impact of pharmacological treatments for gestational diabetes on neonatal anthropometry independent of maternal glycaemic control: A systematic review and meta-analysis. PLOS Med. 2020, 17, e1003126. [Google Scholar] [CrossRef]
- Sukumar, N.; Saravanan, P. Investigating vitamin B12 deficiency. BMJ 2019, 365. [Google Scholar] [CrossRef] [PubMed]
- Rafnsson, S.B.; Saravanan, P.; Bhopal, R.S.; Yajnik, C.S. Is a low blood level of vitamin B12 a cardiovascular and diabetes risk factor? A systematic review of cohort studies. Eur. J. Nutr. 2011, 50, 97–106. [Google Scholar] [CrossRef] [PubMed]
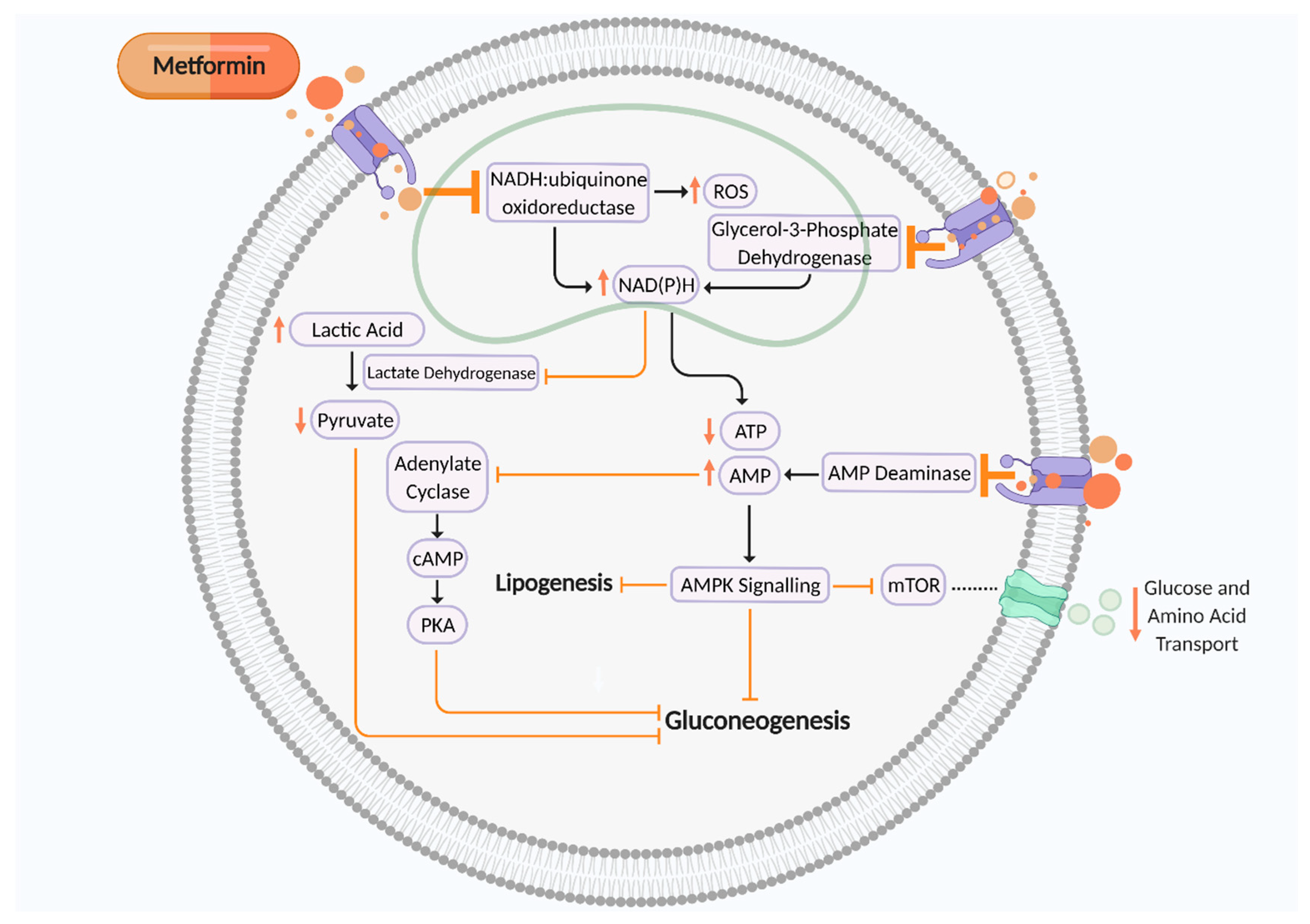
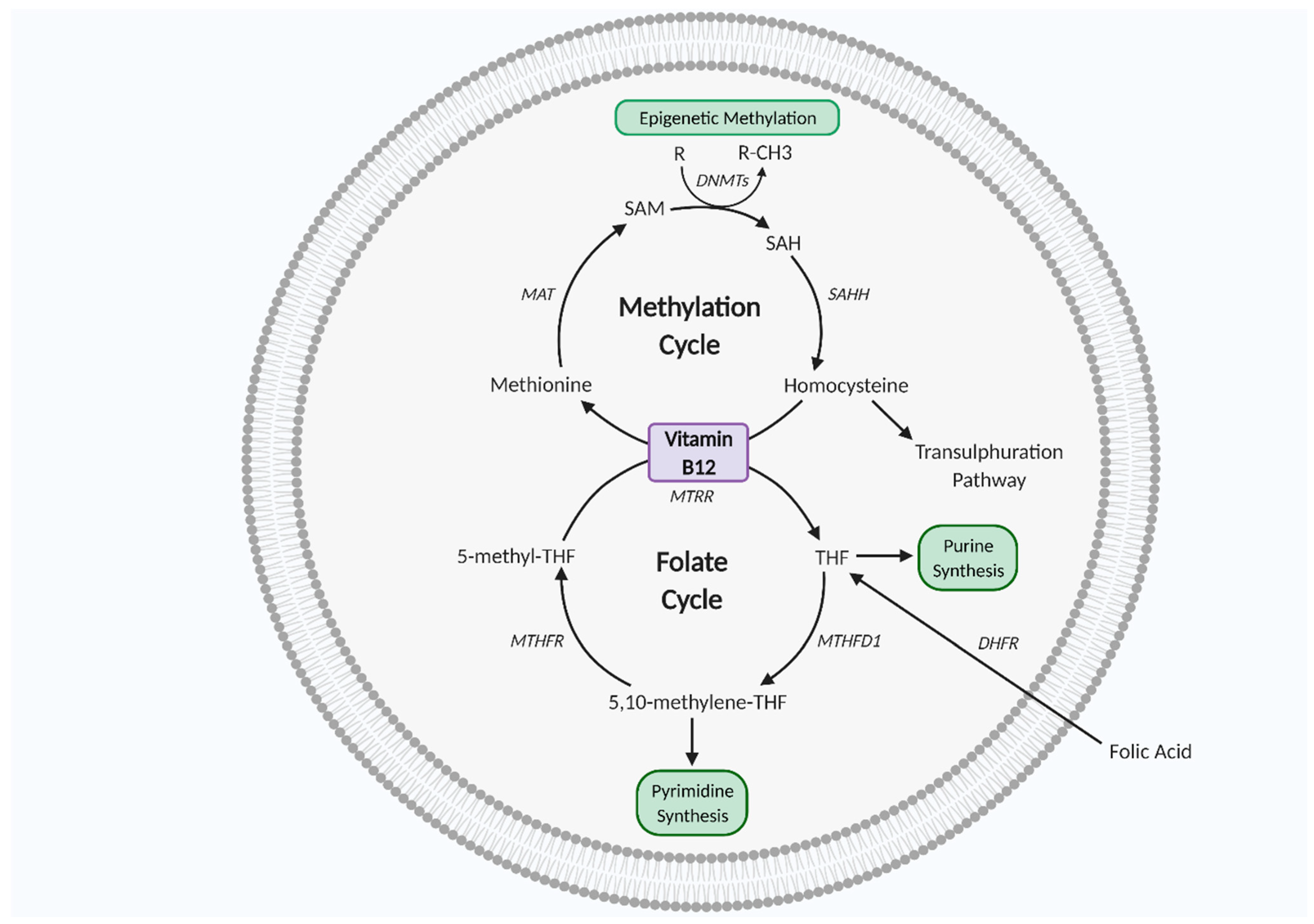
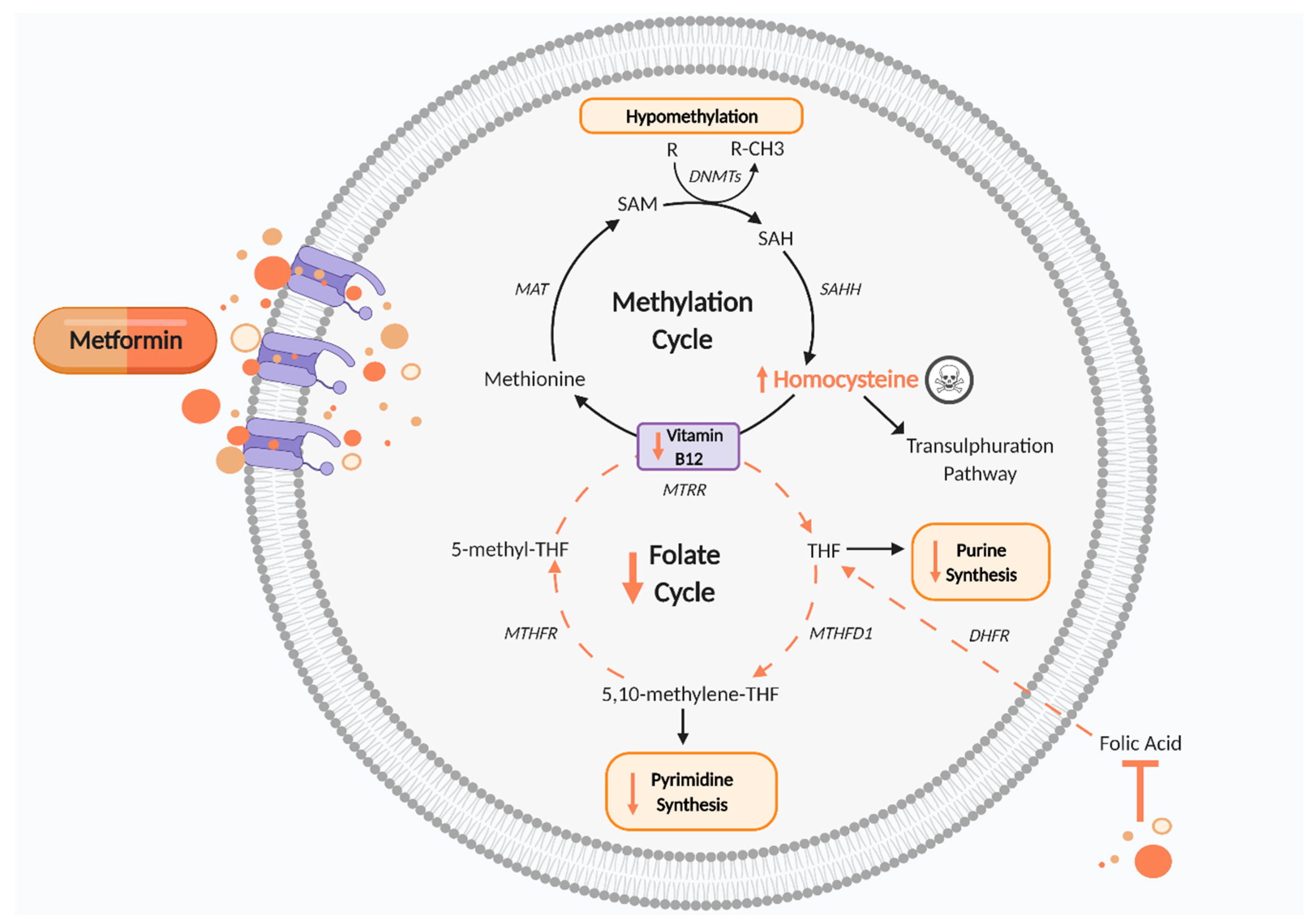
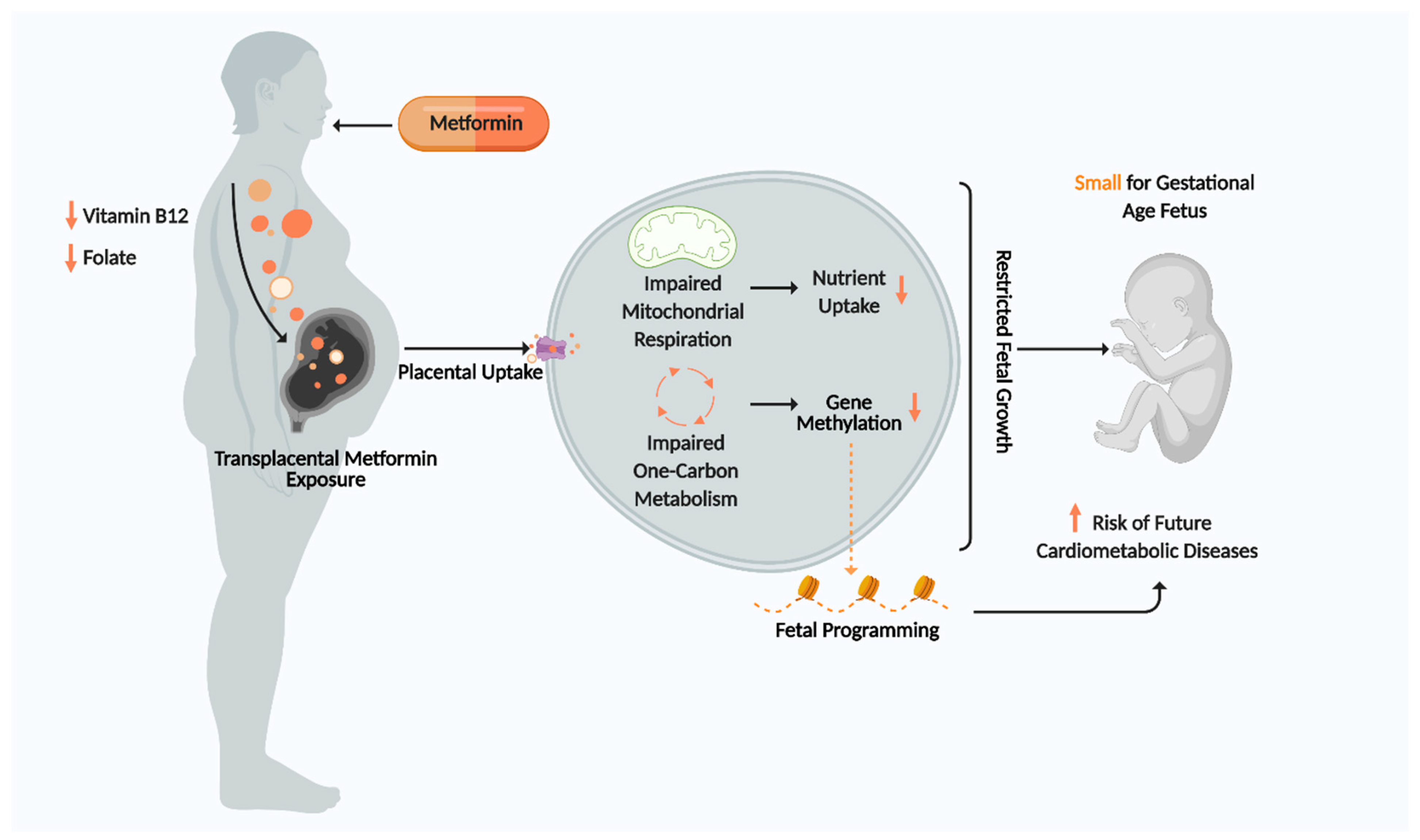
| Reference | Model | Effects Demonstrated by Metformin | Significance |
|---|---|---|---|
| Clinical studies | |||
| Jamal et al. 2012 [32] | Pregnant women with PCOS treated with metformin | - ⇔ on birth weight - ↓ uterine artery pulsatility index | Metformin adversely affected uteroplacental circulation |
| Ex vivo or in vitro human placental studies | |||
| Jiang et al. 2020 [33] | Human GDM and T2DM placental explants cultured and treated with metformin (ex vivo) | Male human placental explants: - AMPK activation - ↑ H3K27 acetylation - ↓ DNMT1 protein abundance - ↓ PGC-1α promoter methylation and ↑ PGC-1α mRNA expression | Effects of metformin may be fetal sex-dependent Metformin may improve placental efficiency by facilitating placental mitochondrial biogenesis |
| Brownfoot et al. 2020 [34] Cluver et al. 2019 [35] Kaitu’u-Lino 2018 [36] Brownfoot et al. 2016 [37] | Human primary tissues exposed to metformin; placental explants, endothelial cells and placental villous explants, whole maternal vessels, maternal omental vessel explants (in vitro and ex vivo) | - ↓ sFlt-1 and sEng secretion from primary endothelial cells, preterm preeclamptic placental villous explants and villous cytotrophoblast cells - ↓ VCAM-1 mRNA expression in endothelial cells - ↑ whole maternal blood vessel angiogenesis - ↓ sFlt mRNA expression - ↓ TNFα-mediated endothelial cell dysfunction | Metformin enhances placental angiogenesis and reduces endothelial dysfunction by decreasing endothelial and trophoblastic antiangiogenic factor secretion via mitochondrial electron transport chain inhibition Metformin is being trialled as a medication for preeclampsia (trial number PACTR201608001752102) |
| Szukiewicz et al. 2018 [38] | Human placental lobules perfused with metformin under normoglycemic or hyperglycaemic conditions (ex vivo) | - ↓ CX3CL1 and TNFα secretion - ↑ placental CX3CR1 protein expression - ↓ placental NFκB p65 protein | Metformin has anti-inflammatory effects in the placenta |
| Correia-Branco et al. 2018 [39] | HTR-8/SVneo extravillous trophoblast cell line exposed to metformin (in vitro) | - ↓ proliferation - ↑ apoptosis - Inhibited folic acid uptake - Inhibited glucose uptake - Effects of metformin were prevented by inhibition of mTOR, JNK, and PI3K pathways | Metformin impairs placental development and nutrient transport via PI3K, mTOR, JNK, and PI3K pathways |
| Arshad et al. 2016 [40] | Human placental explants; from healthy pregnancy, non-treated diet-controlled GDM pregnancy, and metformin-treated GDM pregnancy (ex vivo) | - ↓ similar morphology in metformin-treated GDM placenta and non-treated healthy placenta, except for increased cord width - ↓ placental width in metformin-treated GDM placenta compared to non-treated GDM placenta - ↓ chorangiosis, placental thickness, and syncytial knots in metformin-treated placenta compared to non-treated GDM placenta | Metformin may improve placental morphology by restoring diabetic placental hallmarks to characteristics similar to healthy placenta |
| Han et al. 2015 [41] | Human first trimester trophoblasts treated with or without metformin (in vitro) | - ↓ trophoblast cytokine and chemokine release in normal and high glucose culture concentrations - No antiangiogenic or antimigratory effects | Metformin may potentially decrease placental glucose-induced inflammatory response |
| In vivo rodent studies | |||
| Jiang et al. 2020 [33] | Mice treated with maternal metformin and high-fat diet | Improved placental efficiency in males: - ↓ PGC-1α promoter methylation and ↑ PGC-1α expression - ↑ TFAM expression Improved glucose homeostasis in male offspring | Metformin may improve placental efficiency by facilitating placental mitochondrial biogenesis Metformin may be protective to the offspring by suppressing epigenetic changes evoked by maternal diabetes |
| Wang et al. 2019 [42] | Pregnant mice fed an isocaloric diet (control), high-fat diet, or high-fat diet plus metformin (in vivo) | - ↓ placental weight compared to control - Partially rescued high-fat diet induced ↓ in placental and fetal weight - ↑ VEGF and MMP-2 protein expression | Metformin improves high fat diet-induced reduction in placental and fetal growth, potentially by modulating placental vasculature |
| Alzamendi et al. 2012 [43] | Pregnant rats fed a normal or high-fructose diet, treated with metformin (in vivo) | - ↓ fetal weight - ⇔ on placental weight or blood vessel area - Improved fructose diet induced ↓ blood vessel area | Metformin reduces fetal weight in mice fed a normal diet Metformin prevents high fructose diet-induced placental dysfunction |
| Reference | Model | Functional Effects/Findings | Significance |
|---|---|---|---|
| Clinical studies | |||
| Mani et al. 2020 [97] | Maternal first trimester B12 status correlated with term placental angiogenesis genes | Vitamin B12 deficiency: ↑ placental ENG and VEGF expression in female births only | Suggests placental adaptation to low maternal B12 by upregulating angiogenic pathways in a sex-specific manner |
| Baker et al. 2017 [65] | Prospective study of folate-deficient pregnant women | Folate deficiency: - ↑ trophoblast proliferation and apoptosis - ↓ amino acid transport - ↓ placental hormones (PAPPA, progesterone, and hPL) - ↑ placental miR-222-3p, miR-141-3p, and miR-34b-5p - ↓ ZEB2, MYC, and CDK6 mRNA expression in placenta | Folate deficiency adversely impacts on placental development and function and this may be via regulation of miRNAs in the placenta |
| Ex vivo or in vitro human placental studies | |||
| Moussa et al. 2015 [98] | JEG3 cells exposed to 2nM (low), 20 nM (normal), or 100nM (excess) levels of folic acid | Low folic acid: - ↓ proliferation - ↓ cell invasion - ↓ cell viability Excess folic acid: - ↑ proliferation | Folate deficiency adversely impacts on placental development but excess folate may increase placental growth |
| Shah et al. 2016 [99] | BeWo and JEG cells exposed to 20ng/mL (normal) or 2000ng/mL (supraphysiological) folic acid | Supraphysiological folic acid - ↓ cell viability - ↓bhCG secretion (only in JEGs) - ↑↓EGFR mRNA - ↑oxidative stress - ↑TNF-a mRNA | Excess folic acid treatment has an adverse impact on placental growth, development, and function. |
| Yin et al. 2019 [100] Carletti et al. 2018 [101] Ahmed et al. 2016 [102] | HTR-8/SVneo, BeWo cell lines exposed to supraphysiological (2000ng/mL) or low (2ng/mL) levels of folic acid for 48hr | Supraphysiological folic acid: - ↓ cell viability in BeWo - ↑ proliferation rate in HTR-8/SVneo - ⇔ on apoptosis or β-hCG release - ↑ tert-butylhydroperoxide (TBH)-induced oxidative stress Low folic acide: - ↓ cell viability - ↓ cell invasion - ↑ autophagy - ↓ apoptosis - ↓ invasiveness | Both low and high levels of folate adversely impact on placental development |
| Rosario et al. 2017 [103] Di Simone et al. 2004 [73] Steegers-Theunissen et al. 2000 [104] | Primary trophoblast (third trimester) exposed to low folic acid | Low folic acid: - ↑ apoptosis - ↓ hCG secretion - ↓ mTOR signalling - ↓ activity of key amino acid transporters | Low folate impacts on trophoblast viability and may alter transport of nutrients to fetus |
| Ahmed et al. 2016 [102] Yin et al. 2019 [100] | Human villous explants (third trimester) exposed to supraphysiological (2000ng/mL) or deficient (2ng/mL) levels of folic acid for 48 hours | Supraphysiological folic acid: - ⇔ in any assessed functions Low folic acid: ⇔ in any functional assessments (Ahmed et al. 2016) [102] - ↑ apoptosis and autophagy (Yin et al. 2019) [100] | Limited effect observed in human placental explants suggests this may not be the optimal model for studying high/low folate |
| In vivo rodent studies | |||
| Mahajan et al. 2019 [105] | Mouse dietary model—effect of the altered dietary ratio of folate and B12 on the expression of transporters, related miRNAs, and DNA methylation in maternal/fetal tissues in F1 and F2 generations | Folate deficiency; folate over-supplementation; vitamin B12 deficiency; vitamin B12 over-supplementation; combination of folate/B12 deficiency/over-supplementation: - Altered placental mRNA for folate transporters, B12 transporters/proteins, DNMT1, DNMT3A, and DNMT3B - Altered placental miR-483, miR-221, and miR-133 expression - Placenta global DNA methylation affected | Demonstrates that altered dietary ratios of folate and B12 can have more severe effects than the individual deficiencies |
| Shah et al. 2017 [106] | Rat dietary model fed normal (400 µg/day) or high (5 mg/day) folate +/- B12 (various forms) | High folate: - ↓ placental weight - ↓ offspring birth weight - ↓ miR-16 and 21 expression - ↑ plasma homocysteine High folate combined with Vitamin B12 supplementation: - Restored miR-16 and miR-21 expression - Prevented ↓ offspring birth weight | High folate reduces placental and fetal growth, potentially via altering miRNA levels in placenta. This is restored by vitamin B12 supplementation |
| Yin et al. 2019 [100] | Mice on folate-deficient diet | Folate deficiency: - ↓ placental size - ↓ endocrine function - ↓ placental vascularisation - ↓ trophoblast differentiation - ↑ oxidative stress - ↑ resorption rates | Folate deficiency reduces placental growth and development |
| Rosario et al. 2017 [107] | Mouse on folate-deficient diet before and during pregnancy | Maternal folate deficiency: - ↓ mTORC1 and mTORC2 signalling - ↓ trophoblast plasma membrane systems A and L amino acid transporter activities - ↓ trophoblast amino acid transporter isoform expression | Folate deficiency reduces amino acid transport to the fetus |
| Reference | Study Description | Subjects | Definition of Serum B12 Deficiency | Rates of Vitamin B12 Deficiency | Dose of Metformin Associated with B12 Deficiency | Duration Associated with B12 Deficiency |
|---|---|---|---|---|---|---|
| Kim et al. 2019 [57] | Investigating B12 deficiency and >6 months of metformin treatment | 1111 T2DM patients | ≤300 pg/mL | Deficiency in 22.2% of patients, n = 247 | >1000 mg/day | No association |
| Aroda et al. 2016 [58] | Investigating long-term effect of metformin use on vitamin B12 deficiency | 1800 patients participating in the Diabetes Prevention Program (DPP)/DPP Outcomes Study (DPPOS) | ≤203 pg/mL | 4.3% at 1 year 19.1% at 5 years 20.3% at 13 years | Metformin 850 mg twice daily | 1 year |
| Ahmed et al. 2016 [131] | Investigating the prevalance of vitamin B12 deficiency in T2DM patients treated with metformin | 121 T2DM patients | <150 pmol/L | 28.1% | 2.4 ± 0.7 g/day | 6 months |
| Beulens et al. 2015 [59] | Investigating B12 deficiency and metformin | 550 T2DM patients | <148 pmol/L | Deficiency in 28.1% of patients | 1 mg daily dose escalation = 0.042 pg/mL reduction in serum B12 | No association |
| Ko et al. 2014 [132] | Investigating B12 deficiency and > 3 months of metformin treatment | 799 T2DM patients | ≤300 pg/mL | Deficiency in 9.5% of patients, n = 76 | >1000 mg/d | >4 years |
| Gatford et al. 2013 [111] | Investigating vitamin B12 deficiency and metformin during pregnancy compared with insulin treatment | 180 GDM patients: metformin (n = 89) vs. insulin (n = 91) | <148 pmol/L | No association | Treated with up to 2.5 g/day | No association |
| Tomkin et al. 1971 [133] | Assessment of vitamin B12 in patients taking long-term metformin therapy | 71 patients with diabetes | <190 pg/mL | 29.6% had vitamin B12 malabsorption | 1.97 g/day | Not assessed |
| Reference | Subjects | Duration of Metformin Treatment | Dose of Metformin | Effect on Hcy, B12, and Folate |
|---|---|---|---|---|
| Esmaeilzadeh et al. 2017 [112] | 18 females with PCOS | 6 months | 500 mg twice daily | Hcy ⇔ Serum folic acid ⇔ Serum vitamin B12 −20% |
| Aroda et al. 2016 [58] | 1800 patients participating in the Diabetes Prevention Program (DPP)/DPP Outcomes Study (DPPOS) - 1217 female - 583 male | 3.2 years plus an additional 9 years in selected cohort | 850 mg twice daily | Vitamin B12: −10% at year 1; ⇔ at year 9 Hcy: + 5% at year 1; ⇔ at year 9 |
| Malaguarnera et al. 2015 [69] | 231 T2DM - 111 female - 120 male | 8.2 ±4.6 years | Not documented | Plasma Hcy + 58.1% Plasma folic acid − 34.1% RBC folate − 37.6% |
| Sahin et al. 2007 [140] | 165 T2DM - 99 female - 66 male | 6 weeks | One to two tablets of 850 mg per day | Plasma Hcy + 19.6% Plasma folic acid − 11% |
| Pongchaidecha et al. 2004 [141] | 152 T2DM | 6 months | Not documented | Plasma Hcy ⇔ Serum folic acid ⇔ Serum vitamin B12 − 27% |
| Wulffele et al. 2003 [142] | 353 T2DM - 186 female - 167 male | 16 weeks | One to finally three tablets of 850 mg per day if tolerated | tHcy + 4% Serum folate − 7% |
| Carlsen et al. 1997 [143] | 60 non-diabetic males with CVD | 12 and 40 weeks | One group received up to 2000 mg metformin per day | tHcy: + 7.2% at 12 wks; + 13.8% at 40 wks Serum folate: ⇔ at 12 wks; − 8% at 40 wks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, M.D.; Baker, B.C.; Scott, E.M.; Forbes, K. Interaction between Metformin, Folate and Vitamin B12 and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies. Int. J. Mol. Sci. 2021, 22, 5759. https://doi.org/10.3390/ijms22115759
Owen MD, Baker BC, Scott EM, Forbes K. Interaction between Metformin, Folate and Vitamin B12 and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies. International Journal of Molecular Sciences. 2021; 22(11):5759. https://doi.org/10.3390/ijms22115759
Chicago/Turabian StyleOwen, Manon D., Bernadette C. Baker, Eleanor M. Scott, and Karen Forbes. 2021. "Interaction between Metformin, Folate and Vitamin B12 and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies" International Journal of Molecular Sciences 22, no. 11: 5759. https://doi.org/10.3390/ijms22115759
APA StyleOwen, M. D., Baker, B. C., Scott, E. M., & Forbes, K. (2021). Interaction between Metformin, Folate and Vitamin B12 and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies. International Journal of Molecular Sciences, 22(11), 5759. https://doi.org/10.3390/ijms22115759






